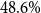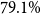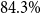1. Introduction
Vast amounts of clinical data are stored in unstructured text, such as doctor’s letters. Natural language processing (NLP) and machine learning (ML) can make their information available for research and clinical routine. While supervised ML approaches rely on large amounts of manually annotated training data, recent developments in NLP showed promising results in text classification tasks using pretrained language models (PLM) and prompting (Brown et al. Reference Brown2020). Prompting exploits the ability of PLMs to make correct predictions if guided through context; in combination with supervised methods, they achieve state-of-the-art results on various text classification tasks (Liu et al. Reference Liu2023).
Doctor’s letters are typically divided into sections, such as anamnesis (patient medical history), diagnosis or medication, containing semantically related sentences. Typically, it is not necessary to consider all sections to obtain specific medical information (Richter-Pechanski et al. Reference Richter-Pechanski2021) or medication information (Uzuner, Solti, and Cadag Reference Uzuner, Solti and Cadag2010). Instead, medical information extraction (MIE) tasks, such as medication extraction or patient cohort retrieval, can be improved by contextualizing the information in a doctor’s letter (Edinger et al. Reference Edinger2017). However, automatic section classification is non-trivial due to a high variability of the structuring of information across physicians and time periods (Lohr et al. Reference Lohr2018).
In close collaboration with physicians from clinical routine, we identified four challenges of MIE projects in the clinical domain (Hahn and Oleynik Reference Hahn and Oleynik2020) (Fig. 1).
-
Ch1 Domain-and-Expert-dependent: Annotation projects often require an active involvement of domain experts for data annotation and model evaluation. This is particularly relevant for lower-resource languages and domains such as the clinics and German language.
-
Ch2 Resource-constrained: Domain experts are costly and have only limited time resources. By contrast, external expert involvement is difficult due to strict data protection measures (Richter-Pechanski et al. Reference Richter-Pechanski2021).
-
Ch3 On-premise: Personal data are confidential, which means that many MIE projects are carried out entirely on premise, that is, in the clinical IT infrastructure. However, computational resources in clinical infrastructures are often a limiting factor (Taylor et al. Reference Taylor2023).
-
Ch4 Transparency: Due to the sensitivity of clinical information, safety standards for using MIE results in clinical routine are high: model predictions must be of high quality, transparent, explainable, and as comprehensible as possible (Tjoa and Guan Reference Tjoa and Guan2020).
We evaluate best-practice strategies to identify an ideal setup to address the multifaceted challenges of conducting a MIE task such as clinical section classification. Specifically, we identify and propose the following solutions:
-
S1 We reduce the demand for clinical knowledge in MIE by exploiting existing domain knowledge available in hospitals, such as clinical routine documents. We evaluate domain- and task-adapted (Gururangan et al. Reference Gururangan2020) general-use PLMs, as well as PLMs pretrained on clinical data from scratch (Bressem et al. Reference Bressem2024) in combination with prompt-based learning methods (Schick and Schütze Reference Schick and Schütze2021a), which require only limited training data.
-
S2 To reduce time investment and costs of manual data annotation through clinical experts, we apply few-shot learning (Lake, Salakhutdinov, and Tenenbaumt Reference Lake, Salakhutdinov and Tenenbaum2015) and context-enriched training data using prompt-based fine-tuning with pattern-exploiting training (PET + PETAL) (Schick and Schütze Reference Schick and Schütze2021a; Schick, Schmid, and SchÃijtze Reference Schick, Schmid and Schütze2020) and compare the results with supervised sequence classification methods. We further evaluate the feasibility of null prompts (Logan et al. Reference Logan2022), which have been shown to alleviate the search for effective prompts while achieving improved results.
-
S3 While large language models (LLMs) have recently shown impressive medical capabilities (Singhal et al. Reference Singhal2023), their demands of compute power, and currently unsolved issues regarding automatic evaluation, faithfulness control, and trustworthiness make their use in clinical contexts often impractical (Parnami and Lee Reference Parnami and Lee2022; Thirunavukarasu et al. Reference Thirunavukarasu2023). We, therefore, focus on smaller PLMs (
 $110$
-
$110$
-
 $345$
million learnable parameters) in a few-shot learning setting. Notably, prompt-based fine-tuning already achieves higher accuracy with smaller, encoder-based PLMs compared to PLMs fine-tuned for sequence labeling with a full-fledged training dataset in German (Schick and Schütze Reference Schick and Schütze2021a).
$345$
million learnable parameters) in a few-shot learning setting. Notably, prompt-based fine-tuning already achieves higher accuracy with smaller, encoder-based PLMs compared to PLMs fine-tuned for sequence labeling with a full-fledged training dataset in German (Schick and Schütze Reference Schick and Schütze2021a). -
S4 To address the need for transparent and trustworthy model predictions in clinical routine, we use well-established masked-language-models. They allow application of state-of-the-art interpretability methods that rely on saliency features computed with, for example, Shapley values (Lundberg and Lee Reference Lundberg and Lee2017), to explain our model predictions.
In what follows we conduct in-depth evaluations of these proposed solutions in a real-world section classification task, applied to German doctor’s letters from the cardiovascular domain. To our knowledge, this is the first in-depth evaluation of a prompt-based fine-tuning method such as PET on real-world clinical routine data in German language.

Figure 1. Challenges for MIE projects in clinics: Our proposed solutions to main challenges for MIE projects in a clinical setting. Abbreviation: “par.” refers to parameters.
1.1 State of research
From fine-tuning to few-shot learning with prompting. Since 2017, most NLP tasks apply a pretrain-then-finetune paradigm: neural models are pretrained with a language modeling objective on large amounts of unlabeled text and then fine-tuned for a down-stream task on a smaller amount of annotated data. However, even fine-tuned PLMs often perform poorly with sparse training data (Gao, Fisch, and Chen Reference Gao, Fisch and Chen2021) and require significant amounts of manually labeled training data to perform well (Liu et al. Reference Liu2023). Especially with low(er)-resource languages and in special domains, we often face a scarcity of high-quality labeled data. With recent scaled-up language models, we observe another shift to a pretrain-then-prompt paradigm, where tasks are formulated using natural language prompts (Shin et al. Reference Shin2020; Schick and Schütze Reference Schick and Schütze2021a; Reynolds and McDonell Reference Reynolds and McDonell2021; Gao et al. Reference Gao, Fisch and Chen2021), revealing impressive zero-shot capabilities of these models (Kojima et al. Reference Kojima2022; Liu et al. Reference Liu2023). While in many applications at least a few training samples are still required to guide model predictions, prompt-based learning soon matched and even surpassed the performance of fine-tuning in various few-shot learning settings (Liu et al. Reference Liu2023; Taylor et al. Reference Taylor2023).
Although model size played a critical role in this development (Chowdhery et al. Reference Chowdhery2023), smaller, encoder-based PLMs have also been successfully applied in few-shot scenarios using prompt-based fine-tuning in combination with a semi-supervised approach (Schick and Schütze Reference Schick and Schütze2021b; Wang et al. Reference Wang2022). Especially, framing text classification tasks as cloze-style problems using pattern-exploiting training (PET) showed promising results for various classification tasks (Schick and Schütze Reference Schick and Schütze2022) (cf. Section 2).
Domain adaptation through further-pretraining. Further-pretraining means training an already pretrained language model further on domain-specific texts using a language model objective. This allows domain-adaptation of general-purpose language models. General-purpose LMs achieve high performance across many tasks (Sun et al. Reference Sun2019), yet performance typically drops in out-of domain settings. Several studies explored further-pretraining on domain-specific data (Zhu et al. Reference Zhu2021), in such cases, demonstrating that further-pretraining even on small-sized task-specific data can improve results in out-of-domain down-stream tasks (Gururangan et al. Reference Gururangan2020).
PLMs for the medical domain. Medical PLMs, pretrained on medical data from scratch and further-pretrained medical PLMs, have outperformed general PLMs in several tasks (Sivarajkumar and Wang Reference Sivarajkumar and Wang2022; Taylor et al. Reference Taylor2023). However, clinical routine texts, as used in this study, have unique textual properties compared to biomedical texts on which such models are trained. This increases the complexity of medical NLP tasks in clinical routine (Leaman, Khare, and Lu Reference Leaman, Khare and Lu2015; Hahn and Oleynik Reference Hahn and Oleynik2020). Also, only a limited number of further-pretrained and clinical PLMs have been published to date, mostly for English, primarily due to strict data protection regulations (Lee et al. Reference Lee2020; Li et al. Reference Li2023; Bressem et al. Reference Bressem2024).
Prompting methods in clinical NLP. Despite extensive research on PLMs for medical domain, previous research has mainly focused on supervised fine-tuning with full-fledged training data approaches that use large amounts of training data (Wu et al. Reference Wu2020; Taylor et al. Reference Taylor2023) with the exception of Taylor et al. (Reference Taylor2023) who investigated prompting on English clinical data. Thus, there is a need to investigate how further-pretraining influences prompting methods in few-shot scenarios.
PET performed well in various downstream tasks in English, i.a. in biomedical text classification, where for adverse drug effect classification it outperformed GPT3 with an
![]() $F1$
-score of
$F1$
-score of
![]() $82.2\%$
versus
$82.2\%$
versus
![]() $68.6\%$
(Schick and Schütze Reference Schick and Schütze2022). This highlights the need for thorough evaluation of PET in clinical routine tasks with medical domain PLMs, particularly for lower-resource languages such as German (Leaman et al. Reference Leaman, Khare and Lu2015; Hahn and Oleynik Reference Hahn and Oleynik2020).
$68.6\%$
(Schick and Schütze Reference Schick and Schütze2022). This highlights the need for thorough evaluation of PET in clinical routine tasks with medical domain PLMs, particularly for lower-resource languages such as German (Leaman et al. Reference Leaman, Khare and Lu2015; Hahn and Oleynik Reference Hahn and Oleynik2020).
Clinical section classification. Identifying sections in clinical texts has been shown to enhance performance on several MIE tasks (Pomares-Quimbaya, Kreuzthaler, and Schulz Reference Pomares-Quimbaya, Kreuzthaler and Schulz2019). However, this research field remains underdeveloped, partly due to the lack of benchmark datasets (cf. comprehensive survey Landolsi, Hlaoua, and Ben Romdhane Reference Landolsi, Hlaoua and Ben Romdhane2023). Therefore, most studies focus on English clinical texts (Denny Reference Denny2008; Edinger Reference Edinger2017). In-depth studies focusing on few-shot learning scenarios and prompting are still lacking (Ge Reference Ge2023). Our work is the first to thoroughly investigate these methods on a freely available clinical German benchmark corpus. Furthermore, we extensively explore German PLMs (Bressem Reference Bressem2024) for clinical domains to detect suitable (further-)pretraining methods for prompting and their effect on section classification.
Interpretability. Given the black-box nature of deep learning architectures, the interpretability of model outputs is challenging and attracts much interest (Fan et al. Reference Fan2021), especially in safety-critical domains such as clinical routine. Various feature attribution methods have been developed to address these issues (Ribeiro, Singh, and Guestrin Reference Ribeiro, Singh and Guestrin2016; Sundararajan, Taly, and Yan Reference Sundararajan, Taly and Yan2017; Lundberg and Lee Reference Lundberg and Lee2017), but we still face challenges in assessing their quality (Jacovi and Goldberg Reference Jacovi and Goldberg2020; Attanasio Reference Attanasio2023). Shapley values provide a theoretically well-founded approach to determine the contribution of individual input features to a model prediction. A computationally optimized implementation called SHAP (Shapley et al. Reference Shapley1953) can be applied out-of-the-box on transformer-based models. To our knowledge, we are the first to study the use of Shapley values for data and model optimization in clinical tasks.
Progress in the area of LLMs. Recently, generative LLMs with billions of parameters deliver impressive results in various general (Brown et al. Reference Brown2020; Scao et al. Reference Scao2022; Chowdhery et al. Reference Chowdhery2023; Touvron et al. Reference Touvron2023) and biomedical and clinical NLP tasks (Singhal et al. Reference Singhal2023; Thirunavukarasu et al. Reference Thirunavukarasu2023; Clusmann et al. Reference Clusmann2023; Peng et al. Reference Peng2023). However, many challenges need to be addressed before LLMs can be applied in clinical tasks (Wang, Zhao, and Petzold Reference Wang, Zhao and Petzold2023): Running them via external APIs is typically prohibited due to data protection regulations. Despite efforts to make LLMs available for use in protected infrastructures (cf. https://github.com/bentoml/OpenLLM), model deployment in clinical infrastructures is often not feasible (Taylor et al. Reference Taylor2023). Moreover, out-of-the-box local GPT and Llama models have shown poor performance in biomedical tasks (Moradi et al. Reference Moradi2021; Wu et al. Reference Wu2023). Finally, due to the generated outputs of autoregressive PLMs, their use in clinical NLP implies unsolved issues concerning automatic evaluation (Guo et al. Reference Guo2023; Chang et al. Reference Chang2024) and judging the faithfulness of model predictions (Parcalabescu and Frank Reference Parcalabescu and Frank2024), which are both critical in the clinical domain.
While evaluation of autoregressive LLMs will mature in the future, our study on encoder-based models serves as a process-oriented guideline for MIE projects in clinical routine tasks for lower-resource languages. All constraints discussed in this study: (1) expert-dependency, (2) data protection regulations, (3) demand for on-premise solutions, and (4) transparency requirements, invariably apply to popular local LLMs such as Llama (Touvron et al. Reference Touvron2023) or Mistral (Jiang et al. Reference Jiang2023), and can serve as guidelines for evaluating these models, too.
2. Methods
2.1 Pattern-exploiting training (S
 $_1$
and S
$_1$
and S
 $_2$
)
$_2$
)
In our experiments, we systematically evaluate methods for few-shot learning, that is, using minimal training data, in a lower-resource domain and language, in our case German clinical routine (Hahn and Oleynik Reference Hahn and Oleynik2020; Jantscher et al. Reference Jantscher2023; Idrissi-Yaghir et al. Reference Idrissi-Yaghir2024). Specifically, we evaluate PET, a semi-supervised prompting method optimized for few-shot learning scenarios (Schick and Schütze Reference Schick and Schütze2021a) which is designed to recast classical text classification or information extraction tasks as a language modeling problem. In our study, we classify paragraphs of German doctor’s letters into a set of nine section categories (Table 1). The objective is, for instance, to accurately categorize a paragraph such as The patient reports pressure pain in the left chest under the section class Anamnese.
Table 1. Distribution of section classes: Number of samples per section class per corpus split. English translation in round brackets.

To conduct PET experiments, we need a pretrained masked language model
![]() $M$
with a vocabulary
$M$
with a vocabulary
![]() $V$
, a few-shot dataset with training instances
$V$
, a few-shot dataset with training instances
![]() $x_i \in X$
and target labels
$x_i \in X$
and target labels
![]() $y_i \in Y$
. We further need a pattern function
$y_i \in Y$
. We further need a pattern function
![]() $P$
that maps instances to a set of cloze sentences (templates)
$P$
that maps instances to a set of cloze sentences (templates)
![]() $P\,:\, X \mapsto V^\ast$
, and a verbalizer function
$P\,:\, X \mapsto V^\ast$
, and a verbalizer function
![]() $v\,:\, Y \mapsto V$
that maps each label to a single token from the vocabulary of
$v\,:\, Y \mapsto V$
that maps each label to a single token from the vocabulary of
![]() $M$
.
$M$
.
The PET workflow contains three basic steps (see Fig. 2): (1) applying
![]() $P$
to each input instance
$P$
to each input instance
![]() $x_i$
and fine-tune a model
$x_i$
and fine-tune a model
![]() $M$
for each template to obtain the most likely token for the
$M$
for each template to obtain the most likely token for the
![]() $MASK$
token
$MASK$
token
![]() $v(y)$
, (2) use the ensemble of fine-tuned models
$v(y)$
, (2) use the ensemble of fine-tuned models
![]() $M$
from the previous step and annotate a large unlabeled dataset
$M$
from the previous step and annotate a large unlabeled dataset
![]() $D$
with soft labels, and (3) train a final classifier C with a traditional sequence classification head on the labeled dataset
$D$
with soft labels, and (3) train a final classifier C with a traditional sequence classification head on the labeled dataset
![]() $D$
.
$D$
.

Figure 2. PET workflow: Three main steps: (a) Apply pattern function P(x) to all few-shot training instances X. Fine-tune a PLM M using a language model objective on each pattern. The output of the PLM is mapped using a verbalizer function v(y). (b) An ensemble of M trained on each pattern is used to annotate an unlabeled dataset D with soft labels. (c) A classifier C with a classification head is trained on D.
2.1.1 Creating templates
Template engineering is a crucial hyperparameter in a PET experiment. For the core experiments, we used four different template types (including examples and English translations (in brackets)):
-
• Null prompt: SAMPLE [MASK] Keine peripheren Ödeme [MASK] (No peripheral edema [MASK])
-
• Punctuation: SAMPLE : [MASK] and SAMPLE - [MASK] Keine peripheren Ödeme : [MASK] (No peripheral edema : [MASK])
-
• Prompt: SAMPLE Sektion [MASK] Keine peripheren Ödeme Sektion [MASK] (No peripheral edema Section [MASK])
-
• Q&A: SAMPLE Frage: Zu welcher Sektion gehört dieser Text? Antwort: [MASK] Keine peripheren Ödeme Frage: Zu welcher Sektion gehört dieser Text? Antwort: [MASK] (No peripheral edema Question: To which section does this text belong? Answer: [MASK])
To minimize engineering costs we also evaluated the feasibility of using exclusively null prompts, by removing all tokens from prompt templates, as proposed by Logan et al. (Reference Logan2022). We defined three null prompt templates: (1) SAMPLE [MASK]; (2) [MASK] SAMPLE; and (3) [MASK] SAMPLE [MASK].
2.1.2 Verbalizer
Defining the verbalizer token can be tedious, because domain knowledge and technical expertise about the used PLM is required. This can be a significant issue, as such a comprehensive knowledge is uncommon in the clinical setting. Moreover, PET restricts the verbalizer token to a single token. Hence, an appropriate and intuitive token may not be applicable for a label mapping, if it is not included in the PLM’s vocabulary. For instance, the word Anamnese is not part of the gbert vocabulary. This makes a verbalizer search for clinicians quite challenging. Therefore, we use PET with automatic labels (PETAL) for all our experiments, except for the zero-shot baselines (Schick et al. Reference Schick, Schmid and Schütze2020). This can reduce engineering costs and makes our experimental setup more comparable and reproducible. As visualized in Suppl. Fig. S2 PETAL calculates the most likely verbalizer token per label, given the few-shot training data for each pattern and given a PLM. We created a separate verbalizer for each few-shot size for each training set.
2.2 Pretrained language models (S
 $_1$
& S
$_1$
& S
 $_3$
)
$_3$
)
To evaluate the feasibility of exploiting existing clinical domain knowledge by further-pretraining, we used a set of three language models, all based on the BERT architecture (Devlin et al. Reference Devlin2019) and available at Hugging Face Hub: (1) deepset/gbert-base (Chan, Schweter, and Möller Reference Chan, Schweter and Möller2020), (2) deepset/gbert-large (gbert), (3) Smanjil/German-MedBERT (medbertde) (Bressem et al. Reference Bressem2024). The largest model gbert-large contains
![]() $340$
million parameters. In our clinical infrastructure, which contains a maximum of two NVIDIA RTX6000 GPUs, we were able to perform all further-pretraining experiments within a reasonable timeframe (cf. Suppl. Section S3). Compared to current foundation models with billions of parameters, we consider these models as lightweight. For both gbert and medbertde, we create medical-adapted variants by further-pretraining, as proposed by Gururangan et al. (Reference Gururangan2020) to assess the impact of different pretraining datasets on section classification results (Fig. 3). We defined datasets for three different pretraining approaches:
$340$
million parameters. In our clinical infrastructure, which contains a maximum of two NVIDIA RTX6000 GPUs, we were able to perform all further-pretraining experiments within a reasonable timeframe (cf. Suppl. Section S3). Compared to current foundation models with billions of parameters, we consider these models as lightweight. For both gbert and medbertde, we create medical-adapted variants by further-pretraining, as proposed by Gururangan et al. (Reference Gururangan2020) to assess the impact of different pretraining datasets on section classification results (Fig. 3). We defined datasets for three different pretraining approaches:
-
1. task-adaptation. Using CARDIO:DE, cf. Section 2.4.1. This dataset contains unlabeled data extracted from the same source as the training and test data of the section classification task. It is relatively small, only
 $5.8\,{\rm MB}$
(megabytes). (PLMs appended with suffix -task)
$5.8\,{\rm MB}$
(megabytes). (PLMs appended with suffix -task) -
2. domain-adaptation. Using 179,000 doctor’s letters from the Cardiology department at the University Hospital, cf. Section 2.4.2. This dataset contains a broad range of texts from clinical routine in cardiovascular domain. With
 $1.3\,{\rm GB}$
(gigabytes), it is significantly larger than the task-adaptation dataset. (PLMs appended with suffix -domain)
$1.3\,{\rm GB}$
(gigabytes), it is significantly larger than the task-adaptation dataset. (PLMs appended with suffix -domain) -
3. combination of both approaches Further-pretrain a domain-adapted PLM on our task specific data (PLMs appended with suffix -comb)

Figure 3. Pretrained language models: We use two publicly available PLMs: gbert and medbertde. We evaluate base and large gbert models. Four pretraining methods are used: (a) publicly available, (b) task-adapted, (c) domain-adapted, and (d) task- and domain-adapted combined.
We performed pretraining using a masked language modelling objective (cf. https://tinyurl.com/5n8bjnbh). For hyperparameters and further training details see Suppl. Section S3.
2.3 Shapley values (S
 $_4$
)
$_4$
)
In many safety-critical domains, in particular in the clinical domain, it is crucial to (1) understand the inner workings of a model (faithfulness) and to (2) evaluate how convincing a model interpretation is for a human observer (plausibility) (Jacovi and Goldberg Reference Jacovi and Goldberg2020). This can increase trust in model predictions (explainable AI) by identifying which token contributed to a specific prediction. Furthermore, if a model makes incorrect predictions, allocating such tokens can help to understand and address these issues.
In recent years, Shapley values became a valuable tool in NLP for local model interpretations using saliency features (Attanasio et al. Reference Attanasio2023). Shapley values offer a systematic approach to attribute the impact of individual textual components (token, token sequences) on a model prediction. In our setup, we apply Shapley values in two ways: (1) From a clinical routine perspective: to make deep learning model predictions more transparent and explainable and (2) from an engineering perspective: to detect biases or errors in the training data and to support choosing the most optimal model architecture. Shapley values, originating from cooperative game theory, allocate the importance of each feature by averaging its marginal contribution across all possible feature combinations in predicting an outcome (Lundberg et al. Reference Lundberg and Lee2017). The Shapley value for a feature
![]() $ i$
is given by
$ i$
is given by
Here
![]() $ f$
is the prediction function,
$ f$
is the prediction function,
![]() $ S$
is a subset of all features without feature
$ S$
is a subset of all features without feature
![]() $ i$
, and
$ i$
, and
![]() $ N$
is the set of all features.
$ N$
is the set of all features.
In our experiments, we use SHAP (SHapley Additive exPlanations) because it offers an optimized algorithm that approximates Shapley values with reduced computational costs, making its application feasible for practical use (Mosca et al. Reference Mosca2022). Furthermore, we conducted experimental explorations and compared several interpretability methods in advance with ferret, a framework for benchmarking popular explainers on transformers (Attanasio et al. Reference Attanasio2023), finding that SHAP was the best-performing method for our setup.
2.4 Data
2.4.1 Annotated corpus
For our experiments, we used a German clinical corpus from the cardiovascular domain, CARDIO:DE, encompassing
![]() $500$
doctor’s letters from the Cardiology Department at the Heidelberg University Hospital. For more details about the dataset, preprocessing steps, data annotation, and data distribution, cf. Richter-Pechanski et al. (Reference Richter-Pechanski2023). The corpus can be accessed via heiData, a public research repository; see Richter-Pechanski and Dieterich (Reference Richter-Pechanski and Dieterich2023). The complete corpus contains
$500$
doctor’s letters from the Cardiology Department at the Heidelberg University Hospital. For more details about the dataset, preprocessing steps, data annotation, and data distribution, cf. Richter-Pechanski et al. (Reference Richter-Pechanski2023). The corpus can be accessed via heiData, a public research repository; see Richter-Pechanski and Dieterich (Reference Richter-Pechanski and Dieterich2023). The complete corpus contains
![]() $993,143$
tokens, with approximately
$993,143$
tokens, with approximately
![]() $31,952$
unique tokens. The corpus was randomly split into CARDIO:DE400 containing
$31,952$
unique tokens. The corpus was randomly split into CARDIO:DE400 containing
![]() $400$
letters (
$400$
letters (
![]() $805,617$
tokens) for training and CARDIO:DE100, containing
$805,617$
tokens) for training and CARDIO:DE100, containing
![]() $100$
letters (
$100$
letters (
![]() $187,526$
tokens) for testing. The corpus was automatically de-identified, by replacing protected health information (PHI) containing patient sensitive identifiers with placeholders using an in-house deep learning model (Richter-Pechanski et al. Reference Richter-Pechanski2019). This was followed by a manual review involving domain experts to fix de-identification errors. To increase readability and semantic consistency and to decrease the chance for re-identification, all PHI placeholders were replaced with semantic surrogates, as proposed in Lohr, Eder, and Hahn (Reference Lohr, Eder and Hahn2021).
$187,526$
tokens) for testing. The corpus was automatically de-identified, by replacing protected health information (PHI) containing patient sensitive identifiers with placeholders using an in-house deep learning model (Richter-Pechanski et al. Reference Richter-Pechanski2019). This was followed by a manual review involving domain experts to fix de-identification errors. To increase readability and semantic consistency and to decrease the chance for re-identification, all PHI placeholders were replaced with semantic surrogates, as proposed in Lohr, Eder, and Hahn (Reference Lohr, Eder and Hahn2021).
We split the corpus by newline characters, which are part of the MS-DOC source documents. Sentence splitting the corpus with publicly available sentence splitting methods or by pattern heuristics showed unsatisfactory results. Furthermore, sequence length of newline split paragraphs rarely exceed
![]() $512$
token (min:
$512$
token (min:
![]() $3$
, max:
$3$
, max:
![]() $599$
, mean:
$599$
, mean:
![]() $30.9$
, median:
$30.9$
, median:
![]() $16$
,
$16$
,
![]() $99{\rm th}$
percentile:
$99{\rm th}$
percentile:
![]() $205$
), thus, comply with most PLM sequence length restrictions. If a paragraph exceeds the maximum sequence length of the PLM, we trim the sample accordingly.
$205$
), thus, comply with most PLM sequence length restrictions. If a paragraph exceeds the maximum sequence length of the PLM, we trim the sample accordingly.
The corpus contains
![]() $116.898$
paragraphs manually annotated with
$116.898$
paragraphs manually annotated with
![]() $14$
section classes: Anrede (Salutation/Greeting), AktuellDiagnosen (Current Diagnosis), Diagnosen (Diagnosis), AllergienUnverträglichkeitenRisiken (AllergiesIntolerancesRisks), Anamnese (Patient Medical History), AufnahmeMedikation (Admission Medication), KUBefunde (Body Findings), Befunde (Findings), EchoBefunde (Echocardiogram Findings), Labor (Laboratory), Zusammenfassung (Summary), Mix (Mix), EntlassMedikation (Discharge Medication), Abschluss (Closing Remarks) (see CARDIO:DE section classes, Suppl. Tab. S1). Manual annotation was conducted on the paragraph level, no nested annotations were allowed. For our experiments, we reduced the section classes to the most significant sections. We removed the Labor section, as it contains flattened tables resulting in a large amount of relatively well structured and short numeric samples. Internal experiments showed that they can be sufficiently identified using regular expressions and patterns. Furthermore, we merged seven semantically similar classes in CARDIO:DE annotations to three meta classes: (1) Diagnosen: (AktuellDiagnosen
$14$
section classes: Anrede (Salutation/Greeting), AktuellDiagnosen (Current Diagnosis), Diagnosen (Diagnosis), AllergienUnverträglichkeitenRisiken (AllergiesIntolerancesRisks), Anamnese (Patient Medical History), AufnahmeMedikation (Admission Medication), KUBefunde (Body Findings), Befunde (Findings), EchoBefunde (Echocardiogram Findings), Labor (Laboratory), Zusammenfassung (Summary), Mix (Mix), EntlassMedikation (Discharge Medication), Abschluss (Closing Remarks) (see CARDIO:DE section classes, Suppl. Tab. S1). Manual annotation was conducted on the paragraph level, no nested annotations were allowed. For our experiments, we reduced the section classes to the most significant sections. We removed the Labor section, as it contains flattened tables resulting in a large amount of relatively well structured and short numeric samples. Internal experiments showed that they can be sufficiently identified using regular expressions and patterns. Furthermore, we merged seven semantically similar classes in CARDIO:DE annotations to three meta classes: (1) Diagnosen: (AktuellDiagnosen
![]() $+$
Diagnosen), (2) Medikation: (AufnahmeMedikation
$+$
Diagnosen), (2) Medikation: (AufnahmeMedikation
![]() $+$
EntlassMedikation), and (3) Befunde: (KUBefunde
$+$
EntlassMedikation), and (3) Befunde: (KUBefunde
![]() $+$
EchoBefunde
$+$
EchoBefunde
![]() $+$
Befunde). Our final dataset contains
$+$
Befunde). Our final dataset contains
![]() $49,258$
paragraphs annotated with
$49,258$
paragraphs annotated with
![]() $9$
section classes (Table 1).
$9$
section classes (Table 1).
During annotation human annotators of CARDIO:DE were presented the whole document (for further annotation details, see Richter-Pechanski et al. Reference Richter-Pechanski and Dieterich2023). To mimic this setup for our automatic section classifiers in this study, we introduced basic information about document structure to the model without introducing additional preprocessing steps or external knowledge. In addition to our training data containing single paragraph samples, we assessed two types of context-enriched datasets for our experiments (Examples Table 2):
-
• no-context (a single paragraph to be classified)
-
• context (previous paragraph + main paragraph + subsequent paragraph)
-
• prevcontext (previous paragraph + main paragraph)
The context-enriched samples still mostly comply with sequence length restrictions of PLMs (minimum
![]() $7$
, maximum
$7$
, maximum
![]() $967$
, mean length
$967$
, mean length
![]() $90.2$
, median length
$90.2$
, median length
![]() $63$
and
$63$
and
![]() $99{\rm th}$
percentile
$99{\rm th}$
percentile
![]() $371$
sub tokens). If the sequence length of the context enriched sample is exceeded, we trim the sequence of the context to fit the maximum sequence length of the PLM.
$371$
sub tokens). If the sequence length of the context enriched sample is exceeded, we trim the sequence of the context to fit the maximum sequence length of the PLM.
Table 2. Contextualized paragraphs: A sample annotated as AllergiesIntolerancesRisks with three different context types, each separated by the [SEP] token. English translation in italics.

2.4.2 Pretraining data
For all pretraining experiments, we used an internal clinical routine corpus containing approximately
![]() $179,000$
German doctor’s letters in a binary MS-DOC format covering the time period
$179,000$
German doctor’s letters in a binary MS-DOC format covering the time period
![]() $2004$
–
$2004$
–
![]() $2020$
. We collected the letters from the Cardiology Department of the University Hospital Heidelberg. The pretraining corpus is disjoint from the annotated corpus. We conducted the following preprocessing steps: each letter was converted into a UTF-8 encoded raw text file using the LibreOffice command line tool soffice (version 6.2.8). We chose LibreOffice, as it best preserved the structure of newlines and blanklines. We automatically de-identified all letters using a method based on a deep learning model trained on internal data, see Richter-Pechanski et al. (Reference Richter-Pechanski2019). We replaced PHI tokens with semantic surrogates, see Lohr et al. (Reference Lohr, Eder and Hahn2021). All doctor’s letters were concatenated into a single raw text file. We separated each new letter by the sequence ###BEGINN. All empty lines and all tables containing laboratory values were removed. The corpus is sentence splitted using NLTK’s (version 3.7) PunktSentenceTokenizer.
$2020$
. We collected the letters from the Cardiology Department of the University Hospital Heidelberg. The pretraining corpus is disjoint from the annotated corpus. We conducted the following preprocessing steps: each letter was converted into a UTF-8 encoded raw text file using the LibreOffice command line tool soffice (version 6.2.8). We chose LibreOffice, as it best preserved the structure of newlines and blanklines. We automatically de-identified all letters using a method based on a deep learning model trained on internal data, see Richter-Pechanski et al. (Reference Richter-Pechanski2019). We replaced PHI tokens with semantic surrogates, see Lohr et al. (Reference Lohr, Eder and Hahn2021). All doctor’s letters were concatenated into a single raw text file. We separated each new letter by the sequence ###BEGINN. All empty lines and all tables containing laboratory values were removed. The corpus is sentence splitted using NLTK’s (version 3.7) PunktSentenceTokenizer.
The doctor’s letters were further supplemented by the GGPONC corpus, which contains German oncology guidelines, with a total of
![]() $2$
million tokens (Borchert Reference Borchert2022). The final corpus covers
$2$
million tokens (Borchert Reference Borchert2022). The final corpus covers
![]() $1.3$
GB of raw text, approximately
$1.3$
GB of raw text, approximately
![]() $218,084,190$
tokens and
$218,084,190$
tokens and
![]() $667,903$
unique tokens.
$667,903$
unique tokens.
2.5 Experimental setup
2.5.1 Metrics
We measure section classification performance with accuracy for per-model results. In a multi-class text classification task, the accuracy is defined as the ratio of text documents correctly classified to their respective classes over the total number of text documents:
where
![]() $ \text{TP}_i$
represents the true predictions for each class
$ \text{TP}_i$
represents the true predictions for each class
![]() $ i$
and
$ i$
and
![]() $ n$
is the total number of classes.
$ n$
is the total number of classes.
To measure section classification performance per-section class, we use the
![]() $ F_1$
-score. It is defined as the harmonic mean of precision and recall given by
$ F_1$
-score. It is defined as the harmonic mean of precision and recall given by
Hence, the
![]() $ F_1$
-score is defined by
$ F_1$
-score is defined by
where TP, FP, and FN represent true positives, false positives, and false negatives, respectively.
We used approximate randomization tests (Yeh Reference Yeh2000) to measure statistical significance for accuracy and
![]() $F1$
-score results. Results are considered significant if
$F1$
-score results. Results are considered significant if
![]() $p\lt 0.05$
, cf. (https://github.com/smartschat/art).
$p\lt 0.05$
, cf. (https://github.com/smartschat/art).
2.5.2 Creating few-shot data
To conduct PET experiments, we created six few-shot datasets. Each dataset contains N paragraphs per section class with size N = 10, 20, 50, 100, 200, and 400 randomly selected from the CARDIO:DE400 data (random seed
![]() $42$
). Each paragraph includes the previous and subsequent context paragraph. All other context types (nocontext, prevcontext) are derived from this dataset. Each few-shot set includes three labeled training files and three unlabeled files with the remaining samples from the CARDIO:DE400 dataset (Suppl. Fig. S3). All experiments were evaluated on the complete CARDIO:DE100 held-out dataset.
$42$
). Each paragraph includes the previous and subsequent context paragraph. All other context types (nocontext, prevcontext) are derived from this dataset. Each few-shot set includes three labeled training files and three unlabeled files with the remaining samples from the CARDIO:DE400 dataset (Suppl. Fig. S3). All experiments were evaluated on the complete CARDIO:DE100 held-out dataset.
2.5.3 Core experiments
We conducted core experiments to assess the performance of different section classification models along three dimensions to compare: (1) fine-tuned sequence classification model variants (SC) to few-shot prompt-based learning with PET (S
![]() $_2$
, Fig. 2), (2) four different pretraining methods for clinical adaptation (S
$_2$
, Fig. 2), (2) four different pretraining methods for clinical adaptation (S
![]() $_1$
), and (3) six different few-shot sizes:
$_1$
), and (3) six different few-shot sizes:
![]() $10-400$
(S
$10-400$
(S
![]() $_2$
).
$_2$
).
The SC model is trained using a BERT-architecture with an additional output layer for a sequence-classification task as described in Devlin et al. (Reference Devlin2019). We use the SC implementation of the PET framework, defined by the parameter — method sequence_classifier.
For all core experiments, we used base-sized BERT models (S
![]() $_3$
) (gbert-base-*
and medbertde-base-*
) using all five templates combined and nocontext samples (Suppl. Tab. S2). To measure standard deviation in core experiments and additional experiments, we used three disjoint training sets including their unlabeled sets for each few-shot set. Furthermore, we conducted all experiments with two random initital seeds (
$_3$
) (gbert-base-*
and medbertde-base-*
) using all five templates combined and nocontext samples (Suppl. Tab. S2). To measure standard deviation in core experiments and additional experiments, we used three disjoint training sets including their unlabeled sets for each few-shot set. Furthermore, we conducted all experiments with two random initital seeds (
![]() $123$
and
$123$
and
![]() $234$
).
$234$
).
2.5.4 Additional experiments
In additional experiments, we investigate the effectiveness of further parameters, using the model that performed best in core experiments, with reduced few-shot sets:
![]() $20,50,100$
, and
$20,50,100$
, and
![]() $400$
. We investigate the impact of (1) model size comparing BERT-large and BERT-base models, (2) null prompt patterns, and (3) contextualization. In core and additional experiments, we further perform class-based evaluations on two primary classes, which were selected with clinical experts: (1) Anamnese (mostly unstructured) and (2) Medikation (semi-structured).
$400$
. We investigate the impact of (1) model size comparing BERT-large and BERT-base models, (2) null prompt patterns, and (3) contextualization. In core and additional experiments, we further perform class-based evaluations on two primary classes, which were selected with clinical experts: (1) Anamnese (mostly unstructured) and (2) Medikation (semi-structured).
Model size (S
![]() $_3$
): We evaluated the impact of adding model parameters, by comparing gbert-base (
$_3$
): We evaluated the impact of adding model parameters, by comparing gbert-base (
![]() $110$
million) vs gbert-large (
$110$
million) vs gbert-large (
![]() $340$
million) PLMs. We limited this setup to gbert PLMs, since a large medbertde was not published.
$340$
million) PLMs. We limited this setup to gbert PLMs, since a large medbertde was not published.
Null prompts (S
![]() $_2$
): Logan et al. (Reference Logan2022) discovered that the usage of null prompts prompts without manually crafted templates achieve competitive accuracy to manually tuned prompt templates on a wide range of tasks. This is of particular interest in the clinical domain, to further reduce costly engineering efforts.
$_2$
): Logan et al. (Reference Logan2022) discovered that the usage of null prompts prompts without manually crafted templates achieve competitive accuracy to manually tuned prompt templates on a wide range of tasks. This is of particular interest in the clinical domain, to further reduce costly engineering efforts.
Adding context (S
![]() $_2$
): To introduce further information to the document structure, we added further context to each input sample to evaluate the effect of adding context paragraphs to each sample. We evaluated three types of context (Table 2).
$_2$
): To introduce further information to the document structure, we added further context to each input sample to evaluate the effect of adding context paragraphs to each sample. We evaluated three types of context (Table 2).
3. Results
3.1 Baselines
We define two baselines to assess model performance in our core and additional experiments: as lower bound we use a zero-shot prompting approach; as upper bound we use a fine-tuned sequence classifier trained on the full size of the training corpus. Fig. 4 shows the accuracy results for both baselines. The upper bound results exceed
![]() $96\%$
accuracy for both models. The further-pretrained gbert models yield a minimal (statistically significant) advance of
$96\%$
accuracy for both models. The further-pretrained gbert models yield a minimal (statistically significant) advance of
![]() $0.4$
–
$0.4$
–
![]() $0.6$
accuracy points above the original gbert-base. For medbertde, no such difference is observed.
$0.6$
accuracy points above the original gbert-base. For medbertde, no such difference is observed.
The zero-shot results are all below
![]() $16\%$
accuracy, except for the public medbert-base that with
$16\%$
accuracy, except for the public medbert-base that with
![]() $28.3\%$
achieves a great advance over gbert-base with
$28.3\%$
achieves a great advance over gbert-base with
![]() $7.2\%$
accuracy. However, the gbert models further-pretrained on both task- and domain-specific data more than double the performance of the original model to
$7.2\%$
accuracy. However, the gbert models further-pretrained on both task- and domain-specific data more than double the performance of the original model to
![]() $15\%$
accuracy, beyond gbert pretrained on domain-specific data only (*-domain). All performance differences for gbert are statistically significant, except gbert-base and gbert-domain.
$15\%$
accuracy, beyond gbert pretrained on domain-specific data only (*-domain). All performance differences for gbert are statistically significant, except gbert-base and gbert-domain.

Figure 4. Section classification baseline results (lower/upper bound): We show accuracy scores in percentage per pretraining method (public, task-adapted, domain-adapted, and combination of both) per model: gbert-base and medbertde-base. (a) Lower-bound: used in zero-shot prompting (b) Upper bound: full training set.
3.2 Core experiments
Fig. 5 presents our core experiment results compared to the baselines introduced above.

Figure 5. Accuracy scores in percentage for core experiments and lower/upper bound: comparing prompting using PET vs. SC, few-shot sizes
![]() $10-400$
and pretraining methods using base BERT models. For reference, lower-bound PET baselines trained with zero-shots (ZERO) and upper-bound SC models trained on complete training set (FULL).
$10-400$
and pretraining methods using base BERT models. For reference, lower-bound PET baselines trained with zero-shots (ZERO) and upper-bound SC models trained on complete training set (FULL).
PET versus SC. The PET model variants significantly outperform SC models at shot sizes
![]() $\le$
100 in 31 out of 32 setups when comparing the same pretraining methods. Only SC medbertde-base-comb outperforms all PET models with shot size 100.
$\le$
100 in 31 out of 32 setups when comparing the same pretraining methods. Only SC medbertde-base-comb outperforms all PET models with shot size 100.
Few-shot size. Both PET and SC models benefit from an increase in few-shot size. We observed statistical significance at shot sizes
![]() $\le$
200. The smaller the shot size, the greater the relative performance gain of PET over SC models.
$\le$
200. The smaller the shot size, the greater the relative performance gain of PET over SC models.
Further-pretraining. We observe notably different results for further-pretrained gbert and medbertde PLMs.
Gbert. PET models benefit significantly from further-pretraining with
![]() $\le$
100 shots. Accuracy gradually increases with task-specific, domain-specific and combined pretraining, in that order. Gbert SC models also benefit significantly from domain-adapted models over all shot sizes (except 10 and 400 shots), but not from task adaptation or their combination. Overall, we observe a more consistent effect of further-pretraining for PET models compared to SC models.
$\le$
100 shots. Accuracy gradually increases with task-specific, domain-specific and combined pretraining, in that order. Gbert SC models also benefit significantly from domain-adapted models over all shot sizes (except 10 and 400 shots), but not from task adaptation or their combination. Overall, we observe a more consistent effect of further-pretraining for PET models compared to SC models.
Medbertde. Further-pretraining shows no consistent performance improvement for medbertde model variants. In particular, with 20 shots, the medbertde-base PET model outperforms the further-pretrained models, achieving a statistically significant
![]() $79.1\%$
accuracy. For few-shot sizes
$79.1\%$
accuracy. For few-shot sizes
![]() $10$
and
$10$
and
![]() $50$
–
$50$
–
![]() $400$
, the best performing model alternates between the medbertde-base-domain and medbertde-base-comb PET models. Similar to gbert models, the relative gain of pretraining decreases with increasing shot sizes. It appears that our pretraining method using cardiovascular doctor’s letters has no impact or may even impair the medbertde model. A possible reason could be that the public medbertde model was only pretrained on
$400$
, the best performing model alternates between the medbertde-base-domain and medbertde-base-comb PET models. Similar to gbert models, the relative gain of pretraining decreases with increasing shot sizes. It appears that our pretraining method using cardiovascular doctor’s letters has no impact or may even impair the medbertde model. A possible reason could be that the public medbertde model was only pretrained on
![]() $10$
G of clinical and medical texts, primarily from the oncology domain. However, future research is needed for further investigation (pretraining data information cf. Fig. 3).
$10$
G of clinical and medical texts, primarily from the oncology domain. However, future research is needed for further investigation (pretraining data information cf. Fig. 3).
Best-performing model variant. According to our core experiments, the overall best-performing model is the gbert domain- and task-adapted model (gbert-base-comb). This model achieved best accuracy scores with shot sizes
![]() $\leq$
100 compared to other pretraining methods and to fine-tuned SC models with shot sizes
$\leq$
100 compared to other pretraining methods and to fine-tuned SC models with shot sizes
![]() $\le$
400. When using only 20 shots, this model outperforms the SC model by
$\le$
400. When using only 20 shots, this model outperforms the SC model by
![]() $30.5$
percentage points (pp.) and the public gbert-base PET model by
$30.5$
percentage points (pp.) and the public gbert-base PET model by
![]() $11.5$
pp. Hence, we select this model for all additional experiments. If not further pretrained medbertde-base outperforms public gbert-base: this is similar to our baseline experiments. However, further-pretraining does not improve the performance of medbertde-base, possibly due to the relatively small pretraining data size of medbertde-base (
$11.5$
pp. Hence, we select this model for all additional experiments. If not further pretrained medbertde-base outperforms public gbert-base: this is similar to our baseline experiments. However, further-pretraining does not improve the performance of medbertde-base, possibly due to the relatively small pretraining data size of medbertde-base (
![]() $10$
G).
$10$
G).
Robustness. Experiments were performed using three training sets and two initial random seeds. For smaller shot sizes (
![]() $\le 50$
shots), standard deviation was low (
$\le 50$
shots), standard deviation was low (
![]() $\sim 2.5\%$
) decreasing to less than
$\sim 2.5\%$
) decreasing to less than
![]() $1\%$
for larger sizes. We observed this for gbert and medbertde with no impact of different pretraining methods.
$1\%$
for larger sizes. We observed this for gbert and medbertde with no impact of different pretraining methods.
3.2.1 Inspecting primary classes
We investigate the impact of shot size on the accuracy of predicting the selected primary section classes (Fig. 6a). Across shot sizes 20–50, the
![]() $F1$
-scores of both classes increase in average by
$F1$
-scores of both classes increase in average by
![]() $9.2\%$
pp. Anamnese, with a lower
$9.2\%$
pp. Anamnese, with a lower
![]() $F1$
-score, benefits more from larger few-shot sizes. However, the SC model trained on the full training set significantly outperforms the 50-shot models. This is especially true for the Anamnese class. Even if shot size is increased to our maximum of 400 shots, the results still differ significantly: (Anamnese:
$F1$
-score, benefits more from larger few-shot sizes. However, the SC model trained on the full training set significantly outperforms the 50-shot models. This is especially true for the Anamnese class. Even if shot size is increased to our maximum of 400 shots, the results still differ significantly: (Anamnese:
![]() $82.4\%$
, Medikation: 97.5%). Results for more semi-structured classes like Medikation are closest to the performance of the full model. For results of all shot sizes cf. Suppl. Fig. S4.
$82.4\%$
, Medikation: 97.5%). Results for more semi-structured classes like Medikation are closest to the performance of the full model. For results of all shot sizes cf. Suppl. Fig. S4.

Figure 6. Core experiments: primary class
![]() $F1$
-score in percentage and selected Shapley values: (a)
$F1$
-score in percentage and selected Shapley values: (a)
![]() $F1$
-score scores per few-shot sizes for primary classes with using gbert-base-comb nocontext. (b) Shapley value analysis for gbert-base-comb nocontext with respect to Anamnese and Zusammenfassung prediction. First column: true label of the sample, second column: predicted label including label probability, third column: selected Shapley values. We used 20 training shots. For readability reasons, we grouped some token sequences. Further details, see Suppl. Fig. S7. Legend: Blue: positive contribution, Red: negative contribution.
$F1$
-score scores per few-shot sizes for primary classes with using gbert-base-comb nocontext. (b) Shapley value analysis for gbert-base-comb nocontext with respect to Anamnese and Zusammenfassung prediction. First column: true label of the sample, second column: predicted label including label probability, third column: selected Shapley values. We used 20 training shots. For readability reasons, we grouped some token sequences. Further details, see Suppl. Fig. S7. Legend: Blue: positive contribution, Red: negative contribution.
While our primary classes benefit from further-pretraining,
![]() $F1$
-score of Anrede slightly decreased. A possible explanation could be that Anrede often contains non-clinical terminology that describes a patient’s place of residence, date of birth and name (Suppl. Fig. S5).
$F1$
-score of Anrede slightly decreased. A possible explanation could be that Anrede often contains non-clinical terminology that describes a patient’s place of residence, date of birth and name (Suppl. Fig. S5).
3.2.2 Inspecting Shapley values
To better understand model predictions in a few-shot setting, we further analyzed Shalpey values of the 20-shot model for the lower-performing class Anamnese. We chose a false positive sample as the running example for the remainder of this study because Anamnese belongs to our primary classes and often suffers from a low precision rate (for 20-shots, gbert-base-comb achieves
![]() $44.6\%$
precision and
$44.6\%$
precision and
![]() $62.2\%$
recall, cf. Suppl. Fig. S6). Table 6b illustrates selected Shapley values per token for the sample: ’Die Aufnahme der Patientin erfolgte bei akutem Myokardinfarkt -LRB- STEMI -RRB- . (English: The patient was admitted due to an acute myocardial infarction -LRB- STEMI -RRB-.)’ toward the classes Anamnese and Zusammenfassung, respectively.
$62.2\%$
recall, cf. Suppl. Fig. S6). Table 6b illustrates selected Shapley values per token for the sample: ’Die Aufnahme der Patientin erfolgte bei akutem Myokardinfarkt -LRB- STEMI -RRB- . (English: The patient was admitted due to an acute myocardial infarction -LRB- STEMI -RRB-.)’ toward the classes Anamnese and Zusammenfassung, respectively.
The model incorrectly classified this sample as Anamnese, with
![]() $76.8\%$
probability, while the correct class is predicted with
$76.8\%$
probability, while the correct class is predicted with
![]() $18.2\%$
probability score. Tokens such as Die (the), Aufnahme (admission), Patient (patient), erfolgte (took place) positively contributed to the Anamnese class, while the tokens Aufnahme and Patient negatively contributed to the correct Zusammenfassung class. Analyzing the 20-shot training dataset, we observe that these keywords occur more frequently in samples for Anamnese (Die (13x), Aufnahme (6x), Patient (7x), erfolgte (8x)) than in samples from Zusammenfassung (Die (5x), Aufnahme (2x), Patient (5x), erfolgte (6x)). The token Myokardinfarkt (acute myocardia) positively contributes to both section classes, and to a higher extent to Anamnese, even though we only observe this token in instances from Zusammenfassung. The token sequences representing brackets -LRB- and -RRB- contribute strongly positively to Anamnese. Analyzing the training data showed a higher frequency of these tokens in Anamnese samples (11x) compared to Zusammenfassung (5x).
$18.2\%$
probability score. Tokens such as Die (the), Aufnahme (admission), Patient (patient), erfolgte (took place) positively contributed to the Anamnese class, while the tokens Aufnahme and Patient negatively contributed to the correct Zusammenfassung class. Analyzing the 20-shot training dataset, we observe that these keywords occur more frequently in samples for Anamnese (Die (13x), Aufnahme (6x), Patient (7x), erfolgte (8x)) than in samples from Zusammenfassung (Die (5x), Aufnahme (2x), Patient (5x), erfolgte (6x)). The token Myokardinfarkt (acute myocardia) positively contributes to both section classes, and to a higher extent to Anamnese, even though we only observe this token in instances from Zusammenfassung. The token sequences representing brackets -LRB- and -RRB- contribute strongly positively to Anamnese. Analyzing the training data showed a higher frequency of these tokens in Anamnese samples (11x) compared to Zusammenfassung (5x).
Note on interpreting Shapley values. Shapley values are additive: they sum up all token contributions along with the base value to yield the prediction probability. Shapley values toward different classes and of different models cannot be compared by absolute value, but only relative to other tokens for the same prediction and the same model.
3.3 Additional experiments
3.3.1 Model size
Given the limited computational resources in clinical infrastructures, we investigated how model size affects performance and investigate its impact with finer-grained analyses. Since there is no medbertde-large model available, we compared gbert-large and gbert-base models.
Larger model size increases accuracy significantly, by an average of
![]() $7.2$
pp. for SC models
$7.2$
pp. for SC models
![]() $\le$
$\le$
![]() $100$
. PET models, by contrast, benefit less from larger model size than SC models. We even observe a slight performance decrease for shot size 20 (Table 7a). The only significant increase, of
$100$
. PET models, by contrast, benefit less from larger model size than SC models. We even observe a slight performance decrease for shot size 20 (Table 7a). The only significant increase, of
![]() $1.1$
points accuracy, we observed for shot size
$1.1$
points accuracy, we observed for shot size
![]() $50$
.
$50$
.

Figure 7. Model size: (a) Accuracy scores in percentage for gbert-comb nocontext PLMs using all templates on four few-shot sizes. (b)
![]() $F1$
-scores in percentage for primary classes for gbert-comb no context PLMs using all templates on various few-shot sizes.
$F1$
-scores in percentage for primary classes for gbert-comb no context PLMs using all templates on various few-shot sizes.
Primary classes: Gbert-large yields an increased
![]() $F1$
-score for Anamense with both shot sizes (
$F1$
-score for Anamense with both shot sizes (
![]() $20, 50$
), by an average of
$20, 50$
), by an average of
![]() $+4.7$
pp. but this is only significant for shot size
$+4.7$
pp. but this is only significant for shot size
![]() $50$
. By contrast, the difference in
$50$
. By contrast, the difference in
![]() $F1$
-score (
$F1$
-score (
![]() $0.1\%-0.4\%$
) for Medikation is not statistically significant (Fig. 7b).
$0.1\%-0.4\%$
) for Medikation is not statistically significant (Fig. 7b).
Shapley values: Both models, gbert-base-comb and gbert-large-comb incorrectly classify our running example belonging to Zusammenfassung as Anamnese. We do not observe significant differences in the respective token contributions (Suppl. Fig. S8).
3.3.2 Null prompts
Inspired by insights of Logan et al. (Reference Logan2022) – who removed all tokens from prompt templates, using null prompts instead, with comparable classification results – we evaluated the gbert-base-comb model using only three null prompt templates (cf. Section 2.1.1).
Null prompts slightly decrease accuracy scores for shot sizes
![]() $\leq$
50 by approximately one percentage point. For shot sizes 100 and 400, we note a slight accuracy increase. We only observed statistically significant differences in accuracy for shot-size 50 (template-based model:
$\leq$
50 by approximately one percentage point. For shot sizes 100 and 400, we note a slight accuracy increase. We only observed statistically significant differences in accuracy for shot-size 50 (template-based model:
![]() $85.6\%$
, null-prompt model:
$85.6\%$
, null-prompt model:
![]() $84.6\%$
) (Suppl. Tab. S3).
$84.6\%$
) (Suppl. Tab. S3).
Primary classes: For our primary classes, we did not observe a consistent pattern. Null prompts have a slightly negative impact on
![]() $F1$
-scores for Anamnese and Medikation with 20 shots. By contrast, with 50 shots, accuracy significantly decreases for Anamnese, but slightly increases for Medikation (
$F1$
-scores for Anamnese and Medikation with 20 shots. By contrast, with 50 shots, accuracy significantly decreases for Anamnese, but slightly increases for Medikation (
![]() $92.4\%$
vs.
$92.4\%$
vs.
![]() $95.9\%$
).
$95.9\%$
).
3.3.3 Adding context
Predicting section classes is difficult for tokens that frequently occur in different classes, as discussed for the example in Fig. 6. To reduce the degree of ambiguity of individual tokens, we experimented with two types of contextualization of classification instances: Adding (1) the previous and subsequent paragraph (context) and (2) only the previous paragraph (prevcontext). Suppl. Fig. S9 shows that across all few-shot sizes, (1) context (with mean
![]() $+2.4$
accuracy points) and (2) prevcontext (with mean
$+2.4$
accuracy points) and (2) prevcontext (with mean
![]() $+1.6$
accuracy points) both achieve significantly higher accuracy than nocontext models (cf. Section 2.5.4).
$+1.6$
accuracy points) both achieve significantly higher accuracy than nocontext models (cf. Section 2.5.4).
Primary classes: Context models improve the
![]() $F1$
-scores for both primary classes (by mean
$F1$
-scores for both primary classes (by mean
![]() $+7.8$
points for Anamnese and
$+7.8$
points for Anamnese and
![]() $+1.3$
for Medikation) (Fig. 8a). For Anamnese, statistically significant improvement is only reached using 50 shots.
$+1.3$
for Medikation) (Fig. 8a). For Anamnese, statistically significant improvement is only reached using 50 shots.

Figure 8. Additional experiments (context) – primary classes
![]() $F1$
-scores and selected Shapley values: (a)
$F1$
-scores and selected Shapley values: (a)
![]() $F1$
-scores in percentage per few-shot sizes for primary classes with nocontext and context using gbert-base-comb. Comparing to gbert-base-comb trained on full training data with nocontext and context. (b) Shapley value analysis for gbert-base-comb nocontext and gbert-base-comb context. First column: true label of the sample, second column: predicted label including label probability, third column: selected Shapley values. We used 20 training shots. For readability reasons, we grouped some token sequences. Further details, see Suppl. Fig. S10. Legend: Blue: positive contribution, Red: negative contribution.
$F1$
-scores in percentage per few-shot sizes for primary classes with nocontext and context using gbert-base-comb. Comparing to gbert-base-comb trained on full training data with nocontext and context. (b) Shapley value analysis for gbert-base-comb nocontext and gbert-base-comb context. First column: true label of the sample, second column: predicted label including label probability, third column: selected Shapley values. We used 20 training shots. For readability reasons, we grouped some token sequences. Further details, see Suppl. Fig. S10. Legend: Blue: positive contribution, Red: negative contribution.
Shapley values: gbert-base-comb context correctly classifies our running example with
![]() $86.6\%$
probability (Table 8b). Most highly contributing tokens belong to the context (previous or following, with Shapley values:
$86.6\%$
probability (Table 8b). Most highly contributing tokens belong to the context (previous or following, with Shapley values:
![]() $0.057+0.596$
), while the main paragraph has an accumulated Shapley value of
$0.057+0.596$
), while the main paragraph has an accumulated Shapley value of
![]() $0.106$
. The previous context contains the sequence: Zusammenfassende Beurteilung, a frequent section-specific title. The subsequent paragraph is the longest paragraph (
$0.106$
. The previous context contains the sequence: Zusammenfassende Beurteilung, a frequent section-specific title. The subsequent paragraph is the longest paragraph (
![]() $37$
tokens). Previously negatively contributing tokens (Aufnahme and Patient) are now positively contributing to the correct class: Zusammenfassung.
$37$
tokens). Previously negatively contributing tokens (Aufnahme and Patient) are now positively contributing to the correct class: Zusammenfassung.
Table 3.
Combining and evaluating best-performing methods: Accuracy scores in percentage for gbert-large-comb context evaluated on few-shot sizes [
![]() $20,50,100,400$
] with base vs. large model sizes in context vs. nocontext settings using PET. Comparison to corresponding SC model fine-tuned on full training set.
$20,50,100,400$
] with base vs. large model sizes in context vs. nocontext settings using PET. Comparison to corresponding SC model fine-tuned on full training set.

3.3.4 Combining best-performing methods
Our core experiments indicated that the gbert-base-comb model performed best of all tested models. The additional experiments showed that models using all five templates (cf. Section 2.1.1), a BERT-large architecture and contextualization often achieved the best performance. Hence, we investigated whether this combination (gbert-large-comb context trained with all templates) could further close the performance gap to a model trained on full training set.
Table 3 shows that gbert-large-comb context significantly outperforms both gbert-base-comb and gbert-large-comb without context. Moreover, gbert-large-comb context statistically significantly outperforms gbert-base-comb context for
![]() $20, 100$
and
$20, 100$
and
![]() $400$
shots. Overall, the gbert-large-comb context outperforms nocontext and base models over all shot-sizes, yielding best results with 400 shots. Yet, PET still lags behind the full SC setting, with a minimal gap of
$400$
shots. Overall, the gbert-large-comb context outperforms nocontext and base models over all shot-sizes, yielding best results with 400 shots. Yet, PET still lags behind the full SC setting, with a minimal gap of
![]() $-5.2$
points accuracy.
$-5.2$
points accuracy.
Primary classes: For our primary classes, gbert-large-comb context now outperforms gbert-large-comb nocontext by large margin (Fig. 9a). Only the 50-shot results for Anamnese are not statistically significant (
![]() $F1$
-score of all shot-sizes cf. Suppl. Fig. S11).
$F1$
-score of all shot-sizes cf. Suppl. Fig. S11).

Figure 9. Additional experiments (combined methods) – primary classes
![]() $F1$
-scores and selected Shapley values: (a)
$F1$
-scores and selected Shapley values: (a)
![]() $F1$
-scores in percentage per few-shot sizes for primary classes with nocontext and context using gbert-large-comb. Comparing to gbert-large-comb trained on full training data with context. (b) Shapley value analysis for gbert-base-comb context and gbert-large-comb context. First column: true label of the sample, second column: predicted label including label probability, third column: selected Shapley values. We used 20 training shots. For readability reasons, we grouped some token sequences. More detailed results, see Suppl. Fig. S13. Legend: Blue: positive contribution}?>, Red: negative contribution.
$F1$
-scores in percentage per few-shot sizes for primary classes with nocontext and context using gbert-large-comb. Comparing to gbert-large-comb trained on full training data with context. (b) Shapley value analysis for gbert-base-comb context and gbert-large-comb context. First column: true label of the sample, second column: predicted label including label probability, third column: selected Shapley values. We used 20 training shots. For readability reasons, we grouped some token sequences. More detailed results, see Suppl. Fig. S13. Legend: Blue: positive contribution}?>, Red: negative contribution.
We also compared the large and base versions of gbert-*-comb context. The
![]() $F1$
-score for Anamnese is significantly increased by
$F1$
-score for Anamnese is significantly increased by
![]() $+14.6$
points with 20 shots and by
$+14.6$
points with 20 shots and by
![]() $+2.6$
points with 50 shots. Performance for Medikation is significantly increased by
$+2.6$
points with 50 shots. Performance for Medikation is significantly increased by
![]() $+2.2$
points with 20 shots, but insignificantly decreased with 50 shots. (Suppl. Fig. S12)
$+2.2$
points with 20 shots, but insignificantly decreased with 50 shots. (Suppl. Fig. S12)
Shapley values: We tested whether the token contributions differ between the large and base gbert-*-comb context models (Table 9b). The large model predicts the true class Zusammenfassung with
![]() $99.2\%$
probability,
$99.2\%$
probability,
![]() $+12.7$
points above the base model. The large context model now also places greater emphasis on the main paragraph, as opposed to the context. The ratio of the accumulated Shapley values (
$+12.7$
points above the base model. The large context model now also places greater emphasis on the main paragraph, as opposed to the context. The ratio of the accumulated Shapley values (
![]() $\frac{{classified\ instance}}{{context\ paragraphs}}$
, higher is better) is
$\frac{{classified\ instance}}{{context\ paragraphs}}$
, higher is better) is
![]() $0.36$
for gbert-large-comb context and
$0.36$
for gbert-large-comb context and
![]() $0.16$
for gbert-base-comb context.
$0.16$
for gbert-base-comb context.
4. Discussion
In this section, we discuss our empirical findings in light of the challenges and proposed solutions outlined in Section 1.
-
S1 Domain- and Expert-dependent. In in-depth evaluations, we compared four pretraining approaches using PET and SC for two public German-language models (Gururangan et al. Reference Gururangan2020): (1) initial pretraining using general German texts with gbert versus exclusively medical and clinical data with medbertde (Fig. 3); and further-pretraining of these PLMs for (2) task-adaption, (3) domain-adaptation, and (4) combined task and domain-adaptation. Finding. Gbert overall accuracy gradually improved with further-pretraining. The task- and domain-adapted gbert-base-comb performs best compared to all models, and with only 20 shots outperforms gbert-base by
 $+11.5$
accuracy points. Also, the positive effect of further-pretraining was more consistent for PET compared to SC models. By contrast, further-pretrained medbertde-based SC and PET models did not achieve consistent performance improvements. Finding. Pretraining from scratch with sufficient clinical and medical data can benefit various MIE tasks. However, when pretraining data limited and/or concentrated on a narrow domain, for example oncology, as in the case of medbertde, further-pretraining was found not to enhance performance. Finding. While medbertde-base without further-pretraining outperformed gbert-base in all shot sizes, and similarly when trained on the full dataset (Fig. 5), it did not improve performance if further pretrained and was outperformed by further-pretrained gbert-base.
$+11.5$
accuracy points. Also, the positive effect of further-pretraining was more consistent for PET compared to SC models. By contrast, further-pretrained medbertde-based SC and PET models did not achieve consistent performance improvements. Finding. Pretraining from scratch with sufficient clinical and medical data can benefit various MIE tasks. However, when pretraining data limited and/or concentrated on a narrow domain, for example oncology, as in the case of medbertde, further-pretraining was found not to enhance performance. Finding. While medbertde-base without further-pretraining outperformed gbert-base in all shot sizes, and similarly when trained on the full dataset (Fig. 5), it did not improve performance if further pretrained and was outperformed by further-pretrained gbert-base. -
S2 Resource-constraints. Prompt-based fine-tuning with PET produces superior classification results in few-shot learning scenarios. Finding. We observed a steady increase in the performance of PET compared to SC models With decreasing few-shot training set sizes (400-10 shots). Using 20 shots, the PET gbert-base-comb nocontext model outperforms the corresponding SC model by
 $+30.5$
pp. The same gbert-base-comb nocontext PET model with 50 shots even rivals the SC model trained on full data, leaving a gap of
$+30.5$
pp. The same gbert-base-comb nocontext PET model with 50 shots even rivals the SC model trained on full data, leaving a gap of
 $-11.1$
pp. Especially semi-structured section classes, such as Medikation, perform close to the full model by
$-11.1$
pp. Especially semi-structured section classes, such as Medikation, perform close to the full model by
 $-6.3$
pp. ( Fig. 6a). Our few-shot models are also robust as measured by standard deviation. Finding. Null prompts exhibit comparable results with no significant difference in performance, especially with few-shot sizes exceeding
$-6.3$
pp. ( Fig. 6a). Our few-shot models are also robust as measured by standard deviation. Finding. Null prompts exhibit comparable results with no significant difference in performance, especially with few-shot sizes exceeding
 $100$
. Finding. Contextualize data with surrounding context paragraphs improved classification results for most section classes, especially primary classes. It allowed our base models to correctly predict our running false-positive sample as Zusammenfassung. However, compared to the base models interpretability analysis using SHAP revealed that the large model places greater emphasis on main paragraph tokens rather than on context paragraphs. Contextualization further reduced the accuracy gap between gbert-*-comb context-based PET models trained on 50 shots to the full SC model to
$100$
. Finding. Contextualize data with surrounding context paragraphs improved classification results for most section classes, especially primary classes. It allowed our base models to correctly predict our running false-positive sample as Zusammenfassung. However, compared to the base models interpretability analysis using SHAP revealed that the large model places greater emphasis on main paragraph tokens rather than on context paragraphs. Contextualization further reduced the accuracy gap between gbert-*-comb context-based PET models trained on 50 shots to the full SC model to
 $-9$
to
$-9$
to
 $-9.5$
pp; for classes such as Medikation even to
$-9.5$
pp; for classes such as Medikation even to
 $-5$
to
$-5$
to
 $-6$
pp. Contextualization does not require complex preprocessing or manual annotation.
$-6$
pp. Contextualization does not require complex preprocessing or manual annotation. -
S3 On-premise: Using smaller models saves computational resources. We therefore compared classification performances of base and large BERT PLMs. Finding. Large PLMs achieve better classification results. However, model size has a lower impact on the performance of PET compared to SC models (Fig. 7a). For classes such as Medikation the further-pretrained gbert-base-comb PLM performs almost on par with gbert-large-comb (Fig. 7b) Finding. For complex sections with free text such as Anamnese, gbert-large PLMs achieved better performance. They also better recognize contextualized instances (Table 9b and Suppl. Sect. S1.2).
-
S4 Transparency. Shapley values (Lundberg et al. Reference Lundberg and Lee2017) an interpretability method based on saliency features and helped identify problems in training data quality and model decisions. We identified tokens that frequently occur in false-positive classes by analyzing model predictions (Fig. 8). Finding. The use of Shapley features is especially beneficial in few-shot scenarios, as it enables data engineers to select few-shot samples with high precision. Shapley values also proved instrumental for identifying problems with contextualization: It became clear that with very small shot sizes, and for section classes with short spans, the model prioritized the context over the instance to be classified. They also provided evidence that our gbert-large-comb model outperforms its base counterpart by focusing on key parts of contextualized samples. Finding. Our analysis of Shapley values showed that gbert-large-comb makes more reliable predictions than gbert-base-comb, by prioritizing features of instances to be classified over context (Table 9b).
5. Conclusions and recommendations
In this work, we have presented best-practice strategies to identify an ideal setup to address the multifaceted challenges of conducting a MIE task, such as clinical section classification, in a lower-resource domain and language such as the German clinical domain. In summary, our best-performing setup used a task- and domain-adapted BERT-large architecture trained with PET on contextualized samples using all five template types.
To reduce the demand for clinical knowledge in MIE we showed in S
![]() $_1$
that few-shot prompting performed particularly well with further-pretrained general-domain PLMs and helped to reduce the demand of clinical expert knowledge for manual data annotation. Our experiments revealed that pretraining data have a strong impact on few-shot learning results (see S
$_1$
that few-shot prompting performed particularly well with further-pretrained general-domain PLMs and helped to reduce the demand of clinical expert knowledge for manual data annotation. Our experiments revealed that pretraining data have a strong impact on few-shot learning results (see S
![]() $_2$
), especially if training data are limited. Specifically, general domain PLMs such as gbert, pretrained on massive amounts of general language, can be effectively domain- and task-adapted by further-pretraining on clinical routine data. In contrast, PLMs pretrained on domain-specific data from scratch, such as medbertde may outperform gbert if not further-pretrained, but may not benefit from further-pretraining. Therefore, if further-pretraining for domain adaptation is not feasible due to IT constraints, we recommend choosing clinical PLMs like medbertde over non-adapted general PLMs.
$_2$
), especially if training data are limited. Specifically, general domain PLMs such as gbert, pretrained on massive amounts of general language, can be effectively domain- and task-adapted by further-pretraining on clinical routine data. In contrast, PLMs pretrained on domain-specific data from scratch, such as medbertde may outperform gbert if not further-pretrained, but may not benefit from further-pretraining. Therefore, if further-pretraining for domain adaptation is not feasible due to IT constraints, we recommend choosing clinical PLMs like medbertde over non-adapted general PLMs.
Our study indicated that prompt-based learning methods improve classification results if annotated data are rare, and effectively reduces time investment and costs of manual data annotation. The larger the amount of annotated data, the higher the efficiency of null prompts, which further save engineering time (see S
![]() $_2$
). Moreover, contextualizing classification instances improves performance, especially for the primary classes, and further closes the gap to full models.
$_2$
). Moreover, contextualizing classification instances improves performance, especially for the primary classes, and further closes the gap to full models.
We found in S
![]() $_3$
that in case of limited computing resources, prompting methods allow practitioners to employ smaller PLMs in a few-shot scenario, while achieving classification results comparable to larger models. However, free-text sections, such as Anamnese, may still benefit from larger model architectures (Fig. 7).
$_3$
that in case of limited computing resources, prompting methods allow practitioners to employ smaller PLMs in a few-shot scenario, while achieving classification results comparable to larger models. However, free-text sections, such as Anamnese, may still benefit from larger model architectures (Fig. 7).
Finally, in S
![]() $_4$
, we addressed the need for transparent and trustworthy model predictions in low-resource German clinical NLP, and possible use cases for interpretability methods. Our study demonstrates that the analysis of Shapley values can help improve training data quality, which is especially important with small shot sizes. Examining Shapley values, or similar interpretability methods, can also inform model selection, by revealing tokens that contribute to classification errors in specific model types. Finally, model interpretability is crucial in safety-critical domains such as clinical routine, to enhance the trustworthiness of model predictions.
$_4$
, we addressed the need for transparent and trustworthy model predictions in low-resource German clinical NLP, and possible use cases for interpretability methods. Our study demonstrates that the analysis of Shapley values can help improve training data quality, which is especially important with small shot sizes. Examining Shapley values, or similar interpretability methods, can also inform model selection, by revealing tokens that contribute to classification errors in specific model types. Finally, model interpretability is crucial in safety-critical domains such as clinical routine, to enhance the trustworthiness of model predictions.
Our study presents strategies and best-practice approaches for optimizing MIE in lower-resource clinical language settings. It highlights the benefits of few-shot prompting with further-pretrained PLMs as a measure to reduce the demand for manual annotation by clinicians. We further demonstrate that prompt-based learning and contextualization significantly enhance classification accuracy, especially in low-resource scenarios, while keeping demands on computing resources low. We are certain that these insights help to advance MIE tasks in clinical settings in the context of lower-resource languages such as German.
6. Declarations
6.1 Ethics approval and consent to participate
The authors state that this study complies with the Declaration of Helsinki. Our task has been performed with respect to Section 46 Abs.2 Nr.2a (LKHG) and Section 13 Abs.1 Landesdatenschutzgesetz BW. In this context, we had the possibility to use the data for the purpose of optimizing internal clinical procedures.
6.2 Availability of data and materials
We used CARDIO:DE, a distributable German corpus containing 500 cardiovascular doctor’s letters from the clinical routine, for all our experiments (available with a signed DUA: https://doi.org/10.11588/data/AFYQDY). Annotations of the held-out datasets are not publicly available as authors of CARDIO:DE use it for shared task competitions. But they are available from the corresponding author on reasonable request. For more details about the dataset, preprocessing steps, data annotation, and data distribution, cf: Richter-Pechanski et al. (Reference Richter-Pechanski and Dieterich2023).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/nlp.2024.52.
Competing interests
The author(s) declare none.