Cardiac malformations are a known association in Turner syndrome with the most common being bicuspid aortic valve and coarctation of the aorta. Reference Sybert1,Reference Gravholt, Landin-Wilhelmsen and Stochholm2 Cardiovascular disease is a cause of mortality in Turner syndrome with aortic dissection being the most serious. Known risk factors for aortic dissection include hypertension, coarctation of the aorta, and bicuspid aortic valve; however, 10% of patients have no obvious risk factors. Reference Sybert1–Reference Lopez, Arheart and Colan8 The risk of aortic dissection is increased with an aortic size index greater than 2–2.5 cm/m2, and these patients require close cardiovascular surveillance. Reference Carlson, Airhart, Lopez and Silberbach4,Reference Matura, Ho, Rosing and Bondy5 The sixfold increased risk of aortic dissection in Turner syndrome has led to recommendations for routine cardiac surveillance echocardiography, CT or MRI at least every 5 years, even if there are no known cardiac abnormalities. Reference Gravholt, Landin-Wilhelmsen and Stochholm2,Reference Gravholt, Andersen and Conway3
Studies investigating the biophysical properties of the aorta have found that Turner syndrome have reduced aortic distensibility and increased stiffness compared to controls even in patients with a normal trileaflet aortic valve. Reference Schäfer, Browne and Truong9,Reference Groenink, de Roos and Mulder10 This has led to the hypothesis that increased aortic stiffness occurs prior to dilatation, as shown in several echocardiography studies. Reference An, Baek and Kim11–Reference Bondy14 Since cardiac MRI forms part of the recommended evaluation tool as per the recent Turner syndrome guidelines, it provides the opportunity to assess associations between aortic dimensions, biophysical properties, and ventricular function in the same study. Reference Gravholt, Andersen and Conway3,Reference Bondy14 Assessment of the aortic properties may provide additional prognostic information about the risks of aortic dilation or dissection and contribute to risk stratification for cardiovascular complications in Turner syndrome. Reference Devos, De Groote and Babin13
The objectives of this study were to assess the biophysical properties of the aorta in patients with Turner syndrome using MRI and their relationship to aortic size index. Our hypothesis was that the biophysical properties of the aorta would be abnormal and correlate with aortic dimensions in a population of paediatric and adolescent patients with Turner syndrome.
Materials and methods
Study population
Patients with Turner syndrome referred for a Cardiac MRI at the Stollery Children’s Hospital and Mazankowski Alberta Heart Institute were recruited for this study. The indications for MRI were screening for aortic dimensions with or without additional cardiac lesions such as a bicuspid aortic valve or coarctation of the aorta as per the most recent guidelines. Reference Bondy15,Reference Saenger, Wikland and Conway16 This is the second part of a previously published study. Reference Somerville, Rosolowsky, Suntratonpipat, Girgis, Goot and Tham17 We included patients aged 8 to 20 years who were able to co-operate for a non-sedated MRI. Exclusions were complex CHD, the usual contraindications for MRI, or large metallic artifacts that precluded analysis. Written informed consent and assent were obtained from parents and participants for the use of their data in the study and to acquire extra images of the aorta at the time of the MRI. The study protocol was approved by the University of Alberta Health Research Ethics Board.
Clinical data that were collected included patient’s age, weight, and height at the time of MRI study, resting systolic and diastolic blood pressure taken at the time of aortic flow measurements, pulse pressure (systolic minus diastolic blood pressure), additional cardiac lesions, karyotype, and any history of growth hormone therapy. Body surface area, body mass index, height, and z scores (AnthroCalc, CPEG) were calculated from these data. End-systolic pressure was calculated as 0.9 × systolic blood pressure. Female controls without Turner syndrome were obtained from our MRI database who underwent screening MRI for specific indications and had a normal study (n = 6), as well as healthy female volunteers recruited for previous research studies (n = 4) who consented for the use of their data in future studies.
Image acquisition
MRI studies were performed with a 1.5 T scanner (Siemens Medical Solutions, Erlangen, Germany) with a 32-channel body array coil with end-expiratory breath-holds. Standard balanced steady-state free precession cines of the heart and aorta were obtained to evaluate the anatomy and volumetric function from a standard short-axis cine stack. Aortic flow was measured using phase-contrast imaging in the ascending aorta and descending aorta at the level of the right pulmonary artery (field of view 250 × 200 mm, voxel size 0.8 × 0.8 × 5 mm, repetition time/echo time = 12/36 ms, 20–30 cardiac phases, and velocity encoding = 150–200 cm/s). Non-contrast, navigator 3-dimensional whole-heart magnetic resonance angiography was performed to evaluate the extracardiac anatomy and visualise the entire aorta in 3 dimensions (Fig. 1). Gadolinium contrast-enhanced magnetic resonance angiography was performed if there were abnormalities that required further anatomical evaluation, e.g. anomalous pulmonary veins.

Figure 1. Aortic measurements showing A: sagittal oblique view of the aorta and B: corresponding perpendicular plane in phase-contrast MRI at the level of the right pulmonary artery. Flow quantification was measured in the ascending aorta and dimensions yellow measured in the ascending red and proximal thoracic aorta green.
The images were analysed using Circle Cardiovascular Imaging software (Calgary, Canada) to calculate the left ventricular end-diastolic and end-systolic volumes, stroke volume, and ejection fraction. Dimensions (luminal edge to luminal edge) of the aortic valve annulus, aortic root, and sinotubular junction were measured from the cine images at end systole. Left ventricular volumes and aortic measurements were indexed to height and body surface area. Phase contrast flow mapping was performed in the ascending and descending thoracic aorta at the level of the right pulmonary artery to obtain aortic flow measurements and cardiac index. The analysis software also provides the maximum and minimum areas from the flow measurement data, allowing for calculation of the relative area change = maximum-minimum area (Fig. 1). We measured the maximum antero-posterior and lateral dimensions from the phase with the maximum cross-sectional area. The largest cross-sectional dimension was used to calculate the aortic size index in cm/m2 and was considered dilated if it was greater than 2 cm/m2.
Biophysical properties were calculated from the data obtained: aortic compliance = relative area change/pulse pressure (mm2/mmHg); aortic elastance = end-systolic pressure/stroke volume (mmHg/ml); aortic stiffness index = ln(systolic blood pressure divided by diastolic blood pressure)/(relative area change/minimum area), where ln = natural logarithm; left ventricular elastance = end-systolic pressure/end-systolic volume (mmHg/ml); ventriculo-arterial coupling = aortic elastance/left ventricular elastance. Aortic distensibility was calculated from the cross-sectional area in the ascending and descending aorta using the following formula Reference Groenink, de Roos and Mulder10,Reference Groenink, de Roos, Mulder, Spaan and van der Wall18 :
where Amax = maximum cross-sectional area of the aorta mm2, Amin = minimum cross-sectional area of the aorta mm2, and PP = pulse pressure
Aortic pulse wave velocity was assessed using 3 locations of the aorta. To determine the aortic segment length, sagittal angulated views of the aortic arch were acquired from multi-planar reconstructed non-contrast imaging. The slice plane intersected the ascending aorta at the right pulmonary artery level and the proximal thoracic aorta, both at an approximate right angle. The first segment was from the ascending aorta to the aortic arch and the second segment from the aortic arch to the proximal thoracic aorta. Aortic pulse wave velocity was calculated as the ratio of distance between the aortic levels and time difference between arrival of the pulse wave at these level. Reference Cavalcante, Lima, Redheuil and Al-Mallah19 Only the ascending aortic pulse wave velocity was available in controls.
Statistical analysis
Results are expressed as median (interquartile range) and indexed to body surface area when appropriate. Correlations between aortic size index with aortic distensibility, compliance, aortic and left ventricular elastance, pulse wave velocity, and stiffness index were performed using Spearman correlation coefficient in Turner syndrome. Mann–Whitney U tests were used to assess for differences between Turner syndrome and controls. Subanalysis was performed between Turner syndrome with a bicuspid aortic valve versus those with a normal trileaflet aortic valve. Analysis was repeated with data indexed to height. Significance was set at p < 0.05.
Results
Clinical characteristics
Among 26 eligible patients with Turner syndrome, flow measurements could not be obtained in 1 patient due to poor cooperation who was excluded from the study. The remaining 25 (28% 45XO, 60% mosaic and 12% unknown karyotype, 60% on growth hormone) had the following cardiac lesions: 6 (24%) bicuspid aortic valve, 3 (12%) left superior vena cava, 1 (4%) status-post coarctation repair with end-to-end anastomosis, and 1 (4%) with partial anomalous pulmonary venous return. No one had significant aortic stenosis, aortic regurgitation, or unrepaired coarctation of the aorta. Although the absolute heights did not differ significantly between Turner syndrome and controls, they had a lower median height z score [interquartile range] (−2.70 [−2.92 – −1.54] versus 0.52 [−0.53 – 1.08], p < 0.001). The rest of the clinical characteristics did not differ significantly between Turner syndrome and controls with respect to age, weight, body surface area, body mass index, systolic blood pressure, and pulse pressure. Heart rate and diastolic blood pressure were significantly higher (p < 0.001) in Turner syndrome compared to controls (Table 1).
Table 1. Clinical characteristics

BMI = body mass index; BSA = body surface area; DBP = diastolic blood pressure; HR = heart rate; SBP = systolic blood pressure.
Data are presented as median (interquartile range).
Cardiac MRI parameters
The MRI parameters of Turner syndrome and controls are displayed in Table 2. Both Turner syndrome and controls had normal median aortic annulus, root and sinotubular junction dimensions. The median aortic size index in Turner syndrome was 1.81 cm/m2 (1.45–2.10) and did not differ from controls, 1.77 cm/m2 (1.41–2.12). There were 7 Turner syndrome and 3 controls with an aortic size index > 2 cm/m2, with 2 Turner syndrome having an aortic size index > 2.5 cm/m2. There were no differences in their maximum or minimum indexed ascending aortic areas. Turner syndrome had smaller indexed ventricular volumes and mass than controls, but their left ventricular ejection fractions did not differ. Although controls had larger stroke volumes than Turner syndrome, the cardiac index did not differ between them, as this corrects for heart rate (Table 2).
Table 2. MRI parameters
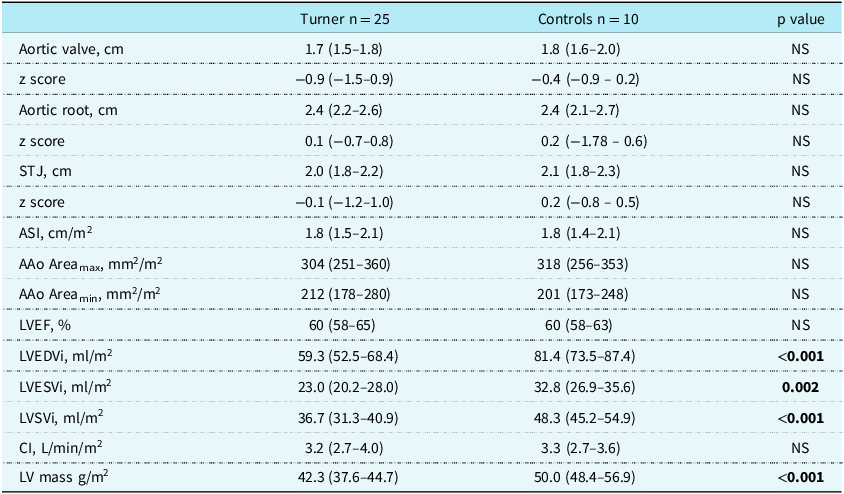
AAo = ascending aorta; ASI = aortic size index; CI = cardiac index; EDVi = indexed end–diastolic volume; EF = ejection fraction; ESVi = indexed end systolic volume; LV = left ventricle; STJ = sinotubular junction; SVi = indexed stroke volume.
Data are presented as median (interquartile range).
Biophysical properties
There were no differences in the aortic properties between Turner syndrome and controls in terms of aortic compliance, distensibility, or stiffness index in the ascending aorta. However, the descending thoracic aorta showed increased compliance and distensibility in Turner syndrome compared to controls, just reaching statistical significance (Table 3). Aortic and left ventricular elastance were greater in Turner syndrome compared to controls. When Turner syndrome with aortic size index > 2.5 cm/m2 were excluded from the analysis, the descending aortic distensibility remained increased in Turner syndrome, but the descending aortic compliance was no longer significantly different between groups. There were no other no differences in aortic properties when excluding the 2 outliers from the analysis. Ventriculo-arterial coupling was normal < 1 and did not differ between Turner syndrome and controls.
Table 3. Biophysical properties

AAo = ascending aorta; DAo = descending aorta; Ea = aortic elastance; Ees = LV elastance; LV = left ventricle; RAC = relative area change; VA = ventriculo–arterial.
Data are presented as median (interquartile range).
In Turner syndrome, larger indexed aortic valve diameters correlated weakly with increased aortic elastance (r = 0.40, p = 0.049) but not with aortic compliance. Similarly, larger indexed aortic root diameters correlated moderately with increased aortic elastance, (r = 0.53, p = 0.007). The aortic size index showed moderate positive correlations with aortic elastance, (r = 0.5, p = 0.01) and left ventricular elastance, (r = 0.59, p = 0.002), but no correlations with aortic compliance (r = 0.32, p = 0.12, Fig 2). The indexed maximum ascending aortic area showed positive correlation with left ventricular elastance (r = 0.49, p = 0.01). Pulse wave velocity did not correlate with aortic size index or other biophysical properties in Turner syndrome.

Figure 2. Correlations between aortic size index and aortic elastance, left ventricular elastance and aortic compliance in patients with Turner syndrome. ASI = aortic size index; Ea = aortic elastance; Ees = left ventricular elastance.
Subanalysis comparing Turner syndrome with a bicuspid aortic valve to those with a normal trileaflet aortic valve found no differences in the aortic dimensions or biophysical properties between the 2 morphologies. When indexing all the above parameters to height, there were no changes in the findings.
Discussion
We found that larger aortic size index correlated with increased aortic elastance and left ventricular elastance in Turner syndrome in the absence of hypertension. Despite Turner syndrome having similar aortic size index to controls, their findings of increased diastolic blood pressure and smaller ventricular volumes may be an indication of increased aortic stiffness while maintaining ventricular function. This study points to the additive role of MRI assessment of not only the cardiac anatomy but also the ability to assess biophysical properties of the aorta in a single test. Furthermore, we describe additional parameters that go beyond a one-dimensional measurement of aortic size index, some of which are not scaled to body size.
The evidence for a primary aortopathy in Turner syndrome has emerged from multiple studies. Reference Pees, Heno, Häusler, Ertl, Gulesserian and Michel-Behnke7,Reference Schäfer, Browne and Truong9,Reference Devos, De Groote and Babin13,Reference Bondy14,Reference Fox, Kang and Potts20,Reference Ostberg, Donald, Halcox, Storry, McCarthy and Conway21 Similar to Marfan syndrome, cystic medial necrosis was found in Turner syndrome on histological specimens. Reference Lin, Lippe and Rosenfeld6 Several echocardiographic-based studies have examined the biophysical properties of the aorta in Turner syndrome, all with similar findings that point to an underlying vessel wall abnormality. Reference An, Baek and Kim11–Reference Devos, De Groote and Babin13 Decreased aortic distensibility and increased aortic stiffness in Turner syndrome have been found by several authors. Reference An, Baek and Kim11–Reference Bondy14 Although stiffness did not correlate to ascending aortic z score diameter, De Groote et al still found that increased stiffness occurred at an early age which was independent of aortic valve morphology pointing to an intrinsic abnormality in the vessel wall. Reference De Groote, Devos and Van Herck12
While many previous studies used ultrasound techniques, there are known limitations with echocardiography due to difficult acoustic windows in older patients with increased body mass index along with limited 3-dimensional capabilities. As shown in our previous study, MRI provides superior diagnostic capabilities and the ability to image the aorta in 3 dimensions, not being limited by acoustic windows. Reference Somerville, Rosolowsky, Suntratonpipat, Girgis, Goot and Tham17 A MRI study by Devos et al found that larger ascending aortic areas, lower distensibility, and higher pulse wave velocity (increased stiffness) occurred in Turner syndrome 13–59 years with a bicuspid aortic valve but not those with normal aortic valves. Looking specifically at the younger age group < 27 years, they found that these functional changes were still present, substantiating other studies that increased stiffness in Turner syndrome occurs at a young age and may precede aortic dilatation. Reference Devos, De Groote and Babin13 In an older population of Turner syndrome (29–66 years) undergoing MRI, the lower aortic distensibility in Turner syndrome was influenced by the presence of aortic coarctation while Turner syndrome without coarctation had similar distensibility to controls. Reference Wen, Trolle and Viuff22
MRI studies have largely focused on older Turner syndrome or a wider age range, likely due to the need for sedation in younger children. An MRI study in adolescences with Turner syndrome aged 16 ± 4 years found increased stiffness (reduced distensibility and relative area change) in the ascending aorta compared to controls, but no differences in the descending aorta. Reference Schäfer, Browne and Truong9 In contrast to their findings, we found increased distensibility and compliance in the descending thoracic aortic in Turner syndrome compared to controls, whereas there were no differences in the ascending aorta. While most studies have concentrated on the ascending aorta, Oberhoffer et al found lower circumferential strain in the abdominal aorta in Turner syndrome compared to controls using speckle tracking echocardiography. Reference Oberhoffer, Abdul-Khaliq, Jung, Rohrer and Abd El Rahman23 Comparatively, we did find subtle differences in the descending aorta of Turner syndrome, with increased compliance and distensibility compared to controls. This implies that the aortic abnormalities in Turner syndrome extend beyond the ascending aorta, which is not surprising as 10% of dissections in Turner syndrome originate in the descending thoracic aorta. Reference Gravholt, Landin-Wilhelmsen and Stochholm2,Reference Carlson, Airhart, Lopez and Silberbach4,Reference Matura, Ho, Rosing and Bondy5 Supporting the notion that the aortopathy in Turner syndrome involves more than just the proximal aorta is the known association between coarctation of the aorta and aortic dissection and a reported increased risk of post-operative haemorrhage after coarctation repair in Turner syndrome. Reference Carlson, Airhart, Lopez and Silberbach4,Reference Pees, Heno, Häusler, Ertl, Gulesserian and Michel-Behnke7,Reference Brandt, Heintz, Rose, Ehrenhaft and Clark24 Thus, routine surveillance in Turner syndrome should include evaluation of the entire aorta.
Some of the variation in results amongst the literature and our study may be due to the method of scaling or indexing used, with some scaling to height, others to body surface area or body mass index, and also various z score measurements. Reference Lopez, Arheart and Colan8 In contrast to previous studies, we indexed our parameters to body surface area when appropriate. When we reanalysed our data by indexing all parameters to height, we found that this did not alter the findings of our study. We chose to focus on the aortic size index due to recent guidelines recommending that this should be the sole index for risk stratification and was found to be associated with measures of aortic stiffness in our study. Reference Gravholt, Andersen and Conway3 In agreement with others, we did not find any differences in the biophysical properties of the aorta between bicuspid and normal aortic valves. Reference Schäfer, Browne and Truong9,Reference Fox, Kang and Potts20 This adds to the argument for an inherent abnormality in the aortic properties of Turner syndrome that is unrelated to congenital heart lesions or aortic valve morphology.
Arterial or left ventricular elastance uses a pressure-flow (arterial) or pressure–volume relationship and are effective measures of vascular or cardiac load as it incorporates all the contributing factors (e.g., peripheral resistance, vascular compliance, cardiac output, stroke work, and ejection fraction). Reference Kelly, Ting and Yang25 Our finding that larger aortas had increased aortic elastance and left ventricular elastance, which were also higher in Turner syndrome compared to controls, was due to the significantly smaller left ventricular volumes in Turner syndrome compared to controls. Accompanying this was our finding of decreased left ventricular mass in Turner syndrome which may be related to the overall smaller heights in Turner syndrome. Previous studies have also found decreased left ventricular volumes in Turner syndrome compared to controls using echocardiography or MRI. Reference Schäfer, Browne and Truong9 The reason for decreased ventricular volumes is unclear and was not discussed in these studies. It may relate to decreased preload from increased arterial stiffness as we also found increased diastolic blood pressure in Turner syndrome compared to controls, a finding also seen in previous studies. Reference An, Baek and Kim11,Reference Devos, De Groote and Babin13,Reference Wen, Trolle and Viuff22 Furthermore, Oz et al found reduced ventricular strain despite normal left ventricular ejection fraction in Turner syndrome, suggesting early myocardial dysfunction. Reference Oz, Cizgici and Ucar26 Higher heart rates and diastolic blood pressure with corresponding decreased pulse pressure, coupled with higher aortic and left ventricular elastance, indicate that increased left ventricular force may be required to eject blood into their stiffer aortas. In the setting of normal ejection fraction and ventriculo-arterial coupling, this may represent an adaptive or altered neurohormonal response to increased aortic stiffness in Turner syndrome.
While increased aortic stiffness has been found in Turner syndrome using pulse wave velocity, we did not find a difference in pulse wave velocity between Turner syndrome and controls. Reference Fox, Kang and Potts20 Although pulse wave velocity is the oldest and most validated method to assess aortic stiffness, it is a regional measure of aortic stiffness compared to other methods which measure the local properties. Reference Cavalcante, Lima, Redheuil and Al-Mallah19 A similar study by An et al found increased aortic stiffness and higher blood pressures in Turner syndrome, but no differences in pulse wave velocity and ascending aortic diameters between Turner syndrome and controls. Reference An, Baek and Kim11
Arterial stiffness is associated with cardiovascular health and risks for cardiovascular morbidity and mortality, so should be evaluated in conditions that affect the aorta. Our findings confirm the current understanding of an underlying aortopathy in Turner syndrome. It also indicates a need for ongoing surveillance using additional parameters that if studied serially, may predict the risk of aortic dissection prior to significant aortic dilatation in patients with Turner syndrome.
The limitations of this study are the small sample size. As we do not routinely perform blood pressure on all patients undergoing MRI, we were only able to collect a small group of normal controls who had blood pressure measurements on the same day as their MRI. While several studies have shown that growth hormone treatment may increase the aortic growth rate, we do not have a sufficient sample size to evaluate the effect of growth hormone on aortic properties, and the long-term effects of growth hormone treatment need to be studied in the future. Reference Dyrka, Rozkiewicz, Obara-Moszynska and Niedziela27
Conclusion
In conclusion, MRI evaluation of paediatric and adolescent patients with Turner syndrome demonstrated increased aortic and left ventricular elastance suggesting an underlying aortopathy. The availability to calculate aortic biophysical properties should lead us to look beyond a one-dimensional measure of aortic size index as the only parameter to evaluate aortas in Turner syndrome, and study the local aortic properties as a potential means to evaluate risk factors for dissection in Turner syndrome.
Acknowledgements
We wish to thank the following people for their support and assistance in this project: Cardiac MRI technicians: Wendy Chu, Rebecca Gray, Melissa Grzeszczak, Kelley Justice, Kam Ma, and Justine Muller.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Health Research Ethics Board at the University of Alberta and adhered to our institution’s research guidelines.








