The production of GM foods is increasing worldwide, regardless of a country’s development level. GM foods are derived from organisms in which the genetic material (DNA) has been altered in a way that does not occur naturally( 1 , 2 ). They are also referred to as ‘transgenic foods’. In Brazil, they have various agronomic characteristics, particularly tolerance to herbicides and resistance to insects( Reference Nodari and Guerra 3 ).
Brazil has the second largest GM cultivation in the world, or the equivalent of 27 % of the world’s production of GM organisms (GMO)( Reference James 4 ), which occupies an area of 49·1 million hectares and takes up approximately 70 % of Brazil’s arable land( 5 ). In addition, of all soyabeans, corn and cotton grown in Brazil, 96·5, 88·4 and 78·3 %, respectively, are GM( Reference James 4 ), without considering the potential for biological contamination from planting to processing( Reference Price and Cotter 6 ). The following crops were approved for cultivation and consumption in Brazil between 1998 and 2018: sixteen varieties of soyabean, forty-four of corn, fifteen of cotton and one of yeast (Saccharomyces cerevisiae), as well as a variety of beans that has not yet been made available for consumption( 7 ).
From these data, it can be inferred that most of the foods sold in Brazil that contain soya, corn or cotton in their composition come from GM plants. Ingredients derived from soyabean, corn and cotton products and by-products are widely used by the food industry due to their large agricultural production, low cost and technical applications( Reference Drewnowski 8 , Reference Singh, Kumar and Sabapathy 9 ), and are increasingly present in the population’s diet( 10 ).
This fact has been evidenced by studies on the laboratory detection of GMO in Brazilian foods, which found that GM ingredients were present in processed meats, hot dog sausages, sausages, ham, bakery products and snack foods, as well as in corn- and soya-based products such as powdered soya milk, soya drinks, biscuits, instant soups, desserts and other foods( Reference Brod and Arisi 11 – Reference Dinon, Treml and de Mello 14 ).
Studies have shown that the consumption of GM foods can be harmful to health, especially when considering the pesticides associated with them( Reference Landrigan and Benbrook 15 ). The following conditions have been observed: hepatic and renal toxicity in animals that were fed GM corn; the appearance of tumours in rats that were fed GM corn( Reference de Vendomois, Roullier and Cellier 16 – Reference Seralini, Clair and Mesnage 18 ); inflammation in the stomachs of pigs that were fed GM corn and soyabeans( Reference Carman, Vlieger and Steeg 19 ); and damage to the mucous membranes of the jejune surface in rats that were fed GM corn( Reference Ibrahim and Okasha 20 ). In man, such harm has been associated with neurological problems, hormonal changes, infertility, cancer, diabetes, obesity, gastrointestinal disorders, depression, heart disease, autism, Alzheimer’s disease and coeliac disease( Reference Shao and Chin 21 – Reference Weintraub 26 ).
In recent years, an increase in products containing GMO and an increase of diseases in the realm of global public health have been observed as well as the increased use of pesticides associated with GM crops( Reference Swanson, Leu and Abrahamson 25 , Reference Antoniou, Habib and Howard 27 , Reference Kim, Kabir and Jahan 28 ). Swanson et al. ( Reference Swanson, Leu and Abrahamson 25 ) showed that the significant increase in the incidence of twenty-two chronic diseases in the USA correlated strongly with the increased cultivation of GM crops and the application of glyphosate-based herbicides, evidencing their effects on human health. Thus, consuming these foods has serious implications for public health, exposure to pesticides and the consequent risks of acute and chronic poisoning, in addition to the diseases mentioned above.
Considering the potential impacts caused by GM food consumption, the precautionary principle should be adopted. According to this principle, in cases where there are threats of serious or irreversible damage, a lack of full scientific certainty should not be used as a reason for postponing cost-effective measures to prevent environmental degradation. In other words, this principle calls for the adoption of measures against potential risks that cannot yet be identified according to current knowledge( 29 ).
To comply with the precautionary principle, it is essential that the population has access to information on the presence of GM ingredients on food labels. In Brazil, the reporting of information on labels aims to guarantee the right to information, which is set forth in the Federal Constitution of 1988 and recommended by the Brazilian Consumer Protection Code, which states that clear and adequate information on the composition of food is a basic consumer right( 30 , 31 ).
According to Biosafety Law No. 11 105/2005 (Article 40)( 2 ), foods and food ingredients intended for human or animal consumption that contain or are produced from GMO or their derivatives must provide this information on their labels, in accordance with Decree No. 4680/2003. This decree states that all foods and food ingredients with more than 1 % of their composition containing or produced from GMO must be labelled. Ordinance No. 2658/2003 of the Ministry of Justice requires the identification of GMO on food labels with the symbol of the letter T in the centre of a yellow triangle( 32 , 33 ). However, Brazilian studies have revealed cases in which GM ingredients have made up more than 1 % of packaged food products without these components being reported on the label, as required by GMO labelling legislation( Reference Greiner and Konietzny 13 , Reference Dinon, Treml and de Mello 14 , Reference Branquinho, Ferreira and Cardarelli-Leite 34 ). Thus, it is evident that the right of consumers to information about the presence of GM ingredients on food labels is not always being guaranteed.
The present study’s hypothesis is that most of the foods consumed by the Brazilian population may contain GM soya, corn and/or cotton derivatives. This is due to the increasing cultivation of GM soyabeans, corn and cotton in Brazil (which represents 96·5 % of soyabeans, 88·4 % of corn and 78·3 % of cotton grown in the country) and the fact that these plants give rise to many by-products used by the food industry. However, presenting these products or by-products in the ingredients list can be confusing to consumers because the terms are complex and difficult to understand or may not make their origin clear (as in the cases of maltodextrin, starch and guar gum). However, we have not identified this type of study in the literature.
Therefore, the objective of the present study was to identify ingredients from products and by-products potentially derived from GM crops on labels of packaged food products sold in a supermarket and to analyse whether these ingredients are present in the foods most commonly consumed by the Brazilian population.
Methods
The present study was a cross-sectional, descriptive and exploratory study, which was conducted in three stages (Fig. 1).
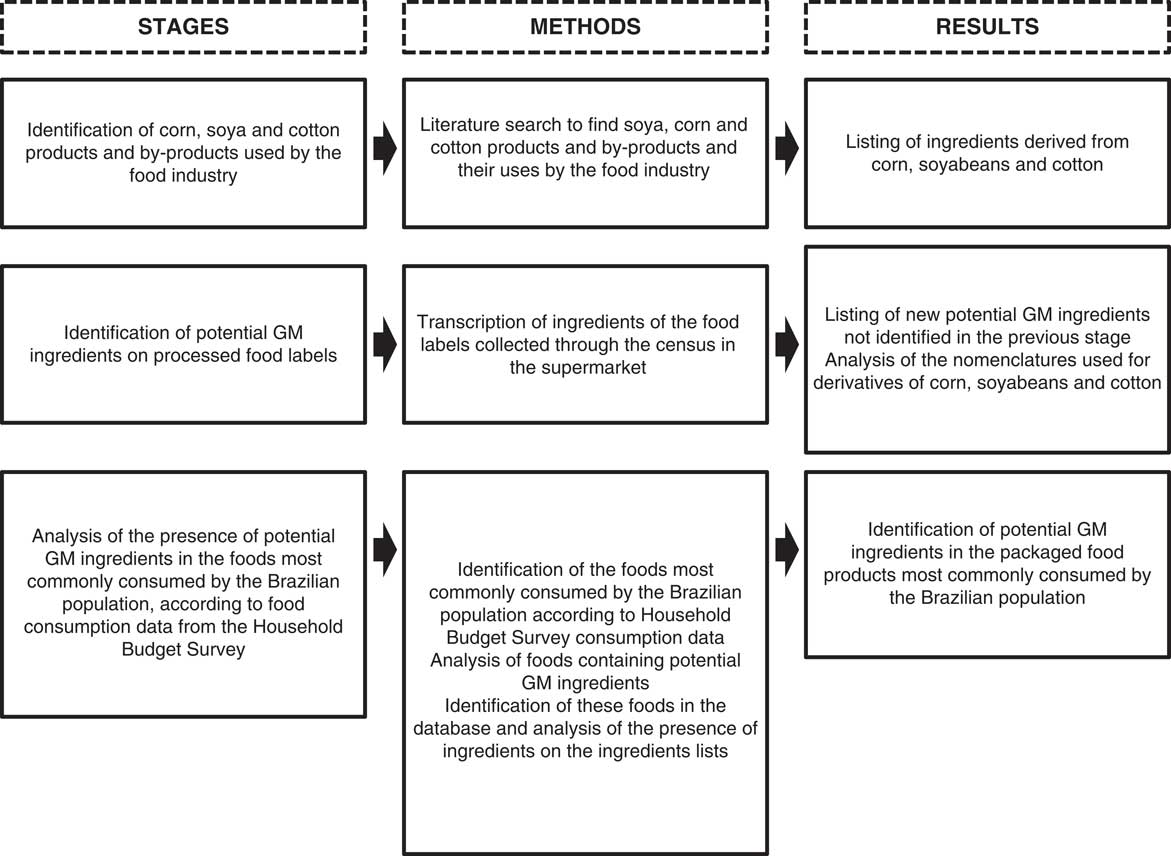
Fig. 1 Study stages of the label survey to identify ingredients potentially containing GM organisms to estimate intake exposure in Brazil
Stage 1
In the first stage, a literature review was conducted in the Scopus and SciELO article databases, Google Scholar, books, websites and documents to identify products and by-products potentially derived from GM crops approved for consumption in Brazil that are used as ingredients by the food industry. The keywords used in the searches were: ‘soybean’, ‘corn’, ‘cottonseed’ and ‘Saccharomyces cerevisiae’, in combination with ‘food industry’ and ‘technological application’. In addition, the snowball technique was used, searching for articles in the references of the studies found. This stage aimed to create an initial list of products and by-products derived from soyabeans, corn and cotton as well as their uses, since many of these may not be recognized as ingredients derived from soyabeans, corn or cotton solely from the nomenclatures used in the ingredients lists. The data were analysed and the results are expressed qualitatively with the technological purposes of the food industry ingredients.
Stage 2
In the second stage, these products and by-products were identified on the labels of packaged foods available for sale in a large supermarket in Brazil. The selected supermarket belongs to one of the ten largest Brazilian chain stores, according to the Brazilian Supermarket Association, with twenty-seven stores throughout the country. We also sought to identify other possible nomenclatures for products and by-products derived from GM crops that were present on the labels but not previously identified in the literature.
All packaged food products that met the criteria established by Brazilian and Mercosur packaged food labelling regulations (No. 259/2002)( 35 ) were included in the study. The analysis performed on all the packaged foods sold raises the possibility that the same foods may be sold in other countries. In addition, few countries have performed labelling analyses that consider all the foods available in a supermarket( Reference Legault, Brandt and Mccabe 36 – Reference Kaur, Scarborough and Matthews 41 ). Foods exempted from mandatory labelling (those packaged in the presence of the consumer) as well as those intended for animal consumption were not included.
The following food product identification information was recorded: name, trade name, brand, manufacturer and country of origin. All labels were photographed for later identification, transcription and ingredients list analysis. The data collectors were trained and the data were collected with the aid of computer tablets, using electronic forms developed in the EpiCollect Plus software program. In total, the information from 5048 food products was collected during a period of five months in 2013–2014.
An analysis was made of the information provided on the ingredients list of all collected food labels. Three researchers exactly transcribed the ingredients and food additives in the order they appeared in the ingredients list on each food label using Microsoft Excel® 2010. For quality control purposes, the data were cross-checked with the data transcribed by three other researchers.
Based on the results obtained in the literature review in Stage 1, the ingredients lists were analysed using the text mining in R technique( Reference Feinerer and Hornik 42 – 44 ). In this way, all terms present on the foods’ ingredients lists were listed. From this listing, all information on the ingredients of all foods was analysed manually to identify the presence of other ingredients and the S. cerevisiae yeast, as well as the nomenclatures used for corn, soyabean and cotton derivatives. Thus, we identified the presence of ingredients derived from products and by-products of these crops that were not initially listed. In the case of ingredients that did not contain the words ‘corn’, ‘soy’ and ‘cotton’, such as guar gum and citric acid, the literature was consulted again to confirm the origin of such ingredients.
It should be noted that a label’s ingredients list is the only way for consumers to identify the presence of potentially GM ingredients in packaged foods sold in Brazil. This is because GMO labelling legislation is often not followed.
Regarding ingredients whose origin was unclear, they were considered potential corn, soyabean or cotton by-products when the scientific literature considered this possibility. For example, one specific ingredient is starch, which can come from corn, manioc or other cereals. When an ingredient’s origin is not specified, it may be a corn by-product and therefore can be considered to potentially contain GMO. An example of a doubtful ingredient is margarine, since complete information on the ingredients used in its production is not made available to consumers. Thus, margarine can originate from sunflower or canola oil, but can also be made from corn, soyabean or cottonseed oil. Thus, when in doubt, margarine that had no designation of origin was considered to potentially contain GMO.
Moreover, as the current study analyses the situation from the point of view of consumer information, it is assumed that if a manufacturer does not provide the complete name of an ingredient, it is leaving its origin in doubt and the analysis should therefore provide for this. That is, if a manufacturer does not use ingredients derived from corn, soyabeans or cotton or that contain the S. cerevisiae yeast, it should make this information clear on the label. Otherwise, it may be considered to be using one or more of these ingredients.
Stage 3
The last stage consisted of identifying the potential presence of ingredients potentially derived from GM crops in the foods most commonly consumed by the Brazilian population. To this end, secondary per capita consumption data from the 2008–2009 Household Budget Survey (HBS)( 45 ) were used. The HBS is a national household survey that is conducted during a period of an entire year every 5 years by the Brazilian Institute of Geography and Statistics in a representative sample of Brazilian households. To obtain information on personal food consumption, data from a randomly selected sub-sample of households (n 13 569) were used. Data on food and beverage consumption over a 24 h period were collected through dietary records that were completed by individuals over 10 years of age (n 34 003) on two non-consecutive days. Details on the sampling plan and study design of the HBS can be found in the Brazilian Institute of Geography and Statistics’ report( 45 ).
The informants cited a total of 1121 foods. These foods were divided into twenty-four groups and 105 subgroups( 45 ). For the present study, all food subgroups defined by the HBS were considered.
From this list of foods most commonly consumed by the Brazilian population, we sought to identify the foods that contain ingredients derived from soyabean, corn and cotton products and by-products and the S. cerevisiae yeast (results of Steps 1 and 2, Table 1), which are potentially GM. To this end, we searched the database of packaged food products collected in the supermarket to find the same or similar foods for each food item in the groups and subgroups of foods most commonly consumed by the Brazilian population. All potential GM ingredients in these foods were analysed and listed, and the results were expressed as ‘may contain’ when any analysed food contained such an ingredient.
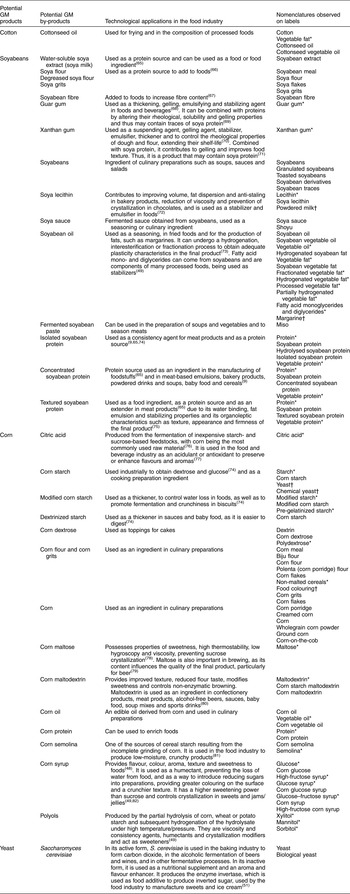
Table 1 Soyabean, corn and cotton by-products used by the food industry, their main technological applications and the nomenclatures found in the ingredients lists of the 5048 packaged food products analysed, Brazil, 2013–2014

* Ingredients that are potentially corn, soya or cotton by-products.
† Composite ingredients listed in the ingredients lists that may contain some ingredients derived from corn, soya or cotton.
Results
In the scientific literature, information was found on one cotton product, thirteen soyabean products and by-products and fourteen corn products and by-products which provide the raw materials for different ingredients used by the food industry for various purposes. From the ingredients lists of the 5048 foods analysed in the supermarket, 101 distinct nomenclatures were identified corresponding to ingredients derived from corn, soyabeans and cotton and referring to the presence of the S. cerevisiae yeast, which may contain GMO. Of these, thirty were terms referring to derivatives of corn, twenty-six to soyabeans, three to cotton and one referred to a yeast. Thirty-two terms did not indicate the ingredient’s origin, possibly being common to the three. For example, vegetable fat and vegetable oil can be produced from corn, soyabean, cottonseed or some other plant source.
Table 1 shows the soyabean, corn and cotton products and by-products or potential by-products, their main technological purposes in the food industry and the nomenclatures found in the ingredients lists of the 5048 food products analysed.
Of the 101 nomenclatures used to designate the ingredients, thirty-two did not specify origin. However, they were considered likely to be soyabean, corn and cotton by-products according to the scientific literature and the criteria explained earlier in the ‘Methods’ section. These ingredients include citric acid, vegetable oil, vegetable fat (fractionated, hydrogenated, processed and partially hydrogenated), guar and xanthan gums, mono- and diglycerides of fatty acids, lecithin, protein, vegetable protein, starch, modified starch, dextrin, polydextrose, maltose, maltodextrin, semolina, glucose syrup, glucose, high-fructose syrup, glucose syrup, glucose–fructose syrup, polyols, xylitol, mannitol and sorbitol, as well as non-malted cereals.
Other examples of doubtful ingredients are food colouring, chemical yeast, powdered milk and margarine, as full information on their production components are not available when these ingredients appear on the packaged food ingredients lists. Nevertheless, such compound ingredients are known to contain soyabean or corn derivatives such as corn starch (which is present in food colouring and chemical yeast), soya lecithin (present in powdered milk) and soyabean, corn and other vegetable oils (which are components of margarine). Biological yeast, in turn, is composed of S. cerevisiae yeast, yet this information is not available to consumers either.
Of the 105 subgroups of foods consumed by the Brazilian population, thirty-eight did not contain any potential GM ingredients. These included foods such as rice, beans, fruits, vegetables, roots, tubers and oilseeds. Table 2 shows the potential GM ingredients present on the ingredients lists of packaged food products collected in the supermarket. It is worth noting that such packaged foods contained at least one of the ingredients listed, but not necessarily all of the ingredients due to variation in the products’ composition.
Table 2 Groups and subgroups of the processed foods most consumed by the Brazilian population according to the 2008–2009 Household Budget Survey, mean per capita amount consumed and potentially GM ingredients present in the packaged food ingredients lists collected in the supermarket survey, Brazil, 2013–2014

It was observed that 63·8 % (sixty-seven food subgroups) of the variety of foods most commonly consumed by the population contain potential GM ingredients. The mean per capita amount of daily food consumption of the Brazilian population was 1587·8g. Of this, 1023·8g (64·5 %) came from foods containing ingredients derived from soyabean, corn and cotton by-products.
It is noteworthy that most of the food items analysed contained three or more ingredients derived from corn and/or soyabeans, which are potential GMO. No cotton-derived ingredients were identified in the packaged food products analysed.
Discussion
From the literature, the present study identified several soyabean, corn and cotton by-products, as well as a yeast, that were identified with 101 distinct nomenclatures on the labels of the packaged food products analysed from one of the largest supermarket chains in Brazil. Therefore, the study’s relevance and scientific contribution is highlighted, since there is not yet any information available or systematized in the literature regarding the food industry’s widespread use of these potentially GM ingredients. In this respect, we must also highlight the study’s methodological rigour, which included the complementary stages necessary to find 101 nomenclatures for potentially GM ingredients. The first stage consisted of a literature search to initially identify products and by-products derived from soyabeans, corn and cotton and their uses by the food industry. We then identified these products and by-products and the new nomenclatures on the labels of more than 5000 packaged foods sold in Brazil and possibly abroad. Without such methodological rigour, the number of potentially GM ingredients could have been underestimated, thus failing to reveal the magnitude of their use and presence in the foods most consumed by the Brazilian population. Our study used current national data and can make a significant contribution to public health actions.
Soyabean-derived products are used as ingredients in various foods. Several by-products are extracted from soyabeans and are mainly used due to their low cost and functional characteristics, since they act as emulsifiers, stabilizers, thickeners and consistency agents and improve the texture and viscosity of foods. They also constitute a source of protein( Reference Singh, Kumar and Sabapathy 9 ). This explains their presence in many of the packaged food products that were investigated in the present study, such as meats, meat-based preparations, processed meats, pâtés, soya-based drinks, breads, pastas, cakes, ready-to-eat farofa (manioc flour), cereals, biscuits, chocolates, frozen pizzas, sandwiches, breaded snacks and ice cream.
Among the by-products derived from corn, starch is the one most used in the food industry as a raw material or food additive( Reference Jobling 46 , Reference Sun, Dai and Nan 47 ). Corn syrup is also widely used by the food industry for the purpose of sweetening and prolonging the shelf-life of food( Reference Goldfein and Slavin 48 ).
The present study’s literature review stage was necessary to identify by-products with nomenclatures that are difficult to recognize as originating from GM ingredients merely by analysing the ingredients lists on the food labels. For example, citric acid is a food additive that is widely used in packaged food products. It is not always derived from fruits and can be obtained from the aerobic fermentation of corn sugar, a fact that the nomenclature does not make clear. Other examples are xanthan gum and guar gum, which are not necessarily derived from soyabeans. However, soya protein can be incorporated during their production process. These gums make it possible to increase viscosity( Reference Goldfein and Slavin 48 , 49 ) and are present in many of the foods we studied, such as beverages, meats, yoghurts, instant noodles, ice cream, chocolate drinks, sauces and condiments.
The stage in which the ingredients lists of 5048 packaged foods were analysed identified 101 distinct nomenclatures to designate twenty-eight by-products derived from soyabeans, corn and cotton as well as S. cerevisiae yeast. One such example is soyabean oil, which was identified with twelve different nomenclatures in the ingredients lists, among them vegetable fat. Glucose syrup, which is derived from corn, appeared with thirteen different nomenclatures in the evaluated ingredients lists, many of which did not use the term ‘corn’. These issues can make identification difficult for consumers who try to avoid consuming these foods for various reasons.
The absence of by-product source specification can make it difficult to identify GMO in the ingredients lists of packaged food products. Of the 101 nomenclatures evaluated, thirty did not specify origin but were considered likely to be soyabean, corn and cotton by-products according to the scientific literature. These by-products included vegetable fat, starch, guar gum, xanthan gum, citric acid, dextrose, glucose syrup, glucose, maltose, maltodextrin, sorbitol, mannitol, xylitol and non-malted cereal.
In the case of starch, corn starch represents more than 80 % of all types used( Reference Liu, Ma and Yu 50 ). However, we highlight the difficulty of identifying the origin of an ingredient on the labels of some of the foods analysed in the present study, possibly because Brazilian food labelling law( 35 ) does not require this information to be provided. For example, an ingredient can be indicated merely as starch instead of corn starch. Therefore, the use of complete ingredient names by the food industry should be mandatory in order to specify ingredient origin.
Likewise, the absence of information on the composition of compound ingredients can make it difficult to identify GMO in the ingredients lists of packaged food products. For example, the present study identified foods that may contain soya or corn derivatives, but do not have their ingredients broken down, such as when they contain food colouring, chemical yeast, powdered milk and margarine. These foods are reported in the ingredients list of a food without indicating their components. This is due to the fact that Brazilian and Mercosur food labelling regulations do not require certain compound ingredients to be reported on food labels if they represent less than 25 % of a food’s composition( 35 ). These limitations of the law may hinder consumers’ access to accurate information, which is vitally important in cases of allergies to soyabeans, corn or cotton or when one wants to avoid consuming GMO derivatives.
Many soyabean and corn by-products are used as additives by the food industry. One example is soya lecithin, which was present in several of the food groups analysed in the present study and acts as an emulsifier in powdered milk, chocolate drinks, biscuits, cakes, breads and soups and as a stabilizer in cereal bars, dairy drinks and candies. As the law does not require additives to be described in descending order of quantity in ingredients lists, lecithin may be listed as one of the last ingredients of a food even though it is present in a greater quantity than ingredients listed before it( 35 ). Thus, the effect of the sum of the quantities of potential GM additives present in the same food is not accounted for, which makes it difficult for consumers to assess and decide which packaged food products to select at the time of purchase.
Besides soyabeans and corn, another GMO that may be present in the Brazilian population’s diet is S. cerevisiae yeast, popularly known as baking yeast, which is widely used in the food and beverage industry in various ways( Reference Yamada, Alvim and Santucci 51 ). It is used during the alcoholic fermentation stage of producing beer, which is the most commonly consumed alcoholic beverage in Brazil. In addition to GM yeast, Brazilian researchers( Reference Mardegan, Andrade and de Sousa Neto 52 ) have also discovered the presence of GM corn as a substitute for barley malt in major Brazilian beer brands. Brazilian law( 53 ) allows up to 45 % of the malt in beer to be substituted with another source of less expensive cereal, and corn is the one most commonly used to manufacture beer.
The analysis of secondary data on the Brazilian population’s food consumption showed that most of the variety (63·8 % of the food subgroups) and quantity (64·5 % of the total daily amount) of the foods consumed daily by Brazilians is liable to contain ingredients derived from GM foods.
In this regard, it seems that the consumption of processed packaged food products may increase the chances of the Brazilian population consuming GM foods. This information is in line with data from the Council for Biotechnology Information( 54 ), which estimates that most processed foods in Brazil contain at least one ingredient derived from soya or corn. In Canada, it is estimated that about 75 % of processed foods contain or are produced from ingredients such as GM corn, soyabeans or canola( 55 ). In addition, according to the present study’s analysis, most of the foods most consumed by the Brazilian population had three or more ingredients derived from potential GM corn and soyabeans.
This fact raises concerns about effects on public health due to a lack of scientific evidence on the safety of consuming these foods. This is because foods derived from soyabeans and corn may also contain residues of herbicides associated with their cultivation, which also pose risks to human health due to the known effects of these substances( Reference Seralini, Clair and Mesnage 18 , Reference Samsel and Seneff 22 – Reference Swanson, Leu and Abrahamson 25 , Reference McDuffie, Pahwa and McLaughlin 56 – Reference Meyer, Sarcinelli and Moreira 59 ).
For example, a main meal such as lunch in Brazil may include rice, beans, ready-to-eat farofa, instant noodles, chicken steak and chips, all of which may contain GM ingredients, either from the ingredients they contain or from the ingredients added during their preparation, such as soyabean, corn or cotton oil used to prepare rice, beans and chips. In addition, ingredients added as seasonings to meals (such as processed sauces) may also contain GM ingredients. Thus, a meal containing foods typically consumed by Brazilians can easily contain several GM ingredients and GM ingredients can be present in all the meals consumed in a single day.
Furthermore, the results of the HBS show that changes in Brazilian food consumption patterns have occurred in the last three decades, with increases of up to 400 % in the consumption of processed food products such as biscuits and soft drinks as well as a decline in the consumption of basic and traditional foods of the Brazilian diet such as rice and beans( Reference Levy-Costa, Sichieri and Pontes Ndos 60 ). Despite this decline, beans were found to be the food with the second highest per capita consumption. In September 2011, the National Technical Commission on Biosafety( 7 ) approved GM beans developed by the Brazilian Agricultural Research Corporation (Embrapa) for sale in Brazil. Although it is not yet available for consumption, this GM crop raises concerns due to a lack of further studies on its impacts and because beans are an integral part of the Brazilian diet, being consumed daily by nearly all Brazilians of all age groups.
Returning to the example of lunch mentioned above, the recommendations of the Dietary Guidelines for the Brazilian Population stand out, as they note that even if a person does not consume ultra-processed foods for lunch (avoiding ready-made farofa, instant noodles, chicken steak and chips), the lunch could still contain GM foods in all of its culinary preparations if, for example, soyabean, corn and/or cottonseed oil were used to cook the food. Thus, even if a person follows the Brazilian dietary guidelines’ recommendations exactly and only eats what he/she prepares at home, he/she may still be exposed to GM foods depending, for example, on the type of fat used to prepare the food (corn, soyabean or cotton oil) or the type of yeast used to bake bread at home. It should also be pointed out that the Dietary Guidelines for the Brazilian Population has not taken a position on GM foods in its recommendations( 61 ).
The present study’s data showed that about 24·5 % of the Brazilian population’s per capita daily food consumption comes from products of animal origin (meat, milk, dairy products and eggs). These results are a cause for concern, since animals fed with feed produced with GM corn and/or soyabeans may also constitute a source of GMO in human food( Reference Aumaitre, Aulrich and Chesson 62 ). Thus, the population can increase its consumption of GM foods by eating beef, pork and poultry from these animals or by consuming foods derived from them, such as dairy products, eggs and pork fat.
In summary, considering the data analysed, the Brazilian population consumes a wide variety of packaged food products that may contain GM ingredients on a daily basis. However, many of these foods do not have their composition clearly identified on their labels. This may be due to Brazilian law, which requires foods to be labelled only if more than 1 % of their composition is comprised of GMO. Thus, foods composed of less than 1 % of GMO are exempted from mandatory labelling, even though this does not mean that they do not contain GMO. In addition, no information has been found on compliance with this regulation. Thus, it can be assumed that the food industry may omit this information and fail to label a product, even if it contains more than 1 % of GMO. In either case, the right of consumers to clear and accurate information about the products they consume is not being guaranteed, as advocated by the Brazilian Consumer Defence Code( 31 ).
Furthermore, food additives are not required to be labelled in decreasing order of quantity and several products do not need to identify the origin of their raw materials, making it even more difficult to identify GMO by reading the labels of packaged food products. For this reason, we highlight the scientific relevance of the present study, which contains a list of potential GM ingredient nomenclatures that was prepared from the identification of these ingredients on the labels of packaged food products available for sale in a Brazilian supermarket. In addition, it should be emphasized that no studies exist that establish a safety percentage for the consumption of these foods. Therefore, even if a product contains less than 1 % of GMO in its composition and is not labelled, it does not mean that it does not pose health risks, since a safe consumption amount has not been established.
In most of the studies available in the scientific literature, the presence of GMO in food is identified through laboratory analysis. Thus, one possible limitation of the present study is the fact that it cannot be determined with certainty whether the food ingredients listed as potential GMO are actually so. However, unlike laboratory studies that analyse samples from a small group of foods, the present study analysed the labels of all foods available in a supermarket. In light of the increasing production of GM foods in Brazil, which corresponds to 96·5 % of the soyabeans, 88·4 % of the corn and 78·3 % of the cotton grown in the country( Reference James 4 ), in addition to the high risk of contamination throughout the production chain, it is assumed to be very likely that the ingredients derived from such foods are GM. Thus, it can be inferred that it is very likely that packaged food ingredients derived from soyabeans, corn and cotton are GM, even if they have not undergone laboratory testing.
This affirmation is supported even when considering the import/export data for these crops in Brazil. In 2017, grain production in Brazil totalled approximately 114 million tons of soyabeans, 98 million tons of corn and 3·8 million tons of cotton( 63 ). Of this total, 68·1 million tons of soyabeans (59·7 %), 19 900 tons of corn (0·02 %) and 450 tons of cotton (0·01 %) were exported. On the other hand, 253 000 tons of soyabeans, 1600 tons of corn and 401 tons of cotton were imported into Brazil in 2017, which corresponds to less than 0·23 % of the total grain remaining in the country (production + imports – exports)( 64 ). Furthermore, Brazil imports soyabeans, corn and cotton mainly from Paraguay, Argentina and the USA( 64 ), which are among the countries that plant the most GM seeds in the world( Reference James 4 ). These data indicate that, even though most of the Brazilian production of these grains is exported, the country is practically self-sufficient in its internal supply.
Therefore, the production that remains in the country together with the amount that is imported contribute to maintaining a large quantity of GM grain in the country, thus maintaining the potential trend of the occurrence of GMO in packaged foods sold in Brazil. We emphasize that the import/export data refer to the grains of products and not their sub-products (e.g. soyabean oil, soya flour, corn starch, corn flour, etc.). However, we point out that only 528 (10·5 %) of the 5048 foods analysed in the present study were imported.
It is also worth noting that the public sector has a responsibility to monitor labelling and perform laboratory tests to identify the presence of GM ingredients in foods to verify compliance with labelling legislation. Furthermore, the present study may contribute to showing the magnitude of the presence of such ingredients in the packaged food products most commonly consumed by the Brazilian population.
In this respect, we emphasize the importance of the method used, which can serve to guide more specific research in the future on the food groups in which the presence of potentially GM ingredients has been identified. In addition, the present study analysed the labels of all foods available for sale in a Brazilian supermarket, which would make it unfeasible to perform laboratory analyses on such a large number of foods. It is also worth noting that the study was conducted from the point of view of the consumer, who only has access to a food’s ingredients list, which is currently the sole source of information available to identify potentially GM ingredients in packaged foods sold in Brazil.
Another limitation refers to the analysis of secondary data obtained from the HBS, whose accuracy cannot be evaluated. However, the use of secondary data was shown to be effective to test the hypothesis about the Brazilian population’s high consumption of potentially GM ingredients. The HBS data in Brazil resemble the national dietary data from several countries and constitute the main source of national information on the Brazilian population’s food acquisition and consumption. Our study was based on the most recent available data on the Brazilian population’s food consumption: the 2008–2009 HBS. Data based on food consumption tend to approximate the population’s actual dietary patterns and have been used to establish food consumption patterns. Household budget surveys reflect the beginning of the consumption chain and enable the formulation of public policies that can modify the population’s food supply and health standards.
In addition, numerous population studies are currently seeking to relate diet to the incidence of various diseases. However, GM ingredients are generally not being analysed. Thus, the current study considers and recommends the attention of researchers to the population’s exposure to foods containing GM ingredients and potentially related diseases.
Conclusion
The present study has shown the high exposure the Brazilian population has to potential GM ingredients through the consumption of packaged food products, which were available in a supermarket. More than half of the foods most commonly consumed by the Brazilian population may contain at least one ingredient derived from potential GM soyabeans, corn or cotton. From the results of the present study, it was possible to demonstrate the difficulty consumers face in identifying the presence of these potential GM ingredients in food, since 101 different nomenclatures were observed on the packaged food labels analysed.
Soya, corn and cotton by-products have been widely used by the food industry for different technological purposes and such use may be underestimated due to the use of ingredients whose origin (derived from soyabeans, corn or cotton) may be unknown, both to health professionals and to consumers. Therefore, we emphasize the importance identifying these nomenclatures that designate potentially GM ingredients, as similar listings have not been found in the scientific literature. This information can be used to support the actions of health professionals with the public, as well as to support discussions about food legislation. In addition, it may help in future studies to investigate the relationship between GM food consumption and health.
Considering the precautionary principle, the present study suggests that regulations be made more restrictive in relation to the approval and production of GM crops in the country, as these crops have not been subjected to an in-depth analysis of their environmental, social and health impacts. In addition, foods derived from soyabeans, corn and cotton may also contain residues of the pesticides associated with their cultivation, adding to the risks to human health caused by the already known effects of these substances and the consequent public health expenditures to treat these diseases.
Competent government agencies should monitor packaged food products to require that information on the presence of GM ingredients be reported on their labels. In addition, it is considered increasingly necessary to act in the legislative sphere to reduce the food industry’s use of by-products derived from GM soya, corn and cotton. This is due to concerns about the effects of consuming these foods on human health, both due to a lack of scientific evidence on the safety of consuming GMO and the use of pesticides associated with the cultivation of these foods.
Furthermore, we cite the adoption of public health actions to guide the population in identifying the presence of GM ingredients in packaged products. Also highlighted is the need for policies that encourage the production and consumption of organic foods and incentives for small agricultural producers that do not use the wide range of by-products that are commonly used by large food corporations for technological purposes.
Acknowledgements
Financial support: The authors thank the Federal Agency for Support and Evaluation of Graduate Education in Brazil (CAPES) for its financial support in the form of scholarships to R.D.M.C., R.F.K. and S.S.M. This analysis was conducted as part of a wider study on the comprehension and use of food labelling in Brazil, which was funded by the National Council for Scientific and Technological Development (CNPq) of the Ministry of Science and Technology in Brazil and by the Brazilian Health Surveillance Agency (ANVISA) (grant number 440040/2014-0), with the aim of filling gaps related to policies, management and organization of the Brazilian National Health Surveillance System. CAPES and CNPq had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare that they have no conflicts of interest. Authorship: R.D.M.C. was responsible for planning the research, collecting, analysing and interpreting data, and drafting the manuscript. R.K.F. and S.S.M. were responsible for analysing and interpreting data and revising the manuscript. R.P.C.P. and S.B.C. were responsible for the design of the original study, planning the research and revising the final manuscript. S.B.C. was responsible for the supervising and drafting the manuscript. All authors approved the manuscript version submitted for publication. Ethics of human subject participation: Not applicable.