Introduction
Nontuberculous mycobacteria (NTM) represent a group of opportunistic human pathogens that are normal inhabitants of the environment, especially of the human-engineered environment [Reference Falkinham1, Reference Falkinham2]. A worldwide increase in the prevalence of human NTM infections has been documented as population-based data in recent decades [Reference Hoefsloot3, Reference Marras4]. According to a recent report, the annual incidence of NTM infection increased from 6.0% to 19% in East Asians from 2008 to 2016 [Reference Lee5]. NTM are important causes of pulmonary and extrapulmonary diseases in at-risk populations, such as immunocompromised hosts, the elderly and in patients with cystic fibrosis [Reference Falkinham6]. Despite the controversy regarding the occurrence of person-to-person spread of NTM-related diseases, recent molecular epidemiological data suggest that pulmonary NTM infections are mainly acquired through transmission of bacteria via fomites and aerosols [Reference Bryant7, Reference Nishiuchi, Iwamoto and Maruyama8], highlighting the need for public health agencies to dedicate additional resources to the control of pulmonary NTM disease transmission.
Recently, increasing numbers of reports from diverse countries and regions have demonstrated that the relative proportions of NTM species isolated from clinical samples differ significantly by region [Reference Stout, Koh and Yew9]. For example, the Mycobacterium avium complex (MAC) has been predominantly detected in patient isolates from North America, East Asia and northern Europe [Reference Stout, Koh and Yew9–Reference Simons11], whereas M. kansasii and M. xenopi have been more frequently isolated within southern Europe [Reference Stout, Koh and Yew9]. Meanwhile, a recent cross-sectional study has revealed that NTM prevalence and distribution vary greatly across China [Reference Pang12]. M. intracellulare, a member of MAC, is predominately isolated in eastern China, whilst M. abscessus is predominant in southern China [Reference Pang12]. In view of intrinsic differences in pathogenicity and drug susceptibility profiles among various NTM species, understanding this diversity is necessary to guide clinical treatment [Reference Aksamit, Philley and Griffith13]. Unfortunately, NTM has not been earmarked as an infectious disease priority worldwide, especially in tuberculosis (TB)-endemic settings [Reference Raju14].
The prevalence of NTM infection is rising in China and currently accounts for approximate one-quarter of mycobacterial isolates according to national population-based data [Reference Wang15, Reference Zhang16]. Despite this growing challenge, reporting of NTM disease to public health authorities is not required in China. As a consequence, only limited studies have been conducted to assess temporal changes in the incidence and prevalence of NTM diseases in this county. To address this concern, we carried out a retrospective study in Hangzhou, located in the eastern coastal region of China. Our objective was to investigate the prevalence of pulmonary NTM diseases among individuals with suspected TB. We also aimed to determine risk factors associated with NTM infections in the population within this region.
Materials and methods
Study design and population
This study was conducted at the Affiliated Hospital of Hangzhou Normal University, a 1500-bed general hospital in Hangzhou, China. We retrospectively reviewed the medical records of individuals with suspected TB seeking care at the hospital between July 2018 and December 2018. Patients presenting with pulmonary TB symptoms who were presumed to have TB disease caused by Mycobacterium tuberculosis (MTB) were each asked to provide two sputum samples for laboratory testing. Routine tests included microscopic examination, mycobacterial culture and/or molecular diagnostic testing. Patients with positive mycobacterial culture test results were recruited for further analysis. This study was approved by the Ethics Committee of the Affiliated Hospital of Hangzhou Normal University.
Laboratory examinations
Microscopy analysis of Ziehl-Neelsen (Z-N)-stained direct smears of sputum samples was conducted based on National Tuberculosis Control Programme guidelines developed in China [Reference Xia17]. Then 1 mL of sputum was analysed using the GeneXpert MTB/RIF (Xpert) assay (Cepheid, Sunnyvale, CA, USA) following the manufacturer's instructions [Reference Tang18]. The remainder of each sputum specimen was decontaminated via the N-acetyl-L-cysteine-NaOH method using the standard recommended final 1% NaOH concentration for 15 min. After decontamination, all specimens were neutralized with phosphate-buffered saline (PBS, pH = 6.8) and centrifuged at 4000 × g for 15 min. Pellets were resuspended in 2 mL of PBS then 0.5 mL of each decontaminated specimen was inoculated into a mycobacteria growth indicator tube (MGIT) supplemented with oleic acid, albumin, dextrose and catalase (OADC) and polymyxin B, amphotericin B, nalidixic acid, trimethoprim and azlocillin (PANTA) (BD Diagnostic Systems, Sparks, MD, USA) [Reference Pang19]. Cultures were assessed for contamination with other pathogens via observation of colony morphologies and via Z-N staining followed by microscopic examination. An immunochemistry-based MPT64 antigen detection test (Genesis, Hangzhou, China) was performed to identify MTB-positive cultures [Reference Pang20]. MTB-positive cultures were subjected to phenotypic drug susceptibility testing (DST) using the proportional method, as previously reported [Reference Pang21].
Species identification
Crude genomic DNA was extracted from positive cultures grown in MGIT tubes [Reference Pang19]. Briefly, an aliquot (500 µL) of sample was pipetted from the bottom of each positive MGIT culture. Next, each sample was centrifuged at 12 000 × g for 15 min then the bacterial pellet was resuspended in 500 µL of Tris-EDTA (TE) buffer. After incubation at 95 °C for 30 min in a water bath, each supernatant was used as the DNA template for species identification. A DNA fragment of the 16S rRNA gene (containing a region of sequence differences between NTM and MTB) was first amplified to distinguish between MTB and NTM. For NTM isolates, multilocus sequence analysis was performed to classify bacterial isolates into various subspecies [Reference Zhang16]. Information on the primers is listed in Table S1. Briefly, the 50 µL polymerase chain reaction (PCR) mixtures contained 25 µL 2 × PCR mixture (TransGen Biotech, Beijing, China), 0.2 µM of each primer set and 5 µL DNA template. The PCR was performed under conditions: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 58 °C for 1 min and extension at 72 °C for 2 min, and final extension at 72 °C for 10 min. For mixed infections, clones were plated to allow the growth of individual colonies on Middlebrook 7H11 agar medium plates (Difco Laboratories, Detroit, MI, USA) then bacteria lifted from individual colonies were cultured at 37 °C before species identification was conducted via multilocus sequence analysis. Amplicons were sent to Tsingke Company (Beijing, China) for DNA sequencing. Nucleotide sequences were aligned to homologous sequences of reference mycobacteria using the online BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST).
Definitions
The definition of pulmonary NTM disease met criteria established by the American Thoracic Society (ATS) in 2007: (i) clinical symptoms and abnormal chest radiograph suggestive of pulmonary TB; (ii) isolation of the same NTM species from two sputum specimens and (iii) exclusion of other differential diagnoses [Reference Griffith22]. The definition of mixed infections with MTB and NTM met the criteria as follows: (i) clinical symptoms and abnormal chest radiograph suggestive of pulmonary TB and (ii) the presence of both MTB and NTM in each sputum sample obtained from each of two separate sputum specimens.
Statistical analysis
Demographic and clinical characteristics were collected from electronic medical records and included gender, age, ethnicity, place of residence, previous TB episode(s) and comorbidities. All collected data were entered into Epi Data version 3.1 (EpiData Association, Odense, Denmark). Careful data entry was carried out and data was cross-checked by two independent researchers to ensure data quality. All variables were tested for association with pulmonary NTM diseases using univariate logistic analysis. Associations between NTM diseases and predictor variables were expressed using odds ratios (ORs) with a 95% confidence interval (95% CI). Differences were considered statistically significant if P < 0.05. All analyses were conducted using SPSS version 20.0 (SPSS Inc, Chicago, USA).
Results
Enrollment of patients
In total, 1208 individuals with suspected pulmonary TB who were retrospectively included in this study sought health care at the Affiliated Hospital of Hangzhou Normal University between July 2018 and December 2018. By reviewing smear, liquid culture and demographic data, 818 (67.7%) were excluded from further analysis, including 15 (1.2%) who failed to produce qualified sputum, 764 (63.2%) with smear-negative/culture-negative results and 11 (0.9%) with contaminated culture results. Ultimately a total of 390 (32.3%) individuals were enrolled in this study. Of these cases, 358 (358/390, 91.8%) were shown to be infected with MTB, 24 (24/390, 6.2%) with NTM and eight (8/390, 2.0%) were coinfected with MTB and NTM (Fig. 1).
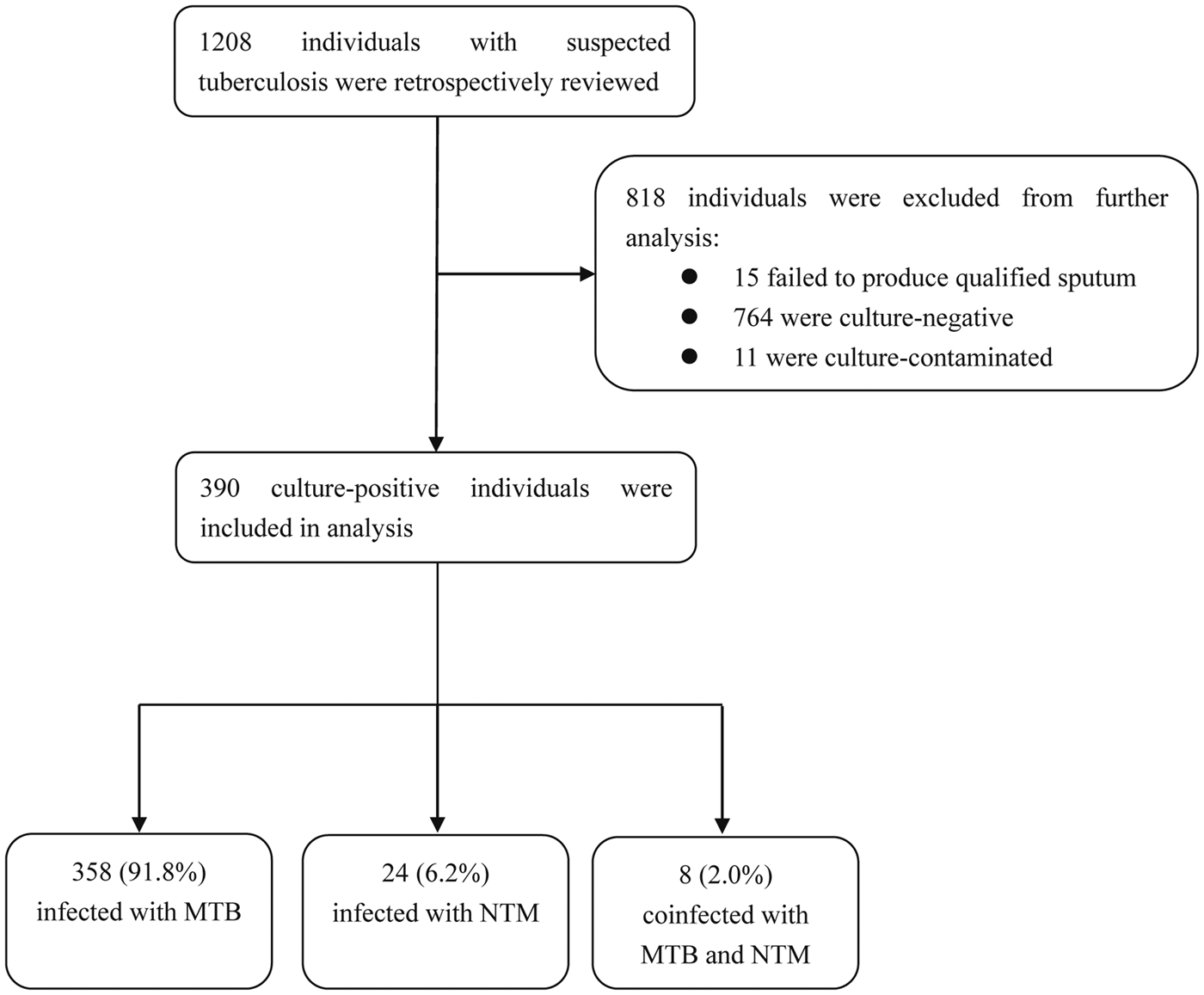
Fig. 1. Enrollment of patients in this study
Distribution of NTM species
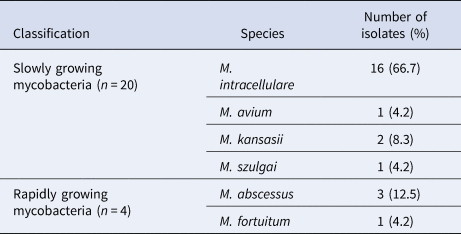
A total of 24 NTM isolates were identified by multilocus sequence analysis using online BLAST tools provided by NCBI. As shown in Table 1, 20 isolates (83.3%) were classified as slowly growing mycobacteria, while the remaining 4 were rapidly growing mycobacteria. The most prevalent species was M. intracellulare (16 isolates), accounting for 66.7% of all NTM isolates in Hangzhou. M. abscessus (three isolates) was the second most frequently isolated species, followed by two M. kansasii isolates, one M. avium isolate, one M. szulgai isolate and one M. fortuitum isolate.
Table 1. Demographic and clinical characteristics of mycobacteria infections in this study
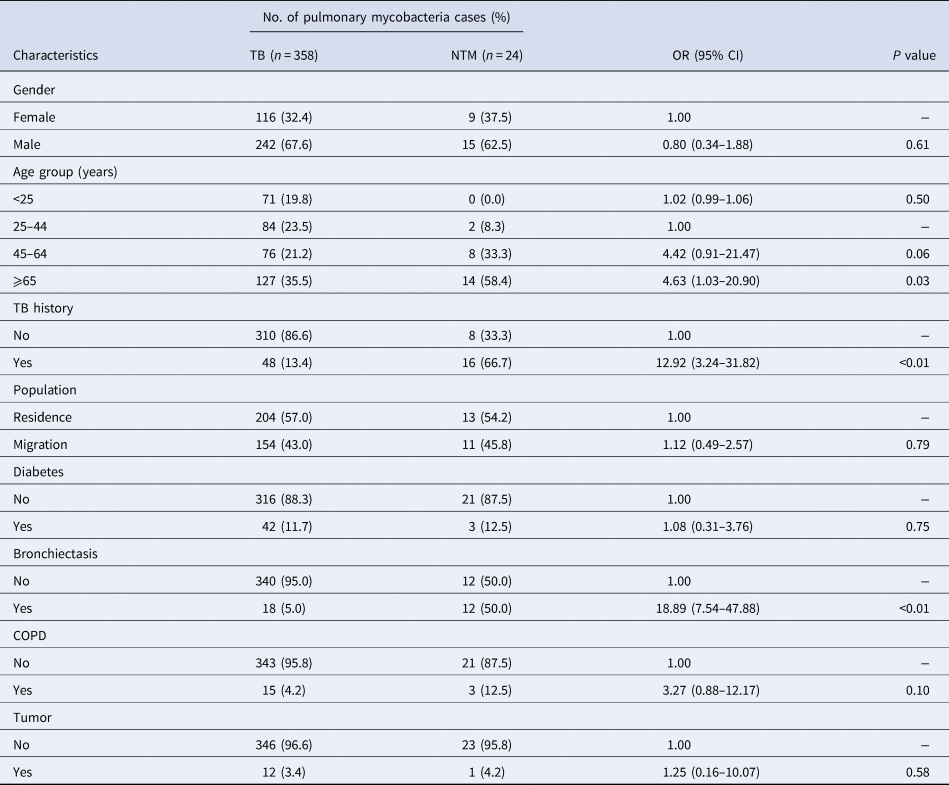
COPD, chronic obstructive pulmonary disease; OR: odds ratio; 95% CI: 95% confidence interval.
Risk factors associated with NTM infections
Comparisons of demographic and clinical characteristics between TB and NTM patients are summarized in Table 2. Using the group of patients aged 25–44 years as a reference, the per cent rate of NTM cases within the ⩾65 years age group significantly exceeded that of TB cases in that group (OR (95% CI): 4.63 (1.03–20.90), P = 0.03), while no significant differences in NTM vs. TB case per cent rates were noted for groups of patients aged <25 years and 45–64 years (P > 0.05). We also found that the prevalence of NTM infection was significantly higher in patients with history of TB disease than in those lacking TB history (OR (95% CI): 12.92 (3.24–31.82), P < 0.01). In addition, patients with pulmonary NTM diseases were significantly more likely to exhibit underlying bronchiectasis than were those with pulmonary TB diseases (OR (95% CI): 18.89 (7.54–47.88), P < 0.01). Meanwhile, no other differences were found based on documented gender, residence or other comorbidities between TB and NTM groups (P > 0.05).
Table 2. Distribution of NTM species isolated from pulmonary NTM patients in Hangzhou
Patients coinfected with NTM and TB
Here, we further analysed the demographic, clinical and laboratory test results of eight patients coinfected with MTB and NTM. Of these patients, the median age at initial diagnosis was 70 years (range 52–88 years) and 75.0% were male. Five patients (62.5%) were local residents and five patients (62.5%) had previous anti-TB treatment history. In addition, half (4/8) of patients exhibited bronchiectasis. Notably, all patient smears were positive for acid-fast bacilli and all were infected with RIF-susceptible MTB as determined from results of the Xpert assay. Further in vitro DST confirmed that seven cases (87.5%) were extensively drug-resistant TB cases. Species identification revealed that six cases were coinfected with MTB and M. intracellulare, one case was coinfected with MTB and M. abscessus and one case was coinfected with MTB and M. kansasii (Table 3).
Table 3. Demographic, clinical characteristics and laboratory examination results of eight patients coinfected with MTB and NTM in this study
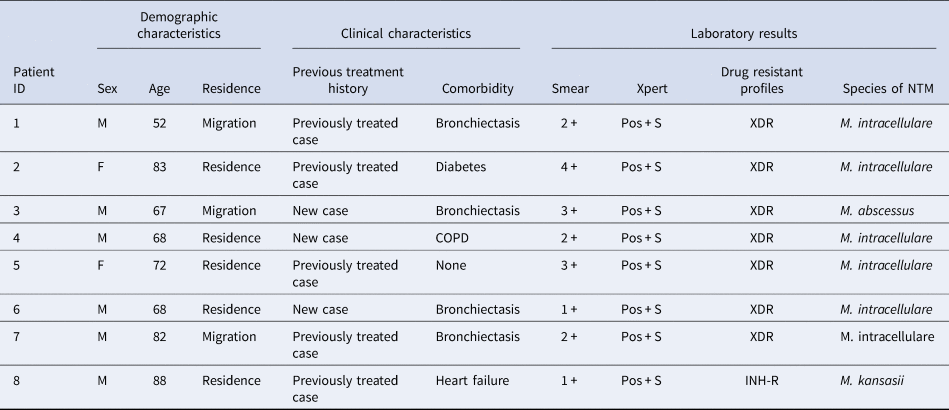
M, male; F, female; COPD, chronic obstructive pulmonary disease; Pos, positive; S, RIF susceptible; XDR, extensively drug-resistance; INH-R, isoniazid-resistance; NTM, nontuberculous mycobacteria.
Discussion
The increasing prevalence of pulmonary NTM disease is a neglected public health concern in China [Reference Zhang16]. In this study, our data indicate that approximately one-tenth of suspected TB patients with positive mycobacterial culture results were infected with NTM, for an overall NTM prevalence of 6.2% among suspected pulmonary TB patients seeking medical care in Hangzhou. In several previous studies, NTM prevalence rates of various regions had been reported as follows: Shandong (1.6%), Jiangsu (3.4%), Shanghai (5.1%), Europe (6.6%) and Israel (10.8%) [Reference Jing23–Reference Levy27]. Such diverse results obtained in those studies indicate that prevalence of NTM disease may differ from one geographic region to another. In China, initial diagnosis and treatment of pulmonary TB is based on positive sputum smear results obtained using microscopic detection of mycobacteria, especially in resource-limited settings [Reference Pang28]. Given that NTM strains are intrinsically resistant to first-line anti-TB drugs, our findings highlight the urgent need to perform species-level identification of bacilli detected in smear-positive patient samples prior to initiating anti-TB treatment, especially in this coastal region of eastern China, a resource-limited setting. However, conventional biochemical methods require about one to two months to produce reliable results, which would delay treatment and thus would fail to meet clinical requirements for optimal TB care [Reference Chimara29]. As molecular diagnostics may provide more rapid identification of MTB and NTM, these techniques should be evaluated for use in resource-limited settings. The ability to achieve bacterial identification in just one day after primary detection of acid-fast bacilli would greatly facilitate the delivery of swift and accurate treatment of MTB and NTM diseases.
Another interesting finding of this study is the occurrence of mixed infections with MTB and NTM among patients with presumptive TB, although coinfections were observed in only a small proportion of cases. This result aligns with the reported results obtained from surveys of TB clinics in Japan conducted by Shigeto and colleagues demonstrating that NTM was a complication in only 1.2% of new registered TB cases there [Reference Kendall30]. In contrast, a population-based study in the United States demonstrated that one in seven confirmed pulmonary TB patients were coinfected with NTM in 2006 [Reference Kendall30]. Notably, in the latter study NTM were often isolated soon after initial TB diagnosis [Reference Kendall30]. As it appears to be difficult to distinguish between coinfection at initial diagnosis and subsequent NTM infection during the follow-up period, the methodology used in that study may potentially explain the extremely high rate of NTM coinfection among active TB cases in the United States. Consequently, our findings also have important clinical implications, especially due to the fact that among our study population, coinfection with MTB and NTM is a major contributor of suspected extensively drug-resistant TB cases. However, current methods in widespread clinical use are unable to distinguish among multiple species of mycobacteria present within a single collected specimen. Fortunately, recent advances in next-generation sequencing (NGS) have ushered in new opportunities for identifying all bacterial pathogens within a sample [Reference Shokralla31]. Nevertheless, further studies are required to evaluate NGS performance in improving the resolution of bacterial species identification in specimens collected from patients coinfected with TB and NTM.
This study demonstrates that the predominant NTM species isolated from prospective TB patients in Zhejiang is M. intracellulare, which aligns with results of recent studies from Shanghai [Reference Pang12]. In contrast, M. abscessus was the most frequently isolated NTM from prospective TB patients in Guangdong [Reference Pang12]. We therefore hypothesize that geographical variations in the prevalence and specific characteristics of causative organisms of NTM infections may reflect environmental NTM diversity across China. In addition, here we identified several risk factors associated with NTM diseases. First, in line with findings of previous studies [Reference Huang32, Reference Dailloux33], pulmonary NTM disease is more common among elderly people, which may result from decreased immune function in older patients [Reference Tan34]. Second, patients previously diagnosed with TB are at greater risk of pulmonary NTM disease. Although the exact reasons for this phenomenon remain unclear, we speculate that lung cavitations due to previous TB activity may provide an infection niche for NTM. Third, NTM infections occur more frequently in patients with bronchiectasis. Numerous previous reports have shown an association between this structural lung disease and pulmonary NTM infections [Reference Winthrop35, Reference Chu36], with the appearance of bronchiectasis cited as an important cause of impaired mucociliary clearance of pathogens from the bronchial tree [Reference Chu36]. The lack of pathogen clearance subsequently increases the risk of NTM colonization within the respiratory tract, thereby contributing to the high prevalence of pulmonary NTM diseases in populations with underlying bronchiectasis.
Numerous weaknesses in this report warrant discussion here. First, this study only analysed mycobacterial isolates from one pilot study site in Hangzhou. Despite being the sole TB designated hospital in that region, the small sample size in this study likely weakens the statistical strength of our conclusions. Second, as HIV status is an important risk factor for NTM infections [Reference Tan34], all patients enrolled in this study were tested for HIV and were HIV-negative, probably due to low-HIV prevalence in Hangzhou. Thus, we could not evaluate the association between HIV status and NTM infection in this population. Third, although we isolated both MTB and NTM from coinfected patients, evaluation of the clinical relevance of these pulmonary NTM isolates is difficult without conducting long-term follow-up of coinfection cases. Future studies focusing on long-term outcomes will provide new insight into the clinical significance of concurrent isolation of NTM and MTB from patient respiratory tract specimens.
In conclusion, our data demonstrate that in the Zhejiang Province of China approximately one-tenth of patients with positive mycobacterial cultures are infected with NTM; of these cases, one-fourth are coinfected with MTB. M. intracellulare is the most frequently isolated NTM from suspected pulmonary TB patients seeking medical care in Zhejiang Province. Notably, elderly people and patients with previous TB and bronchiectasis are at greater risk of pulmonary NTM disease than other patient groups in this population. Ultimately, more attention should be paid to achieve more timely identification of MTB and NTM after primary detection of acid-fast bacilli in patient samples in order to improve patient care in NTM-endemic areas.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268819001626
Acknowledgements
This study was partially supported by the Tongzhou District Science and Technology Committee (KJ2019CX016) and National Major Project (2018ZX10103001-004).
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.






