Anaemia is a public health problem in Peru, with a national prevalence of 33 % among children under 5 years of age, and even higher rates among the youngest (59 % in 6–8-month-old and 60 % in 9–11-month-old children), those living in rural areas (40 %) and from households (HH) in the lowest wealth quintile (41 %)( 1 ). On the other hand, stunting in children under 5 years of age has decreased from 20 % in 2011 to 14 % in 2015; however, much higher rates are observed among rural children (28 %) and those with non-educated mothers (36 %)( 1 ).
Meta-analyses of home fortification with micronutrient powder (MNP) have indicated that this approach is an effective strategy for reducing anaemia among infants and young children( Reference De-Regil, Suchdev and Vist 2 , Reference Dewey, Yang and Boy 3 ), but its effect on growth has not yet been demonstrated( Reference de Pee and Bloem 4 ). In Peru, the government initiated a national supplementation programme in 2009 providing MNP for infants and young children starting at 6 months of age; initially the dose provided was one sachet every other day, but it was later changed to one daily sachet for 6 months followed by a break for the next 6 months. However, programme evaluations have used inadequate study designs (i.e. without a proper control group) and provided inconsistent results. An evaluation with no comparison group reported a significant reduction in anaemia in children 6–35 months of age( Reference Munayco, Ulloa-Rea and Medina-Osis 5 ). Another evaluation with children of similar age, where those who reported not consuming the MNP (n 41) were used as a comparison group, indicated no significant differences in anaemia prevalence; however, among those who consumed the MNP, a lower prevalence of anaemia was observed in children whose supplement consumption was optimal (defined as consuming ≥60 MNP sachets and eating all the food mixed with MNP all or most of the time)( Reference Huamán-Espino, Aparco and Nuñez-Robles 6 ). Moreover, several barriers for MNP consumption have been identified not only among caregivers but also among the government health staff who distribute the supplement( Reference Creed-Kanashiro, Bartolini and Abad 7 ).
A more novel home fortification approach for the prevention of malnutrition is the provision of a small quantity (~20 g) of lipid-based nutrient supplement (LNS), which contains macronutrients (~460 kJ or 110 kcal) and several micronutrients. In a randomized trial conducted in Ghana where 313 children received one of three types of supplement (LNS, MNP or Nutritab) from 6 to 12 months, children’s mean Hb in the LNS group (but not in the MNP group) was significantly higher than that in a comparison group that did not receive any supplements( Reference Adu-Afarwuah, Lartey and Brown 8 ) and children in the three supplemented groups showed improved motor development, although positive effects on growth were observed only in the LNS group( Reference Adu-Afarwuah, Lartey and Brown 9 ).
We have previously tested the use of LNS among infants in Huánuco, Peru and found high levels of adherence (>90 %), with few cases of intra-HH supplement sharing and adverse events not related to the supplements (e.g. vomiting, diarrhoea) reported( Reference Vargas-Vásquez, Bado and Alcázar 10 ). Thus, the present study was conducted to determine the effectiveness of LNS on Hb, linear growth and development in children who received the supplement from 6 to 12 months of age, when compared with MNP supplementation, the current standard of care for nutritional supplementation in infants and young children in Peru.
Methods
Study design
The study was a randomized controlled trial designed to evaluate the effects of LNS for children when compared with the standard of care in Peru. Participants were enrolled at 6 months of age and randomly selected to receive either: (i) LNS containing macronutrients and nineteen micronutrients; or (ii) MNP (also known as Sprinkles™), containing five micronutrients. LNS products are based on peanut paste and other ingredients such as soyabean oil, powdered milk and sugar( Reference Arimond, Zeilani and Jungjohann 11 ). Table 1 shows the nutritional composition of both supplements. The study was conducted by the Peru-based office of the international humanitarian organization Action Against Hunger (ACH; from its initials in Spanish), in collaboration with the Regional Health Division of Huánuco, the Analysis Group for Development (GRADE; from its initials in Spanish) and the University of Cádiz, between July 2013 and December 2015. The MNP was provided by the Peruvian Ministry of Health (MOH) as part of its national nutrition supplementation programme and the Regional Health Division of Huánuco assured the continuous supply of MNP in the MOH health centres (HC) where the study was conducted throughout the study period. The LNS for the present study, the commercial name of which is Nutributter®, was donated by Nutriset (Malaunay, France). Both supplements were distributed by the MOH HC staff according to the individual-level randomization plan developed by ACH-Peru, following a standard protocol. The delivery of a one-month supply of the corresponding supplement occurred during the children’s monthly well-being appointments known as the CRED programme (from the Spanish words for growth and development: Crecimiento y Desarrollo). Caregivers were instructed on how to use the supplements and given a written material with such instructions.
Table 1 Nutritional composition of the study supplements
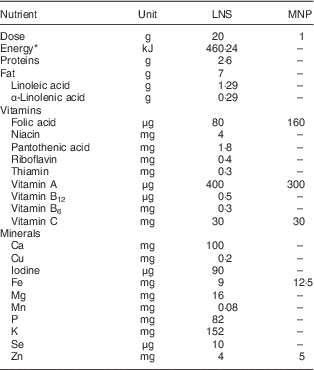
LNS, lipid-based nutrient supplement; MNP, micronutrient powder.
* 110 kcal.
Due to unexpected problems in the process of customs clearance of the LNS product, the supplement was unavailable for a period of two months (September and October 2014). During that time, the probability of being enrolled in each group differed, but those already enrolled in the LNS group continued receiving their monthly LNS supply.
We calculated a target sample size of 204 per arm to be able to detect an effect size (difference between groups, divided by pooled sd) of ≥0·28 on Hb concentration on the basis of the findings from the Ghana trial( Reference Adu-Afarwuah, Lartey and Brown 8 ), with power=80 % and α=0·05, and allowing for up to 20 % attrition by the end of the study.
Study area and population
The study was implemented in five rural districts (Cayna, Colpas, Huacar, San Francisco, San Rafael) in the Province of Ambo in the Department of Huánuco, Peru. These districts were selected based on their high chronic child malnutrition risk( 12 ) and similar poverty levels, rurality, housing conditions, access to water and drainage, electricity, literacy rates and altitude. In 2014, 40 % of the population in Huánuco lived in poverty( 13 ); in 2015, 16 % of women of reproductive age were illiterate and their median years of education was 8 years, and 74 % of children under 3 years of age attended the CRED programme at the HC( 1 ).
Children were screened at 5 months of age, but enrolled when they turned 6 months old. Enrolment took place at the nineteen HC located in the five study districts. Exclusion criteria included: (i) chronic, congenital or severe disease that affects growth and metabolism; (ii) not a singleton; (iii) low birth weight; and (iv) not a permanent resident in the study area.
Data collection procedures
Data were collected by four ACH research assistants who were not involved in supplement distribution. Two of them had an associated degree in Nursing, and the other two were midwifes with a bachelor degree in Obstetrics. They were supervised by a study coordinator who was a nutritionist. Data collection occurred at the HC and at the participant’s home at three different time points: baseline (6 months of age) and at two follow-up visits (9 and 12 months of age). All outcome data, except for language development, were collected at the HC. Research assistants were trained and standardized to conduct the anthropometric measurements by the study coordinator based on standard procedures( Reference Contreras Rojas and Valenzuela Vargas 14 ) and were trained on the administration of the child development assessments by a psychologist. The initial three-day training for child development testing included: (i) morning lectures on child development, the developmental assessment tools and role playing of the maternal report assessments; (ii) afternoon practical sessions administering the tests to several mother–infant dyads in an HC nearby the study office; and (iii) practice sessions administering the tests to more mother–infant dyads at an HC in the study area, plus video recording for later review and discussion. Data collectors needed to pass a written test and achieve a minimum level of agreement with the trainer (85 %) during practice sessions to be ready for independent administration of the child development tests. A one-day refresher training was conducted (over the Internet) using pre-recorded PowerPoint presentations followed by question-and-answer sessions via Skype.
Children’s anthropometric measurements were taken in duplicate (weight) or triplicate (length), and were then averaged for each individual. Digital scales with measurements to the nearest 100 g and portable stadiometers with measurements to the nearest 0·1 cm were used. We used the 2006 WHO growth references to determine Z-scores standardized by age and sex of the child. Hb was measured following standard procedures( Reference Jordan Lechuga 15 ) in capillary blood (heel prick) using portable HemoCue® equipment.
To assess motor development, we used the Gross Motor Development (GMD) milestones tool developed and validated by the WHO( Reference Wijnhoven, de Onis and Onyango 16 ) which consists of six gross motor milestones: (i) sitting without support; (ii) crawling; (iii) standing with support; (iv) standing without support; (v) walking with assistance; and (vi) walking without assistance. These gross motor milestones were assessed through maternal report and confirmed through direct testing. Before starting data collection, we piloted the GMD tool with twenty-four local mother–child dyads (children’s age: 5–13 months). Psychometric analysis of pilot data indicated that the tool had good internal consistency (Cronbach’s α coefficient was 0·75 for maternal report and 0·77 for direct testing). Furthermore, the number of GMD milestones achieved correlated significantly with children’s age, as expected. Also, the number of milestones achieved assessed by maternal report correlated significantly with the number of milestones achieved when the child was tested directly (Spearman’s ρ=0·86; P<0·0001).
We measured language development using an adaptation of the MacArthur–Bates Communicative Development Inventories (CDI) for children 8–15 months of age( Reference Fenson, Marchman and Thal 17 ). The CDI for children aged 8–15 months have three sections: (i) Pre-linguistic Vocalizations; (ii) First Words; and (iii) Gestures, with several sub-domains each. For the current study, we used the Spanish adaptation of the First Communicative Gestures( Reference López-Ornat, Gallego and Gallo 18 ) sub-test (part of the CDI Gestures section), with some minor further adaptations. In that sub-test, the mother was asked whether the child could do each of twelve first communicative gestures; this sub-test assessed pre-verbal language. We also used a Vocabulary inventory based on the Vocabulary sub-domain in the CDI (as part of the First Words section). However, the Vocabulary inventory we used was developed specifically for the study based on the same structure used in the CDI (i.e. categories of words) but including words compiled through semi-structured interviews with mothers (n 17; children’s age: 11–23 months) in the study area and selected based on pilot testing with more mothers of young children in Huánuco (n 32). The initial list of words compiled included 245 words, while the final version of the Vocabulary inventory included 100 words that varied on level of difficulty: easy (twenty-seven words), moderate (fifty-five words) and difficult (seventeen words). Due to low education levels in this population, administration of the Vocabulary inventory was completed by interview, instead of asking the mother to complete it on her own (as done in the original version of the tool). Test–retest reliability in a two-week period was good (n 32; Spearman’s ρ=0·99; P<0·0001).
Further cognitive development assessment was done by testing the child directly, using a two-step means–end problem-solving test adapted from the methodology developed by Willatts( Reference Willatts 19 ), which measures the infant’s ability to execute a planned sequence of steps to achieve a goal. In this test, the child must take two intermediate steps to achieve the ultimate goal. Thus, the infant was shown an attractive toy which was placed out of reach on a cloth and the toy was then covered under a smaller piece of cloth. To retrieve the toy, the infant had to pull the larger cloth towards him or her to bring the (smaller) cover within reach, lift that cover (these are the two steps, the means) and pick up the toy (the end). Scoring was based on the child’s behaviour while s/he took the steps and focused on three specific behaviours at each of the two steps, with possible scores between 0 (no intention) and 2 (clear intention); thus, there was a maximum score of 12 points at each of the three attempts included in the test, and a maximum total score of 36 points for this test was possible. We pilot tested the test with nine children between 10 and 13 months of age who attended an HC in the study area, and adapted the administration procedures and scoring system as needed.
Data on sociodemographic information, infant feeding and dietary practices, morbidity and supplement consumption were also collected through structured interviews. Information on food security was collected at the 9-month follow-up visit using the Escala Latinoamericana y Caribeña de Seguridad Alimentaria (ELCSA)( 20 ). To correct Hb values for altitude above sea level, we measured the altitude of each rural community using a SUUNTO® altimeter. Maternal height was measured (in duplicate) at baseline; but for those who were not measured at that time point, data were then collected in the follow-up visits. Maternal weight was measured in a similar manner. Information on eligibility for a national programme called Cuna Más was collected; the programme targeted low-income populations and included home visits and educational group sessions to promote appropriate caring and early learning practices for children under 36 months of age.
To measure the level of stimulation for child development and learning provided at home, we developed a questionnaire based on the Family Care for Development tool, part of the UNICEF Multiple Indicator Cluster Surveys( Reference Kariger, Frongillo and Engle 21 ), which has been adapted and validated in Bangladesh( Reference Hamadani, Tofail and Hilaly 22 ). This home stimulation questionnaire had two questions about reading materials available at home and five questions about activities that can be done with the child. The total score was obtained by summarizing the answers, giving 1 point for each answer indicating greater stimulation (e.g. if someone played with the child last week v. no one did); thus the total score ranged between 0 and 7.
Because season can affect food consumption, we created a variable reflecting the agricultural periods based on the month in which the child was enrolled. This variable included four three-month categories( Reference Ayala and Vilchez 23 ): sowing (October–December), weeding (January–March), harvest (April–June) and post-harvest (July–September).
Data quality control procedures included: (i) daily checking of study forms for completeness and consistency by the data collectors at the data collection site; (ii) weekly checking of forms by the study coordinator at the study office; and (iii) monthly unannounced field visits by the study coordinator to verify completion of scheduled visits by data collectors. A quality control form, with ten potential error codes, was designed and completed for any incomplete or incorrect study form, which triggered proper follow-up procedures. In addition, during these visits the study coordinator checked the supplement stocks, and verified that the randomization scheme was followed and the supplement distribution by the MOH staff was done according to protocol. Data were double entered using CSPro v 5·1 software.
Outcome variables
Hb concentration was measured in g/l and was corrected for altitude using the formula adapted by Jordan( Reference Jordan Lechuga 15 ). Anaemia was defined as Hb concentration less than 110 g/l. Stunting was defined as a height-for-age Z-score<−2·0; wasting was defined as a weight-for-height Z-score<−2·0; and underweight was defined as a weight-for-age Z-score<−2·0.
Three types of language scores were calculated: (i) pre-verbal language score (the number of gestures the child can do); (ii) receptive language score (the number of words the child could understand); and (iii) expressive language score (the number of words the child could say).
We selected the GMD milestone ‘Walking without assistance’, which is expected to be achieved by at least 50 % of children aged 12 months( 24 ), as the gross motor development (dichotomous) outcome to be analysed and used maternal report of achievement because it was highly correlated with direct testing and had less missing data than the variable based on direct testing.
We defined two other cognitive development outcomes: (i) problem-solving total score (the sum of the scores in each of the three attempts in the two-step means–end task); and (ii) successful problem solving (defined as having achieved the highest score in at least one of the attempts).
Statistical analysis
Because the analytic sample did not include all children randomized to the treatment groups, we compared the analytic sample with the sample of children enrolled at baseline with respect to sociodemographic characteristics using the Student t test (for continuous variables) and the χ 2 test (for categorical variables). Similarly, baseline characteristics of participants in the analytic sample were compared by treatment group to determine whether the groups were similar. Categorical variables were summarized as frequencies and percentages and continuous variables were summarized as means and sd.
The analysis of the effects of the intervention was based on intention to treat; that is, the study results were analysed using all available data on participants regardless of any protocol violation, such as imperfect adherence or contamination of treatment. It was conducted using either ANCOVA (continuous outcomes) or logistic regression (dichotomous outcomes): first without adjustment for covariates, but taking account of the child’s age, in the case of the development outcomes (unadjusted models); and second with adjustment for pre-specified covariates, including the corresponding reference value (i.e. value of the same variable outcome at baseline), when available (adjusted models). Hypothesis testing was two-tailed and a level of significance of 5 % (P<0·05) was used.
Pre-specified covariates were: baseline value (except for language development and problem solving), child’s age, sex and morbidity, maternal characteristics (i.e. age, education and height), HH characteristics (i.e. floor and wall materials, toilet availability, number of HH members <5 years of age and developmental stimulation) and services (i.e. HC and Cuna Más programme beneficiary). Covariates selection for adjusted models was based on the bivariate association between each covariate and each outcome; only covariates associated with the outcome variable (at P<0·10) were included in the adjusted analysis for that outcome variable. The variable season reflected the differential enrolment rate by treatment group due to the LNS disruption, and therefore was included in all adjusted models. Also, because child development is dependent on the child’s age, we included the variable age (at time of outcome assessment) in all analyses (i.e. unadjusted and adjusted models) modelling developmental outcomes.
Unadjusted and adjusted results for continuous outcomes are presented as means and sd. Mean differences with corresponding 95 % CI are also reported. We assessed continuous outcomes and residuals for normality using the Shapiro–Wilk test and values >0·97 were considered acceptable. When needed, continuous outcome variables were transformed appropriately. Logistic regression results are reported as unadjusted and adjusted OR with corresponding 95 % CI and P values. All statistical analyses were performed using the statistical software package SAS version 9.3.
Results
Characteristics of participants
A total of 422 children were enrolled in the study, of whom twenty-five were lost to follow-up due to moving out of the study area and one was not found within the time window of follow-up assessment. In addition, data for thirty-five participants who were reached at the 12-month follow-up visit were excluded because they were falsified by the research assistant at either the baseline or 12-month visit. Figure 1 shows the participant flow. Thus, the analytical sample included a total of 361 children, which corresponded to 86 % of the sample that was enrolled at baseline. Comparison of the analytic sample with that of children enrolled but not included in the analysis indicated that the samples were similar with respect to several sociodemographic characteristics, including maternal age (P=0·55), maternal education (P=0·76), maternal height (P=0·22), number of HH members (P=0·98) or children <5 years of age in the HH (P=0·81), access to toilet with drainage in the HH (P=0·64), as well as child’s sex (P=0·19) and age (P=0·93).
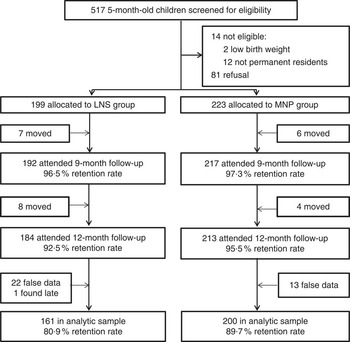
Fig. 1 Flow diagram of study participation (LNS, lipid-based nutrient supplement; MNP, micronutrient powder)
Overall, children were enrolled at 6·1 (sd 0·1) months of age and their mothers were, on average, 23 (sd 8) years old at enrolment. Fifty-two per cent of children were male and 48 % were female. All but one of the participants were being breast-fed; 13 % of children were beneficiaries of the Cuna Más programme. Only 26 % of children lived in houses with access to toilets with drainage, 10 % of houses had some type of floor (e.g. cement, tiles) as opposed to dirt, and 6 % of houses had brick or cement walls. At the 9-month follow-up visit, 47 % reported to be food secure, and among the food insecure, 43 % reported mild, 10 % reported moderate and two participants reported severe food insecurity.
Table 2 shows the baseline nutritional and sociodemographic characteristics of the participants by treatment group. No significant differences were observed by treatment group. In addition to the variables shown in Table 2, we also compared motor development at baseline (measured as maternal report of achievement of the milestone ‘Sitting without support’) and found a marginally significant difference by treatment group (P=0·06). Furthermore, the distribution of children by severity of anaemia (P=0·59) or stunting (P=0·75) at baseline did not differ by treatment group.
Table 2 Baseline characteristics of the 6-month-old children (n 361) by treatment group, Huánuco, Peru, July 2013–December 2015
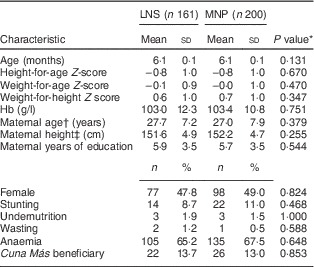
LNS, lipid-based nutrient supplement; MNP, micronutrient powder.
* Obtained using Student’s t test (continuous variables), the χ 2 test or Fisher’s exact test (categorical variables).
† n 353.
‡ n 336.
Based on caregivers’ report, adherence to supplement consumption recommendations was as follows: 96 % of children in the LNS and 82 % in the MNP group consumed the supplement either every day or every other day (P<0·001). Morbidity rates during the previous two weeks, based on caregivers’ reports at the 12-month interview, were similar between groups: 14 % of children in the LNS and 17 % in the MNP group had fever (P=0·93); 9 % of children in the LNS and 12 % in the MNP group had cough (P=0·98); and 14 % of children in the LNS and 17 % in the MNP group had diarrhoea (P=0·93).
Effects on nutritional outcomes
The effects of the intervention on continuous nutritional outcomes are shown in Table 3. Children in the LNS group had a mean Hb concentration significantly higher than those in the MNP group, and this difference remained significant after controlling for covariates. We did not find significant differences between treatment groups regarding any of the anthropometric indicators.
Table 3 Continuous nutritional and developmental outcomes by treatment group among the children (n 361) supplemented from 6 to 12 months of age, Huánuco, Peru, July 2013–December 2015
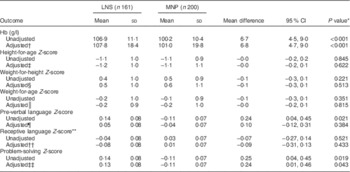
LNS, lipid-based nutrient supplement; MNP, micronutrient powder.
* Two-sided significant levels.
† Adjusted for baseline value, age, maternal education, type of toilet, house floor and wall materials, season, Cuna Más beneficiary and health centre; n 361.
‡ Adjusted for baseline value, sex, maternal education, maternal height, season, type of toilet and health centre; n 335.
§ Adjusted for baseline value, sex, morbidity, maternal height, season and health centre; n 334.
║ Adjusted for baseline value, sex, maternal height, season, type of toilet and health centre; n 336.
¶ Adjusted for age, sex, Cuna Más beneficiary, maternal education, number of household members, type of toilet, house wall material and season; n 361.
** P value and estimations based on transformed variable.
†† Adjusted for age, Cuna Más beneficiary, home stimulation, maternal education, maternal height, season and health centre; n 336.
‡‡ Adjusted for age, home stimulation, maternal education, number of household members, house wall material, season and health centre; n 360.
The unadjusted prevalence of anaemia in the MNP group was higher than in the LNS group (83·5 v. 60·3 %; P<0·001). However, no differences by treatment group were observed in the unadjusted prevalence of stunting (16·2 % in the LNS and 14·1 % in the MNP group; P=0·583), underweight (3·1 % in the LNS and 1·5 % in the MNP group; P=0·475) and wasting (0·6 % in the LNS and 0·0 % in the MNP group; P=0·447). Table 4 shows the results of the logistic regression analyses for anaemia and stunting. Due to low variation in wasting and underweight we did not analyse those outcomes further. Consistent with what was observed for the continuous outcomes, the LNS was associated with lower odds of anaemia and that association persisted after adjustment for covariates. LNS supplementation did not reduce the odds of stunting, when compared with MNP supplementation.
Table 4 OR for the dichotomous nutritional and developmental outcomesFootnote * among children (n 361) supplemented from 6 to 12 months of age, Huánuco, Peru, July 2013–December 2015
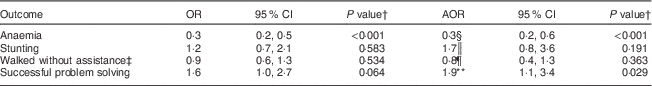
AOR, adjusted OR; LNS, lipid-based nutrient supplement; MNP, micronutrient powder.
* Effect of LNS, MNP group is the reference.
† Two-sided significant levels.
‡ Based on maternal report.
§ Adjusted for baseline value, age, maternal education, number of children <5 years of age in the household, Cuna Más beneficiary and season; n 361.
║ Adjusted for baseline status, maternal education, morbidity and season; n 359.
¶ Adjusted for motor development at baseline, age, morbidity, number of children <5 years of age in the household, Cuna Más beneficiary, home stimulation, season and health centre; n 361.
** Adjusted for age, sex, maternal education, number of household members and season; n 361.
Effects on child development outcomes
The effects of the intervention on continuous development outcomes are presented in Table 3. We observed a higher pre-verbal language score among children in the LNS group compared with those in the MNP group (P=0·021, adjusted for the child’s age), but such difference lost significance (P=0·384) after adjusting for covariates. No significant differences were observed in receptive language scores; in both groups, the mean number of words that the children understood was nineteen (back transformation to original scale).
A significant difference by treatment group was observed in problem-solving scores (P=0·019, adjusted for the child’s age), and such difference remained significant after adjustment for covariates (P=0·043).
The proportion of children who achieved the gross motor milestone was similar in both groups (36·0 % in the LNS and 39·5 % in the MNP group; unadjusted P=0·499). In contrast, the proportion of children who successfully solved the problem (reached the toy after taking two intermediate steps) was higher in the LNS group than in the MNP group (28·0 v. 18·5 %, respectively; unadjusted P=0·033). Table 4 shows the results of the logistic regression analyses for achieving the gross motor milestone and the problem-solving task. LNS supplementation had no effect on gross motor development in the unadjusted (P=0·534) or adjusted analysis (P=0·223). On the other hand, children in the LNS group had higher odds of achieving the problem-solving task than children in the MNP group, although this effect was more evident in the adjusted analysis (unadjusted analysis: P=0·064; adjusted analysis: P=0·029).
Discussion
The intervention of nutritional supplementation with LNS for children for 6 months significantly increased the levels of Hb and reduced the prevalence of anaemia at 12 months compared with MNP supplementation. This finding is quite interesting as the MNP had more Fe than the LNS (12·5 v. 9 mg). Our analysis was based on intention to treat, which is the preferred approach for randomized trials. To rule out compliance as a potential explanation for these results, we re-ran these analyses in a sub-sample of children who consumed the supplements as recommended (i.e. daily); findings in this sub-sample were similar (data not shown). One can also speculate that the relative bioavailability of Fe from the supplements relates to the observed differences in Hb concentrations and anaemia rates. Both products included encapsulated Fe, as ferrous sulfate in LNS and as ferrous fumarate in MNP, and several factors could have influenced the bioavailability of Fe from these encapsulated products( Reference Zimmermann and Windhab 25 ). However, comparison of our findings with those from another LNS study seems to dismiss a bioavailability explanation. Our results contrast with those in a randomized trial conducted in Ghana comparing three types of supplements (i.e. LNS, MNP and Nutritab), where children’s mean Hb concentration and prevalence of anaemia at 12 months did not differ between the LNS v. the MNP group( Reference Adu-Afarwuah, Lartey and Brown 8 ), even though both products included the same amount and chemical form of encapsulated Fe as the supplements we used in the present study. Another potential alternative explanation for our findings may be linked to the vitamin B12 content in the LNS, a nutrient not included in the MNP. Data on the prevalence of vitamin B12 deficiency in Latin America are scarce, but a recent review of the nutritional situation in this region reported a national prevalence of 22·5 % among young Guatemalan children( Reference Galicia, Grajeda and de Romaña 26 ). Vitamin B12 deficiency can result in anaemia due to ineffective erythropoiesis( Reference Koury and Ponka 27 ). Data from young children (aged 12–59 months) from two other areas in Peru (Huancavelica and Ucayali) revealed that between 11 and 30 % of those who had anaemia were also vitamin B12 deficient( Reference Gonzales, Huamán-Espino and Gutiérrez 28 ). Since LNS provided vitamin B12 in the amount considered the Adequate Intake for this age range( Reference Arimond, Zeilani and Jungjohann 11 ), it could have potentially contributed to reducing anaemia related to vitamin B12 deficiency. It is also important to note that although the LNS was associated with a reduction in anaemia in the present study, a concerning high proportion of children in the LNS group (60 %) were still anaemic after receiving the supplement for 6 months. This is likely related to parasitic infections which are prevalent and coexist with anaemia among young Peruvian children( Reference Gonzales, Huamán-Espino and Gutiérrez 28 , Reference Cabada, Goodrich and Graham 29 ). Thus, longer supplementation and/or more comprehensive approaches (e.g. LNS plus increased access to nutritious food and/or deworming treatment) may be needed to further reduce anaemia in this population.
With regard to anthropometric indicators, LNS supplementation had no effect on the height-for-age, weight-for-height or weight-for-age Z-score, or on the risk of stunting, when compared with MNP supplementation. These results may relate to the small amounts of macronutrients that LNS provided, only ~460 kJ or 110 kcal and 2·6 g of protein daily, and the relatively short supplementation period (6 months). Similar results were observed in the study in Ghana where height-for-age, weight-for-height and weight-for-age Z-scores did not differ when children who received LNS were compared with those who received MNP( Reference Adu-Afarwuah, Lartey and Brown 9 ). However, in another randomized controlled trial in Haiti where 6–11-month-old children (n 589) received LNS for either 3 or 6 months, LNS supplementation for 6 months increased height-for-age and weight-for-age Z-scores, compared with the control group( Reference Iannotti, Dulience and Green 30 ). These results suggest that LNS may improve growth when no other nutritional supplementation is available, possibly only by slowing down the dramatic decrease in Z-scores typically observed among young children in resource-limited populations. Wasting and underweight were rare in the study sample, which limited our ability to assess any effects of LNS on these outcomes.
Regarding child development, the LNS intervention showed significant effects on children’s problem-solving skills but no effects were observed on motor or language development. In the randomized trial conducted in Ghana, 39 % of children who received MNP were able to walk independently at 12 months( Reference Adu-Afarwuah, Lartey and Brown 9 ), a percentage quite similar to what we observed in our study in the MNP group (39·5 %). However, in the group of children who received the LNS supplement in Ghana, 49 % achieved the motor milestone( Reference Adu-Afarwuah, Lartey and Brown 9 ). This proportion was very close to what is expected for that age since, according to the WHO references, ~50 % of children can walk without support at 12 months of age or earlier( 24 ). It is noteworthy that in the Ghana study the observed difference in the percentages of children who achieved this gross motor milestone did not reach statistical significance( Reference Adu-Afarwuah, Lartey and Brown 9 ), which is consistent with our findings. Similarly, in the randomized controlled trail in Haiti, LNS supplementation for either 3 or 6 months did not affect the achievement of gross motor milestones, compared with a control group( Reference Iannotti, Dulience and Green 30 ).
There were no significant differences between the treatment groups with respect to children’s language. Protein, Fe and iodine are critical nutrients for myelination during infancy( Reference Wachs, Georgieff and Cusick 31 ) and myelination in several language-correlated brain regions reaches maturity at about 1·5 years of age( Reference Su, Kuan and Kaga 32 ). Consistently, children’s vocabulary increases dramatically during the second year of life( Reference McMurray 33 ). Thus, it is possible that the effects of LNS supplementation on children’s language were not detected because the assessment was conducted at a very early age.
A positive effect of LNS supplementation on children’s problem-solving skills was observed. Children in the LNS group had higher scores and were more likely to successfully accomplish the problem-solving task than children in the MNP group. To our knowledge, this is the first report of an effect of LNS supplementation on problem solving at this young age, which, besides being novel, is an important finding because of the association between problem solving during infancy and intelligence in later childhood( Reference Slater 34 ). A potential explanation for the improved problem-solving skills may relate to the fatty acids content in the LNS. DHA, a long-chain PUFA, is found in high concentrations in the brain and is associated with information processing speed( Reference Willatts, Forsyth and DiModugno 35 ). Moreover, based on the higher Hb concentrations and reduced anaemia prevalence observed in the LNS group, one can also speculate that improved Fe status played a role in this finding, as Fe is needed for brain development and myelination( Reference Wachs, Georgieff and Cusick 31 ). However, we did not assess Fe status directly, and recent estimations indicate that the proportion of anaemia due to Fe deficiency may be lower than what was previously assumed( Reference Petry, Olofin and Hurrell 36 ). Still, long-term follow-up assessment of cognitive skills would allow us to determine the functional importance of the observed effect, as well as further developmental effects that may become more evident when children are older.
The current study has some limitations, including the disruption of LNS for a couple of months due to logistical problems, which affected the randomization plan during that time. We attempted to control for this issue by including a covariate measuring season; this variable reflected the different distributions per treatment group (at enrolment), which were driven by the LNS disruption. Another important limitation relates to the different appearance of the two supplements which made it impossible to keep participants blind. Evaluators were kept blind to the extent possible, but we could not rule out the possibility of exposure to the supplements during contacts with participants. Furthermore, attrition could have introduced selection bias; however, comparisons between those included v. those not included in the analysis dismissed that possibility. Nevertheless, the present study also has strengths, such as a rigorous study design (i.e. randomized controlled trial) and the assessment of several potential confounding variables. In addition, the present report is the first on the effects of a novel nutritional supplementation approach (LNS) in Peru, when compared with the standard of care (MNP) in this country. Moreover, as opposed to a tightly monitored efficacy trial, the study was an effectiveness trial conducted in collaboration with Peruvian MOH authorities and the Regional Health Division whose staff distributed the supplements as part of their regular preventive care services, and therefore provides relevant information on the feasibility and potential impact of an LNS intervention at scale.
Conclusion
In summary, a 6-month nutritional supplementation intervention with LNS delivered through the government health-care system increased Hb concentration, reduced anaemia prevalence and improved problem-solving skills, but had no effect on anthropometric indicators, motor or language development among 12-month-old Peruvian children, when compared with MNP supplementation. Further analyses taking account of the levels and modes of supplement consumption could provide information of programmatic relevance. Evaluation of potential long-term effects, as nutritional supplementation occurred during the critical first 1000 d, is warranted.
Acknowledgements
Acknowledgements: The authors thank Juan Pablo Aparco from the National Center for Food and Nutrition (CENAN; from its initials in Spanish), Patricia Delgado from the Regional Division of Health in Huánuco and Mary Penny from the Institute for Nutritional Research (IIN; from its initials in Spanish) for their technical contributions; the research field staff for their dedication; and the authorities and staff at the Regional Division of Health in Huánuco and Health Micro Network in Huacar and San Rafael for their assistance in the implementation of the study. Financial support: This study was funded by the UBS Optimus Foundation (grant #02) and the Action Against Hunger (ACH; from its initials in Spanish) Foundation. The lipid-based nutrient supplements (Nutributter®) were donated by Nutriset (Malaunay, France). UBS Optimus Foundation and Nutriset had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: S.L.M. contributed to study design, data collection training, analysed the data and wrote the first draft of the manuscript. A.V.-V. contributed to study design, data collection training and supervision, and data processing. R.B.P. contributed to study design and supervised data collection. L.A.V., O.A.V., A.R.M. and J.P.N.R. contributed to study design. All authors read and approved the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Ethics Committee of the Asociación Benéfica Prisma (Peru). Written informed consent was obtained from all participants.








