Introduction
One of the key limiting factors in female fertility is oocyte quality, which reflects the developmental potential of an oocyte and plays a vital role in subsequent fertilization and embryonic development. Abnormal oocytes are characterized by multiple morphologic aspects, such as abnormal zona pellucida (ZP), irregular ooplasm, dark ooplasm, oocyte fragments and large perivitelline space, which may affect the developmental potential of preimplantation embryos and even lead to early miscarriage (Bartolacci et al., Reference Bartolacci, Intra, Coticchio, Dell’Aquila, Patria and Borini2022; Nikiforov et al., Reference Nikiforov, Grøndahl, Hreinsson and Andersen2022).
The human zona pellucida is a highly ordered structure composed of four glycoproteins named zona pellucida sperm-binding proteins 1–4 (ZP1–ZP4), which are secreted by oocytes and granulosa cells during the process of follicle formation (Wassarman and Litscher, Reference Wassarman and Litscher2021). The zona pellucida is seen initially as isolated extracellular deposits of nascent ZP fibrils that are gradually merged to form a thickening matrix surrounding the oocyte. During the fertilization process, sperm must penetrate the ZP to fuse with the oocyte; when a sperm and oocyte fuse, the ZP can play a role in preventing polyspermic fertilization (Litscher and Wassarman, Reference Litscher and Wassarman2020).
After fertilization, the ZP protects the cleavage-stage embryo from being transported from the fallopian tube into the endometrial cavity (Litscher and Wassarman, Reference Litscher and Wassarman2020). The morphology of the ZP is one of the predictors of oocyte and embryo quality (Lunn and Wright, Reference Lunn and Wright2013). Moreover, the density, structure, or interaction of ZP proteins are related to the quality of oocytes and can also be used to predict embryo implantation and abortion rates (Sauerbrun-Cutler, Reference Sauerbrun-Cutler, Vega, Breborowicz, Gonzales, Stein, Lederman and Keltz2015; Zhou et al., Reference Zhou, Ni, Wu, Chen, Xu, Zhang, Mu, Li, Yan, Fu, Wang, Zhao, Dong, Sun, Kuang, Sang and Wang2019; Cao et al., Reference Cao, Zhao, Zhang, Zhang, Lu, Wang, Hu, Ling, Zhang and Huo2020). Therefore, the ZP is closely related to the prognosis of oocyte maturation, fertilization, embryo development and pregnancy outcome. Recently, many studies have reported abnormalities of the ZP in the assisted reproductive treatment cycle and retrospectively analyzed the effects of abnormal ZP on oocyte maturation, embryo development and pregnancy outcomes (Li et al., Reference Li, Ma, Yang, Wu, Zhong, Yu and Chen2014; Shi et al., Reference Shi, Xu, Wu, Jin, Luan, Luo, Zhu, Johansson, Liu and Tong2014; Sousa et al., Reference Sousa, Teixeira da Silva, Silva, Cunha, Viana, Oliveira, Sá, Soares, Oliveira and Barros2015; Pan and Zhang, Reference Pan and Zhang2020; Yang et al., Reference Yang, Yang, Yang, Wang, Zhu, Wang, Ding, Rao, Xue, Peng, Wang, Cao, Zou, Chen and Zhang2022). However, there is still a lack of consensus among different laboratories regarding the definition of ZP abnormalities, the causes of abnormal ZP, and the causes of infertility led by ZP abnormalities.
According to the severity of ZP abnormalities, this study divided 245 cases of oocyte retrieval cycles into three categories (mild group, moderate group and severe group). In addition, we enrolled 345 cycles with normal ZP as the control group and analyzed the effects of ZP abnormalities on oocyte quality, fertilization, embryo development and clinical outcomes among four different groups.
Materials and methods
Study design
This study retrospectively analyzed patients who underwent in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment and had abnormal ZP morphology of retrieved oocytes at the Reproductive Center of Drum Tower Hospital Affiliated with Nanjing University Medical College from January 2015 to December 2020. The exclusion criteria were preimplantation genetic testing (PGT), testicular epididymal sperm aspiration (TESA)/percutaneous epididymal sperm extraction (PESA), uterine pathology, and female age over 38 years. The total number of included oocyte retrieval cycles with abnormal ZP was 245, among which 67 cycles were cancelled because of no usable embryos. There were 345 cycles selected as the control group in which oocyte retrieval occurred on the same day. This retrospective study was approved by the Medical Ethics Committee of Drum Tower Hospital Affiliated with Nanjing University Medical College (Nanjing, China; Approval No. 2021-484-01).
Definition of abnormal ZP
ZP abnormality is defined as the zona pellucida morphology of the retrieved oocyte being transparent and dense (Figure 1). The detailed criteria are as follows: the ZP is thinned or thickened, the colour is transparent, the outer edge is jagged or smooth, and the shape is sometimes irregular, accompanied by a small or no perivitelline space. Abnormal ZP will be defined according to ZP morphology at the time of fertilization (intracytoplasmic sperm injection, ICSI) or 5 h after fertilization (conventional IVF).
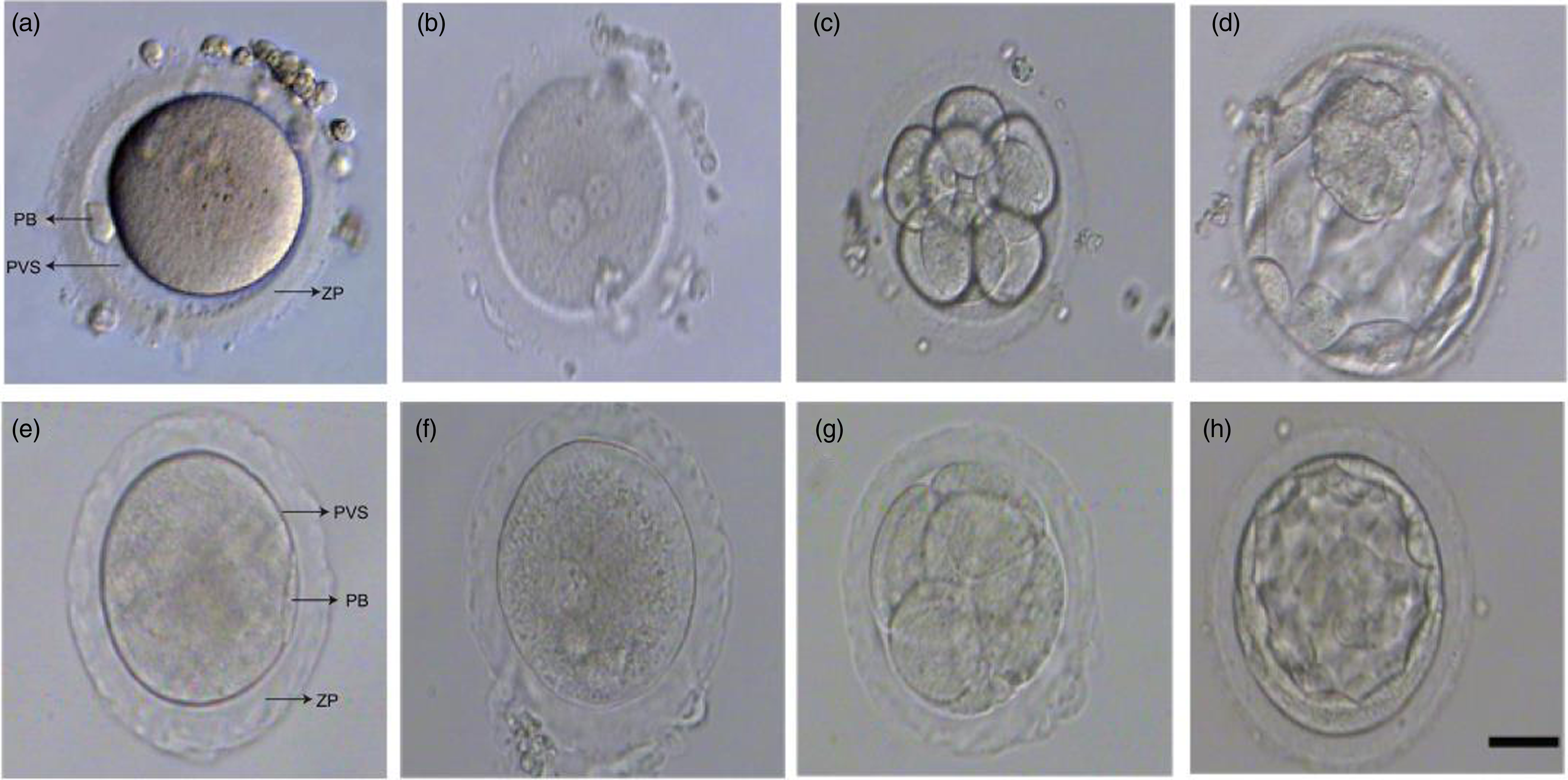
Figure 1. Representative images of early embryo development from human oocytes with normal and abnormal ZP. (a, e) Human mature metaphase II oocyte (MII) with normal and abnormal ZP. (b, f) Human zygote with normal and abnormal ZP. (c, g) Cleavage embryo (D3) with normal and abnormal ZP. (d, h) Blastocyst (D5) with normal and abnormal ZP. Scare bar represents 50 µm. PB, Polar body; PVS, Perivitelline space; ZP, Zona pellucida.
Classification of abnormal ZP oocytes
Three oocyte morphological parameters, namely, perivitelline space, percentage of immature oocytes and percentage of abnormal morphology oocytes, were subjectively scored (Table 1). The classification of ratings was graded according to the final score, and the patients with abnormal ZP oocytes were assessed in three grades: Grade 1 was the mild group, Grade 2 was the moderate group, and Grade 3 was the severe group (Table 1).
Table 1. The degree of ZP abnormality

The numbers in parentheses represent scores based on different parameters. The classification of ratings was graded according to the final score.
Ovarian stimulation
Controlled ovarian hyperstimulation (COH) was performed using the conventional protocols in our centre, which were adapted according to oestrogen, progestogen, and luteinizing hormone levels and vaginal ultrasound results. When the average diameter of the largest follicle was greater than or equal to 17 mm, 10,000 IU of hCG was injected that night, and 30–36 h later, the oocytes were collected through vaginal ultrasound guidance in strict accordance with the requirements of sterility.
Oocyte insemination and embryo culture
Following oocyte retrieval, oocytes were fertilized using conventional IVF or ICSI according to the sperm parameters of the patient. In the IVF, oocyte–cumulus complexes were collected in 0.5 ml of IVFplus (Vitrolife, Sweden) medium and inseminated with capacitated sperm for 5 h. A fertilization check was performed under an inverted microscope after insemination and confirmed by the presence of the second polar body. ZP morphology was assessed according to the above criteria. For ICSI-fertilized oocytes, ZP morphology was assessed during injection, and fertilization was confirmed by identification of pronuclei 16–18 h after insemination. The fertilized oocytes were cultured in G1plus (Vitrolife, Sweden) for 3 days and changed to G2plus (Vitrolife, Sweden) for another 2 or 3 days for embryo culture.
Fresh embryo transfer
On the third or fifth day after oocyte retrieval, patients with an endometrial thickness of more than 7 mm and without any physical complaints were scheduled for fresh embryo transfer. Progesterone and dydrogesterone tablets were used for corpus luteal support. The number of embryos transferred was one or two. On the day of embryo transfer, the embryo was transferred into the uterus guided by ultrasound.
Embryo cryopreservation
A routine process is to transfer or vitrify one or two good-quality cleavage-stage embryos on the third day or a good blastocyst on the fifth day after ovum pick-up and to culture the remaining embryos for 2–3 more days for blastocyst vitrification. The morphological assessment of cleavage-stage embryos was performed according to the Istanbul consensus (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011). The morphological assessment of blastocysts was performed according to Gardner’s scoring criteria (Gardner and Schoolcraft, Reference Gardner and Schoolcraft1999). Embryos were cryopreserved using the vitrification method. Cleavage-stage embryos were first equilibrated in equilibration solution (ES) (KITAZATO, Japan) at room temperature for 8–10 min, while blastocysts were equilibrated for 10–12 min and then placed in a vitrification solution (VS) (KITAZATO, Japan). Then, the embryos were transferred onto the tip of straws for less than 60 s. After being submerged in liquid nitrogen, the straw was transferred into a liquid nitrogen tank for long-term storage.
Frozen–thawed embryo transfer (FET)
For frozen–thawed embryo transfer cycles, endometrial preparation was performed through hormone replacement therapy (HRT). Cleavage-stage embryos were thawed and transferred after they were supplemented with oestrogen and progesterone for 18–19 days, or frozen–thawed blastocyst transfer was performed 20 days later.
Clinical outcome measures
The serum hCG level was measured 14 days after embryo transfer and considered positive if it exceeded 10 IU/ml. Hormone therapy was terminated if the serum hCG level was negative. A clinical pregnancy was defined as the presence of an intrauterine gestational sac on ultrasound 30 days after embryo transfer.
Analysis of data and statistics
The statistical analyses were conducted using SPSS 23.0 software. The results were expressed as the mean ± standard deviation, and intergroup comparisons were made using one-way analysis of variance (ANOVA). Count data are expressed as ratios and were compared using the chi-squared test or Fisher’s exact probability method. A significance level of 0.05 was applied to all tests, and multiple testing was corrected according to Holm–Bonferroni.
Results
Basic characteristics of participants
The baseline characteristics of the 590 oocyte pick-up (OPU) cycles are shown in Table 2. The results showed no significant differences in female age, body mass index (BMI), basic follicle-stimulating hormone, basic luteinizing hormone, basic estradiol or basic testosterone level among the four groups. However, all three abnormal ZP groups had a longer infertility duration than the control group (3.76 ± 2.53 years), and there were no significant differences among the mild (4.79 ± 2.37 years), moderate (4.63 ± 3.03 years) and severe groups (4.87 ± 3.23 years) (Table 2). The rates of primary infertility were similar among the mild group (84.15%), moderate group (82.61%), and severe group (81.69%) but higher than that in the control group (71.30%, P < 0.05). Moreover, there were no significant differences in the rate of the infertility diagnosis of pelvic inflammatory disease among the four groups (Table 2). Although the proportion of ICSI regimen was lower in the abnormal ZP groups than in the control group (84.35%, P < 0.001), and there were no significant differences among the mild (47.56%), moderate (63.04%) and severe groups (65.85%) (Table 2). Therefore, the proportion of the R-ICSI regimen was higher in the abnormal ZP groups than in the control group (15.65%, P < 0.001), there were no significant differences among the three abnormal ZP groups (Table 2). Furthermore, all three abnormal ZP groups had a higher proportion of repeated oocyte retrieval cycles than the control group (11.59%), but there were no significant differences among the mild (30.49%), moderate (34.78%) and severe groups (45.07%) (Table 2).
Table 2. General information of enrolled patients

BMI: Body mass index; E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; ICSI: Intracytoplasmic single sperm injection; OPU: Ovum pick-up; R-ICSI: Early rescue ICSI; T: Testosterone. Primary infertility refers to couples who have never successfully achieved pregnancy.
Measurement data are presented as the means ± standard deviations (SD); comparisons between two groups were performed using the t-test. Enumeration data are reported as percentages and were compared by the χ2 test. Letters a and b represent significant differences (Bonferroni test).
The degree of abnormal ZP and embryo development
Compared with the control group, the abnormal ZP groups produced significantly fewer retrieved oocytes, and the average number of oocytes obtained in the severe group was the lowest at 7.62 ± 4.29 (Table 3). However, the mild and moderate groups were not significantly different in terms of retrieved oocytes (9.48 ± 5.28 vs. 9.76 ± 5.32). Regarding mature oocytes, with increasing abnormal ZP severity, the number of mature oocytes decreased significantly, with the number in the three abnormal ZP groups being significantly lower than that in the control group (P < 0.001). Moreover, the severe group had an average number of mature oocytes of only 1.66 ± 2.08 (Table 3). The fertilization rate did not differ significantly among the four groups. Compared with that in the control group, the cleavage rate, day-3 high-quality embryo rate, and blastocyst formation rate of the abnormal ZP groups were significantly lower. Moreover, both the cleavage rate (90.43%) and day-3 high-quality embryo rate (41.18%) in the severe group were the lowest among the four groups (Table 3). Although it was significant that the blastocyst formation rate was lower in the abnormal ZP groups than in the control group (59.63%), there were no significant differences among the mild (51.25%), moderate (52.02%) and severe groups (47.62%; Table 3).
Table 3. The effect of the degree of zona pellucida abnormality on oocyte and embryo development

High-quality embryo: 7–12 blastomeres at equal size on day 3, with no fragmentation or less than 15%. Measurement data are presented as the means ± standard deviations (SD); enumeration data are reported as percentages and were compared by the χ2 test; Letters a, b, c and d represent significant differences (Bonferroni test).
The degree of abnormal ZP and clinical outcome of fresh embryo transfers
Compared with the control group, the abnormal ZP groups had a significantly lower implantation rate, clinical pregnancy rate and live birth rate in fresh embryo transfer cycles (Table 4). Among the three abnormal ZP groups, there were no significant differences in the implantation rate, clinical pregnancy rate, live birth rate or spontaneous miscarriage rate after fresh embryo transfer (Table 4). The number of fresh embryo transfer cycles in the severe group was 15; only four patients achieved clinical pregnancies, and three of them obtained live births (Table 4). The spontaneous miscarriage rates in fresh embryo transfer cycles were not significantly different among the four groups (Table 4).
Table 4. The effect of the degree of zona pellucida abnormality on the clinical results of fresh embryo transfer

Enumeration data are reported as percentages and were compared by the χ2 test. Letters a and b represent significant differences (Bonferroni test).
The degree of abnormal ZP and cumulative pregnancy
Further examination of the cumulative clinical pregnancy rate and the cumulative live birth rate from the different groups revealed that they were much lower in the abnormal ZP groups than in the control group (Table 5). Similarly, there were no significant differences among the ZP abnormality groups in terms of the cumulative clinical pregnancy rate or the cumulative live birth rate (Table 5). No significant differences were found in the rate of spontaneous miscarriage among the four groups. However, the cycle cancellation rate in the severe group was as high as 66.20%, which was significantly higher than those in the mild group (8.54%), the moderate group (14.13%) and the control group (5.22%). The cycle cancellation rates of the mild group and the control group were comparable (Table 5). Moreover, the cause of the cycle cancellation for three groups (the control group, the mild and the moderate groups) was mainly due to early embryo development arrest. However, the cause of the cycle cancellation for the severe group was not early embryo development arrest but immature and abnormal oocytes (63.83%, 30/47; Table 5).
Table 5. The effect of the degree of zona pellucida abnormality on the clinical results of cumulative embryo transfer

Enumeration data are reported as percentages and were compared by the χ2 test. Letters a, b and c represent significant differences (Bonferroni test).
The degree of abnormal ZP and neonatal outcomes
Neonatal outcomes were comparable among the four different groups (Table 6). In total, 267, 41 and 42 babies were born in the control group, the mild group and the moderate group, respectively, and only eight babies were born in the severe group. Gestational age and the rates of preterm birth, birth weight, and low birth weight were not significantly different among the groups (Table 6).
Table 6. Neonatal outcomes among the different groups

Measurement data are presented as the means ± standard deviations (SDs); enumeration data are reported as percentages and were compared by the χ2 test.
Discussion
In this study, we developed a scoring system based on three parameters (perivitelline space, percentage of immature oocytes and percentage of oocytes with abnormal morphology) to address the severity of ZP abnormalities. The present study shows that the different degrees of ZP abnormalities did not significantly affect blastocyst formation or clinical or live birth outcomes. However, severely abnormal ZP led to the lowest number of retrieved oocytes and mature oocytes and the highest cycle cancellation rate.
Previous studies have explored whether the ZP plays a crucial role during oocyte maturation. A study by Sousa et al., showed that oocytes with an indented ZP were associated with low maturity(Sousa et al., Reference Sousa, Teixeira da Silva, Silva, Cunha, Viana, Oliveira, Sá, Soares, Oliveira and Barros2015). According to their further ultrastructural analysis, the outer ZP layer had an indented surface with protuberances, and in the inner ZP, there was an obliteration of the perivitelline space and a zona pellucida structure with large empty electrolucent regions on the outside (Sousa et al., Reference Sousa, Teixeira da Silva, Silva, Cunha, Viana, Oliveira, Sá, Soares, Oliveira and Barros2015). de Almeida Ferreira Braga et al. reported that immature oocytes have higher levels of birefringence (de Almeida Ferreira Braga et al., Reference de Almeida Ferreira Braga, de Cássia Savio Figueira, Queiroz, Madaschi, Iaconelli and Borges2010). Another study also revealed that significant inner layer (IL) ZP thinning occurred during oocyte maturation, with significant reductions in IL-ZP thickness and area, and there were no relevant changes in total ZP thickness (Canosa et al., Reference Canosa, Adriaenssens, Coucke, Dalmasso, Revelli, Benedetto and Smitz2017). According to the results of the present study, the number of retrieved oocytes from the abnormal ZP groups was significantly lower than that from the control group and was the lowest when the degree of ZP abnormalities was severe. Moreover, our results demonstrated that the degree of ZP abnormalities indeed affected oocyte maturity and that the severe group had an average number of mature oocytes of only 1.66 ± 2.08.
In this study, the proportion of the R-ICSI regimen was higher in the abnormal ZP groups than in the control group, however there were no significant differences among three abnormal ZP groups and the fertilization rate of oocytes was not affected by ZP abnormalities. In clinical practice, oocytes with abnormal ZP from some patients will be morphologically recognized by cumulus–oocyte complexes, and then they will be fertilized by ICSI. However, most of them were difficult to identify during the process of oocyte retrieval, and the R-ICSI method was passively chosen to complete fertilization. In addition to low cleavage rate, severe ZP abnormalities caused a reduced good-quality embryo rate. However, the rates of blastocyst formation, clinical pregnancy and live birth were similar among the three abnormal ZP groups.
It is currently unclear what may be causing different degrees of ZP abnormalities. In the current state of evidence, it appears that an accurate conclusion cannot be reached. In light of our study, several baseline characteristics of enrolled patients contributing to the cause of different degrees of ZP abnormalities might be excluded. The physical condition of the patients, including female age, BMI, and basal hormone levels, may not be an important reason for the different degrees of ZP abnormalities in our study. A previous study reported that pelvic inflammatory disease might be one of the causes of ZP agar-like oocytes (Yang et al., Reference Yang, Yang, Yang, Wang, Zhu, Wang, Ding, Rao, Xue, Peng, Wang, Cao, Zou, Chen and Zhang2022). The present result is not consistent with this, and there was no significant difference in the cause of patient infertility by pelvic inflammatory disease among the four groups.
In addition, ZP abnormalities can also be caused by genetic mutations in ZP genes. Mice with ZP gene knockout showed that maintenance of ZP structure relies on all three murine ZP proteins, and deleting any of these three ZP genes leads to abnormal ZP (Liu et al., Reference Liu, Litscher, Mortillo, Sakai, Kinloch, Stewart and Wassarman1996; Wassarman et al., Reference Wassarman, Qi and Litscher1997; Rankin et al., Reference Rankin, Talbot, Lee and Dean1999; Rankin et al., Reference Rankin, O’Brien, Lee, Wigglesworth, Eppig and Dean2001; Wang et al., Reference Wang, Lv, Huang, Zeng, Yi, Tan, Peng, Yu, Deng and Xiao2019). Additionally, in human oocytes, sequence variations in four human ZP genes are associated with a variety of abnormal ZPs, particularly thin and thick zona, oval zona or zona splitting (Huang et al., Reference Huang, Lv, Zhao, Li, He, Li, Sha, Tian, Papasian, Deng, Lu and Xiao2014; Yang et al., Reference Yang, Luan, Peng, Chen, Su, Zhang, Wang, Cheng, Zhang, Wang, Chen and Zhao2017; Zhou et al., Reference Zhou, Ni, Wu, Chen, Xu, Zhang, Mu, Li, Yan, Fu, Wang, Zhao, Dong, Sun, Kuang, Sang and Wang2019; Cao et al., Reference Cao, Zhao, Zhang, Zhang, Lu, Wang, Hu, Ling, Zhang and Huo2020; Sun et al., Reference Sun, Zeng, Chen, Zhou, Fu, Sang, Wang, Sun, Chen and Xu2021; Zhang et al., Reference Zhang, Zhu, Liu, Ren, Yang, Li, Luo, Peng, Zhou, Jia, Hou, Li, Jin and Zhang2021; Wei et al., Reference Wei, Li, Liu, Liu, Yan, Zhu, Zhou, Tian, Zhang, Li and Lu2022). In this study, abnormal ZP groups had a higher number of repeated oocyte retrieval cycles than the control group, however there were no significant differences among the three abnormal ZP groups. Moreover, the patients with more than one cycle of abnormal ZP were accompanied by immature oocytes or oocyte abnormalities. For this reason, genetic screenings are generally recommended for these patients before their next cycles in clinical practice. Therefore, this strategy is important both to avoid repeated ovulation induction failures and to save costs for these patients. According to the published literature, the definition of ZP abnormalities differs between studies. The names in the previous studies include indented ZP (Sousa et al., Reference Sousa, Teixeira da Silva, Silva, Cunha, Viana, Oliveira, Sá, Soares, Oliveira and Barros2015), agar ZP (Yang et al., Reference Yang, Yang, Yang, Wang, Zhu, Wang, Ding, Rao, Xue, Peng, Wang, Cao, Zou, Chen and Zhang2022) and heterogeneous ZP (Pan and Zhang, Reference Pan and Zhang2020); however, they did not classify the degree of ZP abnormalities. Our study will contribute to the classification of ZP abnormalities.
In conclusion, the appearance of ZP abnormalities impairs the maturity and developmental competence of human oocytes. Moreover, ZP abnormalities show various degrees of severity, and in all patients regardless of the degree of ZP abnormalities who achieve available embryos, there will be an opportunity to eventually give birth.
Acknowledgements
We are grateful to the patients and their families for providing detailed information in the follow-up.
Funding information
This study was supported by funding from the Nanjing Medical Science and Technology Development Foundation (Grant No. YKK22092).
Competing interests
The authors state that no conflict of interest exists in this research.










