INTRODUCTION
Haemorrhagic fever with renal syndrome (HFRS), a rodent-borne disease caused by hantaviruses, is characterized by fever, haemorrhage, headache, back pain, abdominal pain, and acute kidney injury [Reference Klein1]. In China, HFRS is mainly caused by two types of hantaviruses, one is Hantaan virus (HTNV) and another is Seoul virus (SEOV), each of which has co-evolved with a distinct rodent host. HTNV is associated with Apodemus agrarius, whereas SEOV is associated with Rattus norvegicus [Reference Fang2]. China has the highest incidence of HFRS worldwide, with reported cases accounting for 90% of total cases globally [Reference Yan3]. Shaanxi, a populous province in central China is one of the main HFRS epidemic areas [Reference Huang4], since the first case was reported in the 1950s.
HFRS is transmitted by contact with rodent urine, faeces, or saliva and is therefore closely associated with rodent population dynamics [Reference Olsson5–Reference Clement7]. Various studies have revealed a strong positive correlation between rodent abundance and HFRS cases worldwide [Reference Kallio8, Reference Olsson9]. In addition, the infection prevalence in host populations directly influences human disease dynamics [Reference Xiao10–Reference Glass12]. Thus, infection surveillance can provide insight into early warnings of potential epidemics of this disease [Reference Viel13]. However, few studies have examined the relationship between hantavirus prevalence and HFRS epidemics in the relative long term in China.
In this study, we conducted a surveillance on rodent hosts, hantavirus infection prevalence in rodents and the number of human HFRS cases between 1984 and 2012.
We aimed to examine the dynamic of endemic HFRS in Shaanxi province, and quantify the relationship between HFRS transmission and the infection of hantavirus reservoir hosts. It is expected that the findings from this study will inform the decision-making process with regard to the prevention and control of HFRS.
METHODS
Study area
The study area covered the whole of Shaanxi province (between 105° 29′ and 111° 15′ E, and 31° 42′ and 39° 35′ N), including 10 cities with an area of 205 800 km2. Shaanxi province is one of the most seriously affected areas in mainland China. Hu county in Shaanxi has been designated as a national surveillance site to monitor the epidemiological relationships between host animals and humans. The highest incidences of HFRS in Hu county and Shaanxi province were recorded as 300/100 000 and 38/100 000, respectively, in 1984.
Human HFRS cases
The data on reported HFRS cases were obtained from the National Notifiable Disease Surveillance System, which included information about sex, age, residential address, and onset date of symptoms for each case. All the HFRS cases were first diagnosed according to the clinical criteria from the Ministry of Health of the People's Republic of China, and then confirmed by detecting antibodies against hantavirus in serum samples obtained. Serum samples were collected from patients with clinical signs of HFRS and sent to the Shaanxi Centre for Disease Control and Prevention (CDC) for detection of hantavirus reactive antibodies.
Sample collection
Data on relative rodent density in spring within the HFRS endemic region have been available since 1984 in Xi'an through monitoring by CDC. There were about 11 fields selected for trapping purposes every year with one study patch per field. More than 200 traps per patch were placed in the field for three consecutive nights (every 5 m in each row, with 50 m between rows). The average distance between trapping fields was 9·05 km. Captured rodents were euthanized with ether, and their sera and lungs were collected and stored in a portable liquid-nitrogen freezer. Virus isolation was then performed in laboratory as described previously [Reference Zhang14]. Relative rodent density = (number of rodents captured/number of traps) × 100%.
Ethical review
The present study was reviewed by the research institutional review board of Shaanxi CDC. The study did not include animal experimentation, it was not necessary to obtain an animal ethics licence from the Animal Experiment Board.
Detection of hantavirus antigen
The antigens of hantavirus in lung tissues (frozen sections) were detected by direct immunofluorescence assay (DFA) between 1984 and 2012 as described previously [Reference Lee, Lee and Johnson15]. Hantavirus antibody in serum samples from captured rodents was tested for immunoglobulin (Ig) G antibodies in 2011 and 2012. The kappa test was used to evaluate the agreement in detections between lungs and serum.
Statistical analysis
Correlation analysis was performed to assess the associations between variables and HFRS cases. To determine the associations between HFRS incidence and relative rodent density, as well as prevalence rate, we performed a generalized linear model (GLM) with a Poisson distribution and a log link [Reference McCullagh and Nelder16] using the R software package (R Foundation, Austria). Adjustment for autocorrelation was also conducted. Our model for HFRS counts (Y) in the presence of overdispersion can be written as:
where i is the lag determined by autocorrelation analysis, η is the fitted model and Po is the Poisson distribution. RD and P represent relative rodent density and hantavirus prevalence rate in rodents, respectively. Prevalence of hantavirus in rodents was estimated as the number of rodents which were positive by the ELISA test divided by the number of rodents captured and tested. A stepwise method was used to include variables, as long as there was significant improvement determined by maximum-likelihood ratio. Model diagnosis was performed by pseudo-R 2 value and the residuals of the model.
RESULTS
From 1984 to 2012, more than 99 000 human cases and 2537 deaths due to HFRS (case-fatality rate 2·55%) were reported in Shaanxi province. During this period, the incidence was highest in 1984, with an average of 38/100 000. Within Shaanxi province, the highest incidence of HFRS was recorded in Hu county at 300/100 000 in 1984. A total of 7186 rodents was captured during 142 955 trap nights. The annual rodent density was 5·03%. Apodemus agrarius accounted for 93% of all captured rodents, the remainder were Cricetulus barabensis and Mus musculus.
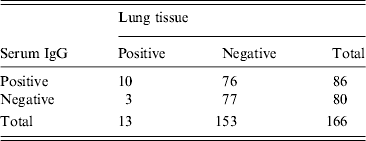
During the study period, 6928 lung tissues were tested and the overall hantavirus prevalence was 4·99% in the rodents sampled. In 2011 and 2012 a total of 366 rodent serum samples were also tested for IgG antibodies. Hantavirus antigen detection of IgG had a sensitivity of 76·92% (10/13) and a specificity of 50·33% (76/153). The detection between serum and lung tissue was in agreement in 54·21% of samples (see Table 1) (87/166) (kappa test = 0·76).
Table 1. Concordance between hantavirus detection in serum and lung tissue

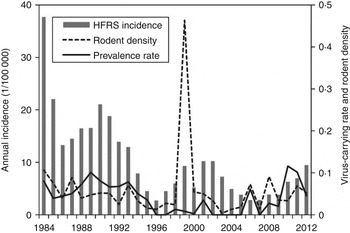
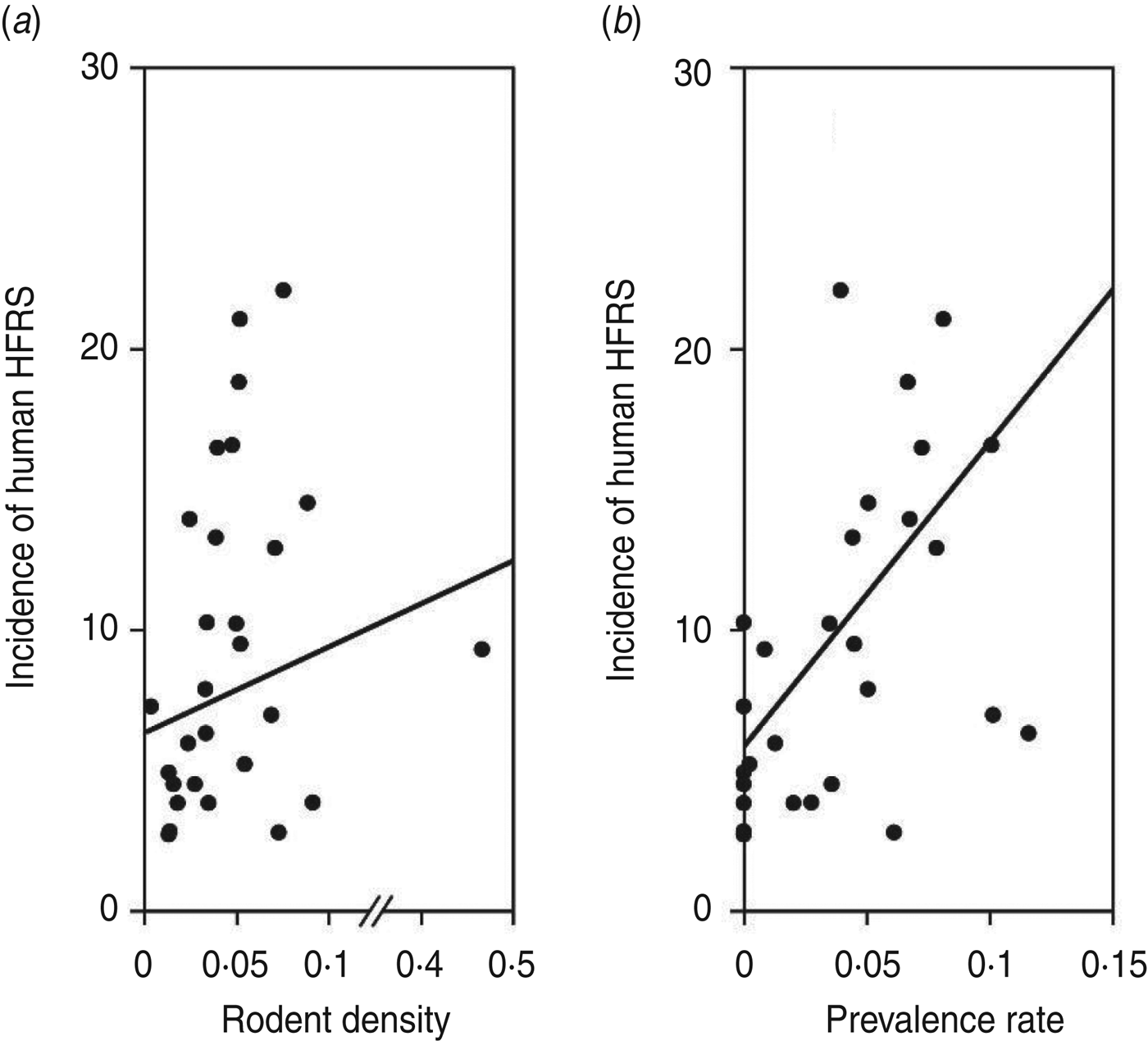
As shown in Figure 1, an 8- to 10-year epidemic cycle was noted, with high incidence lasting for 2–3 years. The incidence fluctuation was associated with the relative rodent density and prevalence rate. The results reveal strong associations between annual HFRS incidence and rodent density (Spearman's ρ = 0·444, P = 0·016), and between annual incidence of HFRS and prevalence rate (Spearman's ρ = 0·584, P < 0·001) (Fig. 2).
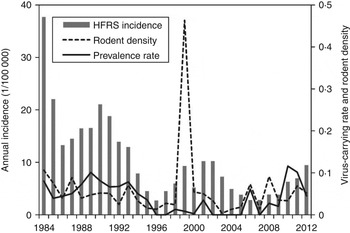
Fig. 1. Haemorrhagic fever with renal syndrome (HFRS) cases, rodent density and hantavirus infection in Shaanxi province. Grey bars denote the annual incidence of HFRS, the solid and dotted lines represent prevalence rate and rodent density, respectively.
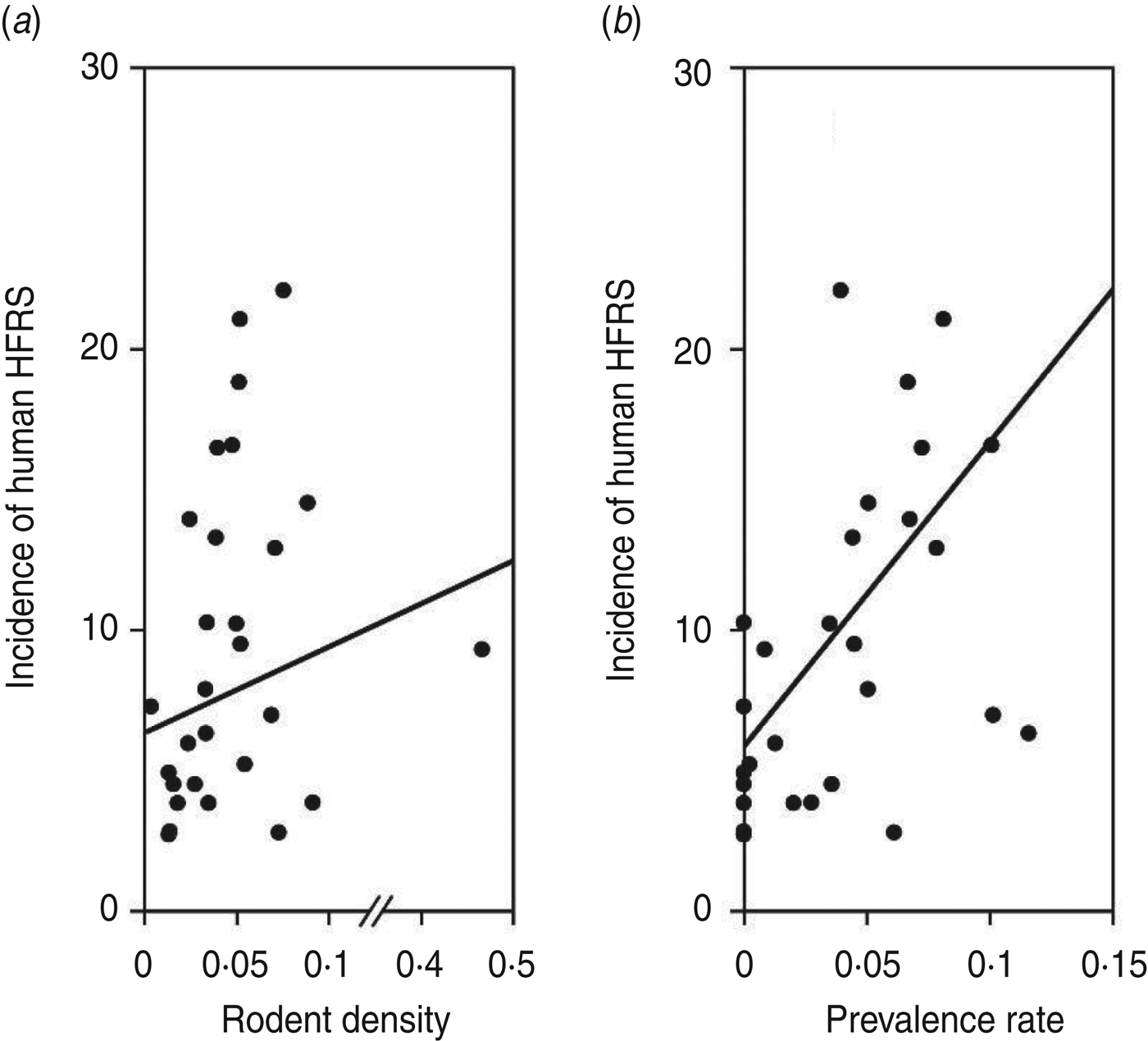
Fig. 2. Annual haemorrhagic fever with renal syndrome (HFRS) incidence, rodent density and hantavirus infection, 1984–2012. (a) Scatterplot of HFRS incidence and rodent density. (b) HFRS incidence and prevalence rate.
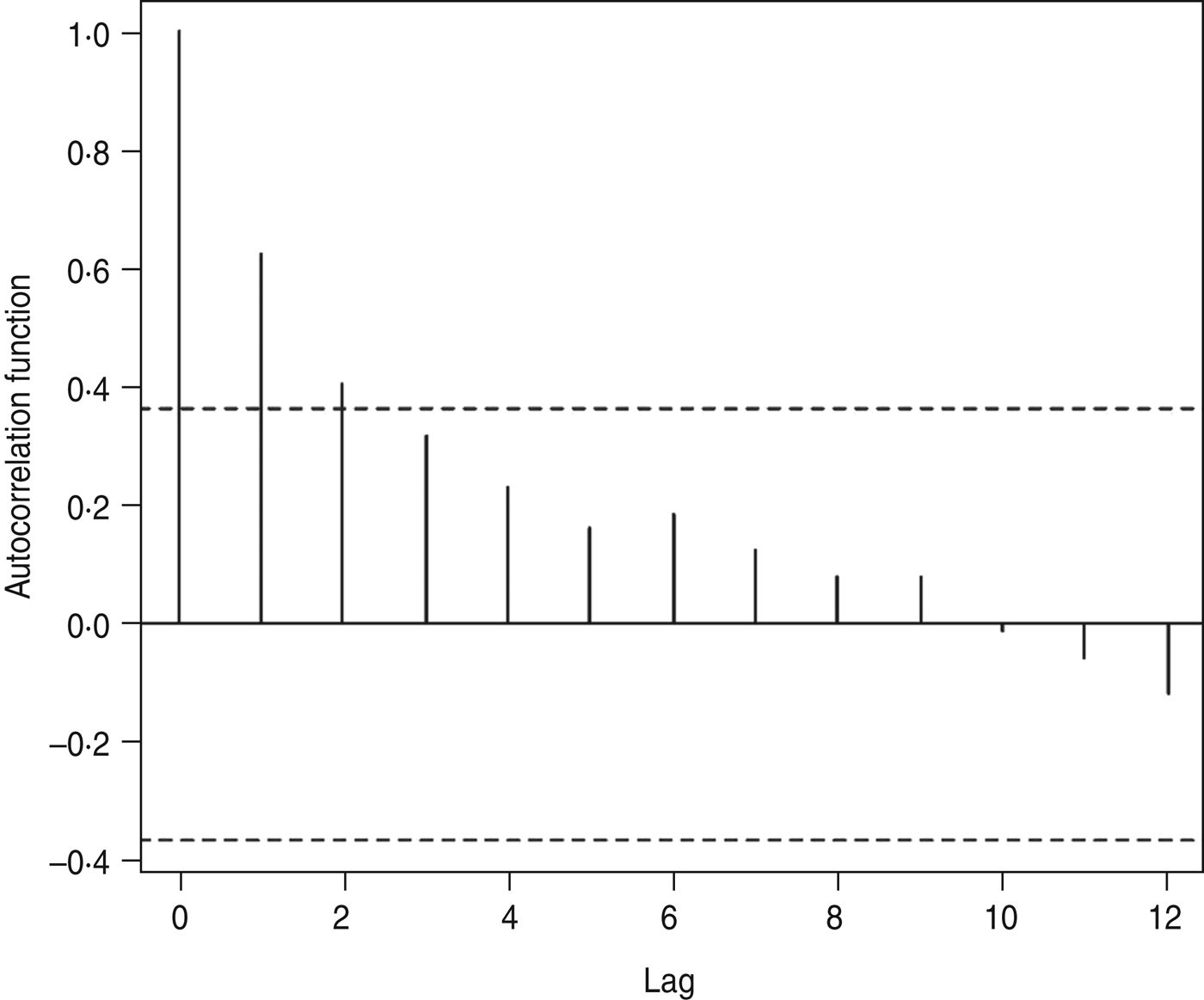
The autocorrelations, by a lag of years 1, 2, … , 12, were respectively 0·62, 0·40, 0·32, 0·23, 0·16, 0·19, 0·12, 0·08, 0·08, −0·01, −0·06, −0·12 and 0·06 for annual HFRS cases (Fig. 3). The high positive correlation was found at a lag of 1 year. Similar patterns were also observed from the plots of the partial autocorrelations.
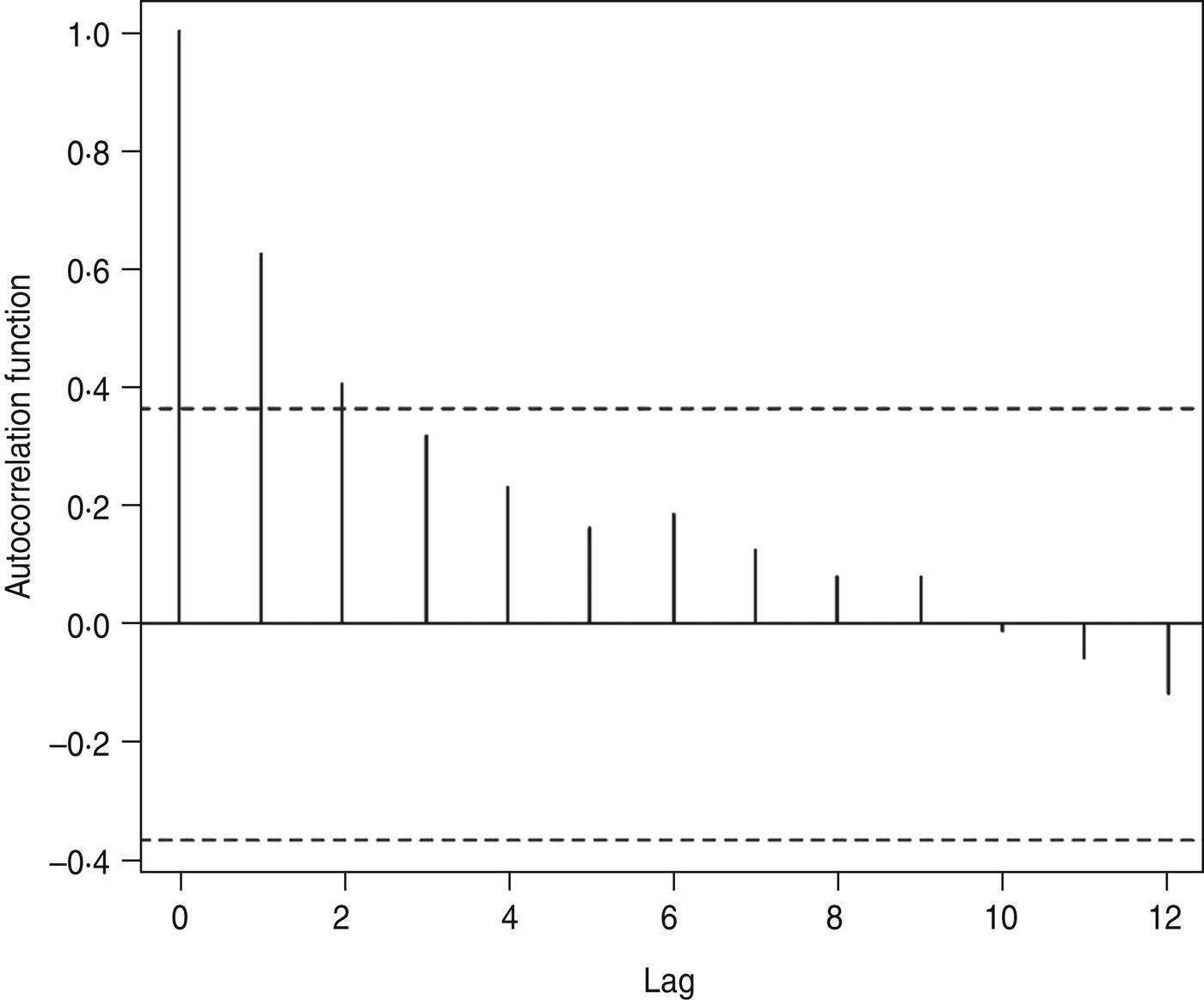
Fig. 3. Autocorrelation coefficients of haemorrhagic fever with renal syndrome.
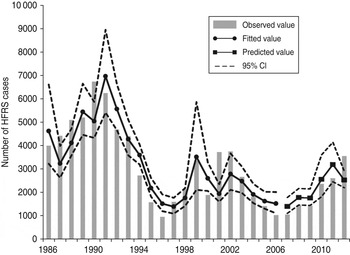
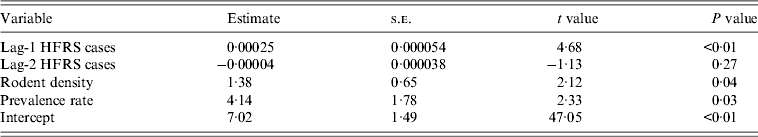
We detected clear effects of relative rodent density and virus prevalence rate on HFRS incidence throughout Shaanxi province (Table 2). The estimated effects translate to a 13·8% increase in incidence with every 10% increase in rodent density and a 41·4% increase for every 10% increase in prevalence rate. As shown in Figure 4, the expected number of cases from the final model fitted very well with the observed number of cases in Shaanxi province over the period 1984–2012, including peak values. The pseudo-R 2 value for the fitted model was 72·60% and for the predicted model was 62·94%. In diagnosis of the residuals of the model, a random distribution was observed, with no autocorrelation among them.
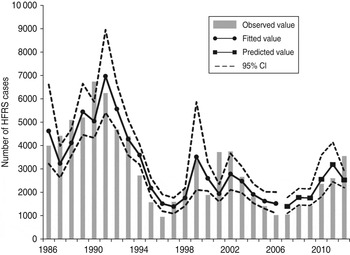
Fig. 4. The time-series plots of the observed haemorrhagic fever with renal syndrome incidence, fitted value and predicted value. CI, Confidence interval.
Table 2. Parameter estimates for the statistically selected best model

HFRS, Haemorrhagic fever with renal syndrome.
DISCUSSION
In HFRS epidemic areas, attempts have been made to predict HFRS outbreaks in order to make effective preventions. An HFRS occurrence was generally considered to be associated with the composition, density, and infection rates of rodent species, as well as human immunity status [Reference Olsson5, Reference Xiao10, Reference Clement17, Reference Guan18]. Among these indicators, rodent density was important, as it could influence the contact between humans and animals. In addition, rodent density was found to be affected by climate, geographical characteristics, vegetation, farming practices and human living activities [Reference Xiao10, Reference Xiao19, Reference Xiao20]. However, the determinants and the epidemic pattern of hantavirus prevalence rate in rodents still remain unknown in Europe [Reference Davis and Calvet21], and China. Human immunity status is affected by the intensity of an HFRS epidemic, apparent infection (infection followed by symptoms of disease) or inapparent infection (presence of infection in a host without the occurrence of recognizable symptoms or signs) of hantavirus, coverage rate of vaccinations and immigration of non-epizootic populations. Vaccine inoculation was found to have a great impact on HFRS occurrence [Reference Wang22]. Tian & Feng used χ 2 test analysis to conduct a comparison on the data of HFRS incidence for 17 years after vaccination in Hu county, Shaanxi province, and the results showed that the vaccines still have strong immune effects after 17 years, which changed the original epidemic pattern and characteristics of HFRS [Reference Tian and Feng23].
Since the 2000s, the Government has paid more attention to public healthcare, and farming methods have become mechanized in epidemic areas, causing significant decline in rodent density. At the same time, rodent surveillance was no longer strictly conducted according to the trap-night method. Moreover, in order to capture rodents, the method of selection of sites and baits, trap numbers and spacing distances between traps was no longer used after 2006. The continuous 29 years’ of observation data after the start of surveillance in 1984 showed that the correlation between human HFRS incidence and hantavirus infection rates in rodents was 0·58. The coincidence between positive serological specificity of IgG and hantavirus infection rate in lung tissues of rodents is 52·41%, thus possibly indicating a good correlation between past hantavirus infection in rodents and human HFRS incidence. As average lifespan of A. agrarius is around 2 years in the study area [Reference Zheng24], the positive rate of antibodies in the sera of rodents and hantavirus infection rate in the lung tissues of rodents were both high, the positive rate of antibodies is especially well reflected in the active status of hantavirus in rodents during these 2 years. Therefore, as we only studied data over 2 years, we were not able to conclude the quantitative relationship between HFRS incidence and previous hantavirus infection.
The HFRS incidence in Shaanxi province showed a three-step fluctuating downwards trend, which was not only influenced by the fluctuations of the infection rate, but also influenced by natural and social factors. The infection rate of rodents peaked at 8·03% in 1984. Scattered farming methods after fixing farm output quotas for each household increased the contact rate between humans and rodents in rural areas, keeping HFRS incidence at a high level. The infection rate of rodents peaked at 10·08% in 1989, and then declined to around 1% after another outbreak peak in 1990. Since the appearance of a vaccine in 1993, most people in serious epidemic areas received vaccination, thus causing HFRS incidence to decline and remain at a low level. The hantavirus infection rate of rodent hosts reached 3·49% in 2001, and another HFRS incidence peaked with low values. With the mechanization of farming methods and the new development of the countryside, the contact between humans and rodents declined, causing HFRS incidence to remain at a low level. The infection rate of rodents increased to 10·15% in 2011, and HFRS incidence peaked in 2012.
Hantavirus is transmitted to people by rodents, thus vaccination can only supply individual protection instead of an immunity barrier for the whole population. Currently, even the accumulative hantavirus apparent infection is only 1·5% in the high-incidence county in Shaanxi, but the human inapparent infection rate is 30%. The total vaccination rate is now <40% and more than 30% of the populations are still susceptible to HFRS infection. The relative density of rodent hosts, the hantavirus infection rate of and the contact between people and rodents are the important factors for HFRS transmission. New infection of hantavirus is characterized as a carrier state and previous infection is confirmed by IgG. The surveillance of hantavirus prevalence in rodent lung tissues and serological specificity of IgG can both predict the incidence of HFRS.
Some limitations of the study should be acknowledged. Population levels of immunity are related to apparent and inapparent infections of hantavirus and vaccination coverage. HTNV is the predominant serotype in Shaanxi province. The peak of HFRS incidence appears every 8–10 years, and HFRS incidence during 2010–2012 was the fifth reported peak. However, the periodicity of relative rodent density was not obvious. Although a total of 7 million people have now been vaccinated in Shaanxi province, there is a lack of information on the continuous immunity status of the population and inapparent infection.
In conclusion, this study found a strong positive correlation and a repeated temporal pattern in relative rodent density, hantavirus infection prevalence and HFRS cases in Shaanxi province. From January 2010 to December 2012, a total of 8511 HFRS diagnoses were reported, representing a new epidemic peak.
ACKNOWLEDGEMENTS
This work was supported by the Chinese National Science and Technology Major Project (grant no. 2013ZX10004202); the Shaanxi Provincial Department of Science and Technology [grant nos. 2013K12-04-02, 2007K12-02(22)]; and the National Research Program of the Ministry of Science and Technology, China (grant nos. 2010CB530300, 2012CB955501, 2012AA12A407, 2013AA122003).
DECLARATION OF INTEREST
None.