INTRODUCTION
Group A streptococcus (GAS) is responsible for a wide range of diseases ranging from simple pharyngitis to life-threatening toxic shock. The last 20 years have seen a global increase in both the incidence and severity of invasive GAS (iGAS) infections [Reference Lamagni1–Reference Ben-Abraham9] particularly in the elderly and the very young [Reference O'Grady5, Reference O'Brien7, Reference Tyrrell10, Reference Lamagni11]. There are few studies of iGAS in Australia [Reference O'Grady5, Reference Delvecchio12, Reference Carapetis13] and none reporting specifically on the paediatric age group. Australian data suggest that there is a higher incidence of iGAS in the Aboriginal population in Northern Australia with up to 82 cases/100 000 [Reference Carapetis13, Reference Norton14]. Queensland (QLD) is the only state in Australia which has mandatory reporting of iGAS despite a recent study emphasizing the need to include these infections as a notifiable condition in Victoria [Reference O'Grady5]. Mandatory reporting in QLD commenced in December 2005 and this generally occurs from laboratories to QLD Health.
QLD includes both tropical and subtropical climates and covers more than 1·7 million km2. The population (4·32 million) is decentralized with the majority living in the southeast corner of the state in large urban centres; there are also remote communities spread across the north and west of the state. The geographic spread of the population is challenging for delivery of healthcare and accurate gathering of health statistics. Recently in QLD there has been a perception of an increased incidence of iGAS in both adults and children, with more frequent admissions and deaths reported. This concern prompted this study to investigate the epidemiology, clinical features and outcomes of iGAS in children in this state.
METHODS
Population data
Population data for QLD were sourced from the Australian Bureau of Statistics (ABS). The data divided into 1-year age groups were available for each of the years of the study (2004–2009) but data for the Indigenous population stratified by year group was only available for 2006. The 2006 Indigenous population data were therefore used to calculate the annualized incidence rates for iGAS in this population for the total study period. The approximate non-Indigenous annualized incidence rates were derived for each of the years by subtracting the Indigenous population for 2006 from the total population for each year. Incidence data were calculated using population data specific to the relevant age group.
Definitions
QLD Health definition of iGAS was used; defined as isolation of GAS from a usually sterile site. Streptococcal toxic shock syndrome (STSS) was defined by criteria as outlined in consensus guidelines from 1993 [Reference Breiman15]. Ethnicity was reported as Indigenous [Aboriginal or Torres Strait Islander (ATSI)] or non-Indigenous. Current practice in QLD hospitals is to only identify ethnicity of patients if they are Indigenous; no ethnic identification is made in hospital records for non-Indigenous peoples.
Data collection
Identifying data were requested from QLD Health for all patients notified with iGAS between January 2004 and May 2009. Medical records were obtained from the treating hospitals for each case aged ⩽18 years. Data obtained included postcode, age at iGAS isolation, ethnicity, sex, risk factors for iGAS infection, date and site of isolation, date of onset of symptoms, length of hospital stay, antibiotic use, surgical interventions, comorbidities, outcome and whether iGAS was acquired in hospital or in the community.
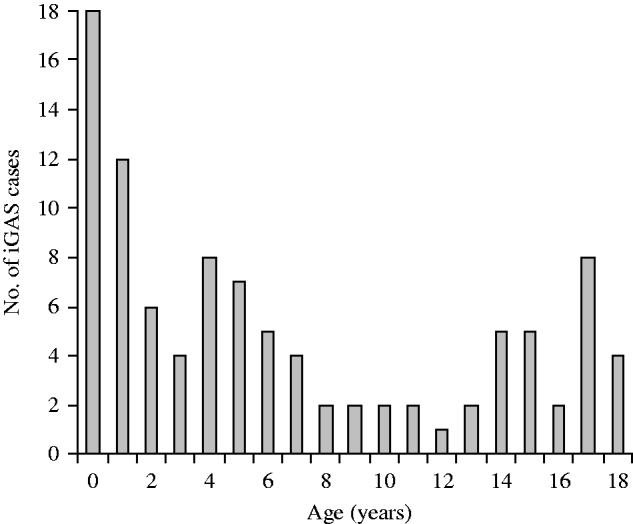
Fig. 1. Age at isolation of invasive group A streptococcus (iGAS).
Laboratory studies
In addition to the requirement for a laboratory to notify iGAS to QLD Health, all isolates of iGAS are required to be submitted to the reference laboratory at Forensic and Scientific Services (FSS) for typing.
Isolates were plated on Columbia horse blood agar and incubated for 24 h in 5% CO2. Colonies were suspended in 400 μl TE buffer for emm gene sequencing and spe exotoxin gene detection. The emm gene sequencing was performed according to the protocols at http://www.cdc.gov/ncidod/biotech/strep/protocols.htm. For spe a, b, and c toxin gene detection, multiplex PCR was performed as previously described [Reference Stanley16], with the minor modification of using AmpliTaq Gold (Applied Biosystems Roche, USA).
Statistical methods
Data analysis was performed in Stata 10.1 (StataCorp, USA).
Ethics
This study received ethical approval from the Research Ethics and Governance Unit Human Research Ethics Committee of Queensland Health and from the Human Research Ethics Committee of Mater Health Services.
RESULTS
Identifying data for 592 patients aged 1 day to 99 years were supplied by QLD Health. One hundred and twenty-five patients were aged ⩽18 years at the time of isolation of iGAS and of these 13 had no record of patient location and were excluded from the study, therefore 112 patient records were requested from the treating hospitals. Two patients were classified as stillborn infants and one notification was considered to be an episode of colonization rather than invasive disease. A further 10 medical records were either not supplied by the treating hospital or supplied with inadequate details for analysis despite repeated communications with these hospitals.
Clinical records of 99 patients aged ⩽18 years were therefore examined. The number of cases notified increased between 2004 and 2008 as outlined below (Table 1). Incidence rates were not calculated for 2004, 2005 and 2006 as numbers reported were likely to greatly underestimate true incidence. Forty-eight patients were aged ⩽4 years, 18 were aged ⩽1 year and 11 were aged ⩽3 months (Fig. 1). Twenty-four cases were of Indigenous origin. In this Indigenous subgroup, 16 (67%) were aged ⩽4 years, 11 (45%) were ⩽1 year and six (25%) were aged ⩽3 months. The annualized incidence of iGAS in 2008 in Indigenous infants aged 0–1 year was 123·34/100 000 and in non-Indigenous infants it was 12·23/100 000. There were regional variations in the distribution of cases of iGAS; 66 were located in South East QLD, 26 in North QLD (including Cape York and Torres Strait) and seven in Central QLD. There were 66 males and 36 females. Cases of iGAS occurred evenly across the year and no seasonal pattern was identified.
Table 1. Annualized incidence of invasive group A streptococcus per 100 000 of population in Queensland
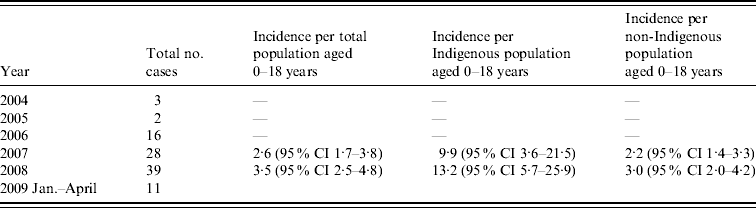
CI, Confidence interval.
Sites of infection were blood (66), joint (15), deep tissue abscess (7), pleural fluid (4), lymph node (2), tonsil (1), muscle (1) cartilage (1), CSF (1) and peritoneal fluid (1). There were six cases of STSS and one of necrotizing fasciitis. Fourteen cases were admitted to intensive care. Comorbidities were identified in 17 patients and included prematurity (2), spina bifida (2), systemic lupus erythematosus, malignancy, end stage renal failure, recurrent respiratory infection, short gut syndrome, cystic fibrosis, failure to thrive, hepatitis B carrier, autoimmune haemolytic anaemia, congenital CMV, newborn, intellectual impairment and renal abnormalities. In five cases comorbidity data were not available.
Potential risk factors were identified in 45 cases; 26 had disruption to the skin including, scabies (6), impetigo (5), preceding varicella infection (4), penetrating trauma (4), non-specific skin lesions (3), eczema (2), and one each of injecting drug use and pressure sores. One case with preceding varicella infection developed STSS and died. Twelve cases had experienced blunt trauma such as a fall or sporting injury prior to the onset of iGAS. Of these five developed bacteraemia, four septic arthritis, two deep tissue abscess and one, necrotizing fasciitis. There were seven cases with concurrent pharyngitis. Of cases with scabies, five were in the 0–1 year age group and four were Indigenous. No nosocomial infections were identified.
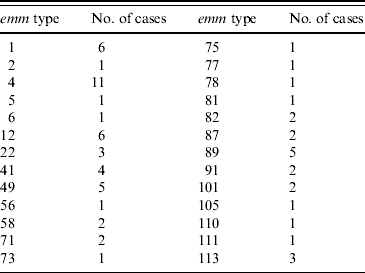
Seventy-two of the 99 isolates had been sent to the reference laboratory for typing. This laboratory had also been sent an additional 47 isolates from patients aged ⩽18 years that had not been notified to QLD Health from the same time period. Sixty-seven isolates had emm typing performed and 26 different emm types were identified. emm type 4 was the most common accounting for 11 (16%) isolates and 12 emm types were each represented by single isolates (Table 2). emm type 4 was not associated with more severe iGAS infection. SPE typing of the same 72 isolates revealed type B (28), BA (13), BC (30) and unidentified (1). In the six STSS cases the following emm types were identified: type 4 (1), type 12 (1), type 1 (1), type 5 (1) and untyped (2).
Table 2. emm types identified in 72 invasive group A streptococcus isolates in Queensland
Antibiotics were given in all cases but precise data on timing and duration of courses was not consistently available. β-Lactam antibiotics were the most commonly prescribed (62 patients); 51 received flucloxacillin and 18 received clindamycin. In seven cases a single antibiotic was used and 18 cases were given antibiotics prior to admission to hospital. In one case STSS antibiotics were not able to be given prior to death. In the remaining cases multiple different antibiotics were used. In particular clindamycin was administered to two STSS patients. Intravenous immunoglobulin (IVIg) therapy was administered in one case of STSS in combination with penicillin and clindamycin.
The overall mortality was 4/99 and all deaths were attributed to STSS which had a mortality of 67%. At the time of discharge from hospital a full recovery was expected in 82% of cases and a partial recovery was anticipated in 4% of cases. One case had a surgical amputation and the remaining three had debridement of necrotic soft tissue. The outcome was unknown in 10% of cases and the average length of stay in hospital was 9·77 days (range 1–84 days).
DISCUSSION
This study provides the first detailed description of the epidemiology of iGAS in a paediatric population in Australia. It is evident from the data that there is marked underreporting of iGAS in QLD as numbers reported from different health districts with similar populations differed markedly and a large number of isolates sent to the reference laboratory for typing during the study period were not reported to QLD Health. In order to report more accurate and complete incidence data it would be necessary to include both review of hospital discharge records and review of reports to both QLD Health and the reference laboratory. Hospital discharge record review would also facilitate identification of iGAS infections such as pharyngeal abscess and necrotizing fasciitis which may not be associated with the isolation of GAS from a normally sterile site and hence may not have been reported to QLD Health. However, using the available data, the annualized minimum incidence rate for the paediatric population, 3·53/100 000 (in 2008) is higher than that published for Victoria during 2002–2004 (2·7/100 000) [Reference O'Grady5] but lower than the rate reported for the Northern Territory (NT) in 1999 [GAS bacteremia 9·3/100 000; Indigenous (23·8/100 000); non-Indigenous (4·7/100 000)] [Reference Carapetis13]. QLD incidence is comparable with internationally reported all-age incidences 3·33–5·0/100 000 [Reference O'Brien7, Reference Tyrrell10, Reference Lamagni11, Reference Laupland17, Reference Moses18] and slightly higher than that reported in Canadian children (1·9/100 000) [Reference Laupland19].
As reflected in previous Australian studies [Reference Steer, Danchin and Carapetis3, Reference Norton14], the rate of invasive disease is higher in the Indigenous population, although not as high as past reports from QLD and NT [Reference Carapetis13, Reference Norton14]. The predominance of iGAS in children aged ⩽4 years (48% of cases) and particularly in infancy (18% of cases) is in accord with previous studies [Reference Davies4, Reference O'Brien7, Reference Lamagni11, Reference O'Loughlin20]. This is probably due to immature immunological defence in combination with a possibly higher carriage rate of GAS. This trend is more marked in the Indigenous population especially in infants (2008 annualized incidence of iGAS in Indigenous infants 123·34/100 000 vs. 12·23/100 000 in non-Indigenous infants.) Further studies are needed to investigate possible causes of this inflated incidence. There are potential links with scabies incidence (in this study four of the 11 children of Indigenous origin had concurrent scabies infection compared to one of seven of non-Indigenous children in the same age group). Preventative strategies should be targeted towards these risk groups.
Surprisingly no seasonal pattern was identified. An increase in cases in winter and spring has been consistently reported in other studies [Reference Lamagni1, Reference Tyrrell10, Reference Laupland17]. The lack of seasonal variation may reflect the tropical and subtropical climate in QLD. It is possible that seasonal influences seen in northern hemisphere populations, such as low humidity and reduced sunlight, do not apply in QLD. Two thirds of the reported cases were male. This is not easily explained but has been previously noted [Reference Lamagni11, Reference Duff21].
The suspected increase in incidence and severity of disease during the past 5 years was not confirmed by our study. The case-fatality rate was 4% rising to 67% for STSS. Of concern is the lack of consistency in clindamycin (2/6) and IVIg (1/6) use in cases of STSS as clindamycin and IVIg have been shown to be beneficial in this situation [Reference Arnholm, Lundqvist and Stromberg22–Reference Mulla, Leaverton and Wiersma25]; this suggests that further education of health professionals is needed in regard to this matter.
There was a low proportion of children (4/99) with varicella-associated iGAS. This low rate is similar to that reported in Victoria [Reference O'Grady5] but much lower than the 15–30% reported elsewhere [Reference Laupland19, Reference Burnett and Domachowske24, Reference Tyrrell26, Reference Ciftci27]. This may be as a result of the introduction of funded universal immunization for varicella in QLD in November 2005 [Reference Patel, Binns and Shulman28].
Blunt trauma preceded 12% of cases which is consistent with the recent study from Florida where blunt trauma was significantly associated with the development of GAS necrotizing fasciitis [Reference Nuwayhid, Aronoff and Mulla29]. The postulated mechanism involves the increased production of a GAS binding protein (vimentin) on the surface of damaged skeletal muscle [Reference Bryant30]. The relationship between blunt trauma and the onset of iGAS warrants further investigation.
Molecular typing of group A streptococcal strains is used as an epidemiological tool in tracking of particular strains. Contrary to initial suggestions, the emm type is not thought to be related to the ability of a particular organism to cause disease [Reference Rogers6]. Further analysis of superantigens using SPE testing is performed in QLD although current testing does not include all existing SPE types. In previous reports from diverse geographical areas throughout the world no emm type has predominated [Reference Rogers6, Reference Norton14]. In this study emm type 4 was the most common; however, this may not be significant as only 72/99 cases were typed. An association between SPE type A and STSS has previously been suggested [Reference Musser31], although not confirmed [Reference Norton14]. We found no association between SPE type and disease severity.
Our study has some limitations. Significant underreporting was revealed, despite the study not being specifically designed to detect this and thus the true incidence of iGAS in children in QLD is underestimated. In addition, not all of the identified cases had accessible clinical records resulting in an incomplete dataset. Many isolates were not submitted for typing making accurate predictions about the relationship between the organism type and severity of disease unreliable.
Nevertheless, we have shown that iGAS infection results in significant morbidity and mortality in children in QLD with infants of the Indigenous population having an increased risk. More complete reporting needs to be encouraged and further studies incorporating methods to identify all iGAS in QLD, such as use of hospital discharge diagnosis, would be of value. Studies aimed at identifying specific risk factors for acquisition of iGAS in the Indigenous population are warranted to inform the design of preventative strategies. Moreover, the male sex preponderance, the lack of seasonal variation and the association with blunt trauma need to be confirmed and further investigated.
ACKNOWLEDGEMENTS
We acknowledge the support of the Mater Research Support Centre in providing statistical and technical assistance in the performance of this study. We also thank Dr Brad McCall and Dr Christine Selvey of Queensland Health for guidance and support.
DECLARATION OF INTEREST
None.





