In addition to its metabolic consequences, type 2 diabetes (T2D) is characterised by an immune dysfunction that leads to increased susceptibility to infection and, in septic patients, high morbidity and mortality. Various alterations in immune defence have been observed in animal models of insulin resistance, notably including an impairment in cytokine and NO production by peritoneal macrophages( Reference Breuillard, Belabed and Bonhomme 1 , Reference Blanc, Moinard and Béziel 2 ). This may be attributed to the progressive decrease in the plasma concentration of arginine, the NO precursor, that develops with the metabolic syndrome and T2D( Reference Belabed, Senon and Blanc 3 ).
Arginine is a pleiotropic amino acid involved in insulin secretion, insulin sensitivity and inflammatory status. On the one hand, from a metabolic perspective, NO synthesis by arginine in endothelial cells is required to improve blood flow and thus insulin and nutrient supply to peripheral organs( Reference Wu, Satterfield and Bazer 4 ), and arginine in adipose tissue has been shown to promote fatty acid oxidation and thus help improve insulin sensitivity( Reference Wu, Satterfield and Bazer 4 , Reference Jobgen, Meininger and Jobgen 5 ). On the other hand, arginine plays a very specific role in macrophage functions. First, arginine is the sole precursor of NO, which plays a central role in immunity via its cytotoxic and cytostatic effects on pathogens( Reference Li, Yin and Li 6 ). Second, as we have shown previously( Reference Breuillard, Belabed and Bonhomme 1 ), supplementation of arginine to the culture medium of macrophages from diabetic obese rats decreases pro-inflammatory cytokine production, which points to the regulatory role of this amino acid and the importance of arginine availability in these cells.
Interestingly, plasma arginine concentration has been shown to decrease in diabetic obese patients as a result of at least two mechanisms: increased plasma arginase activity( Reference Kashyap, Lara and Zhang 7 ) and defective renal arginine synthesis from citrulline with progressive deterioration in kidney function. This is demonstrated by the moderate increase in plasma citrulline concentration that leads to the development of the metabolic syndrome( Reference Sailer, Dahlhoff and Giesbertz 8 ). Given the regulatory properties of arginine, alterations in arginine availability and metabolism may be deeply involved in diabetes-associated immune deficiency.
These data suggest that supplying extra arginine could be useful as a nutritional therapy in diabetic obese patients. However, research on the metabolic features of arginine has also suggested that supplementation may well not be the best choice for improving macrophage functions in vivo ( Reference Curis, Nicolis and Moinard 9 ). For example, a study by our group in endotoxaemic diabetic obese rats has shown that excessive arginine supply is associated with increased mortality( Reference Bonhomme, Belabed and Blanc 10 ), while a graded arginine supply has no effect on macrophage functions( Reference Breuillard, Darquy and Curis 11 ).
Citrulline, the precursor of arginine through the two enzymes argininosuccinate synthase and lyase( Reference Curis, Nicolis and Moinard 9 ), might offer a safe alternative to arginine for improving macrophage function under conditions of diabetes and obesity. Studies have shown that citrulline transport is effective in macrophages, and argininosuccinate synthase and argininosuccinate lyase are expressed in these cells( Reference Baydoun, Bogle and Pearson 12 , Reference Bryk, Ochoa and Correia 13 ). Evidence suggests that NO production by macrophages is dependent on the recycling of citrulline into arginine( Reference Nussler, Billiar and Liu 14 ), and that exogenous citrulline can be used for NO synthesis( Reference Baydoun, Bogle and Pearson 12 , Reference Bryk, Ochoa and Correia 13 ).
Only a few studies have evaluated the specific effect of citrulline on macrophage function in an arginine-free medium( Reference Baydoun, Bogle and Pearson 12 , Reference Bryk, Ochoa and Correia 13 ), and the contribution of citrulline under conditions of diabetes and obesity has not yet been investigated.
We hypothesised that in a context of decreased arginine availability, citrulline could be an efficient substitute for arginine to improve macrophage functions under conditions of diabetes and obesity.
The aim of the present in vitro study in peritoneal macrophages from diabetic obese rats was to determine whether increased citrulline availability influenced arginine metabolism and cytokine production. Zucker diabetic fatty rats were used as the model for T2D and obesity. An in vitro approach was required to identify the direct effects of citrulline on these cells, as in vivo citrulline administration has been quantitatively associated with increased plasma arginine concentration, which, in turn, influences macrophage function( Reference Faure, Raynaud-Simon and Ferry 15 ).
Materials and methods
Animals
All animal experiments were performed in accordance with the French and European Community regulations governing animal care and experimentation (Official Journal of the European Community L 358, 18 December 1986). The study protocol was approved by the local Île-de-France Regional Ethics Committee (no. P2.CC.109.09).
In the present study, 11-week-old male Zucker diabetic fatty rats (Charles River Laboratories) were used, which were divided into two experimental groups: diabetic obese group (n 5) or control group (lean littermates; n 5). After a 1-week acclimatisation period( Reference Breuillard, Belabed and Bonhomme 1 ), the rats were anaesthetised by isoflurane (Aerrane®; Baxter) inhalation, and then killed by decapitation. Blood samples were collected in tubes containing sodium heparinate (25 000 IU/5 ml, Heparine Choay®; Sanofi-Aventis) and rapidly centrifuged. Plasma was obtained, and rapidly frozen and stored at − 80°C until analysis. Peritoneal macrophages were collected by washing the peritoneal cavity with 20 ml of Dulbecco's modified Eagle's medium (Sigma-Aldrich), as described previously by Breuillard et al. ( Reference Breuillard, Belabed and Bonhomme 1 ).
Measurement of plasma glucose and insulin concentration
Plasma glucose concentration was measured using an automated enzymatic method on a Cobas 6000 analyser (Roche Diagnostics) with standard reagents. Insulin was determined using a rat insulin RIA kit (RI-13K; Linco Labodia). The plasma insulin:glucose ratio was calculated as a marker of insulin sensitivity.
Experimental design
Peritoneal macrophages were isolated from the rats( Reference Breuillard, Belabed and Bonhomme 1 ) and cultured in six-well polystyrene culture plates (Becton Dickinson) at 2 × 106 cells/well. Given the limited number of macrophages obtained from one rat, only eight wells (two wells per citrulline concentration) were seeded for each rat. Non-adherent cells were removed after incubation for 2 h, and macrophages were cultured in arginine-free Dulbecco's modified Eagle's medium (Eurobio) for 6 h in the presence of 0·1, 0·5, 1 or 2 mm-citrulline. The lowest concentration corresponded to the physiological plasma concentration of citrulline in Zucker diabetic fatty rats (about 100 μmol/l for diabetic obese and lean rats)( Reference Belabed, Senon and Blanc 3 ), and the other concentrations were in the range of peak plasma concentrations observed previously after in vivo citrulline administration in rats( Reference Faure, Raynaud-Simon and Ferry 15 , Reference Ventura, Noirez and Breuillé 16 ). At the end of the incubation period, the culture media were collected and stored at − 80°C until analysis( Reference Breuillard, Belabed and Bonhomme 1 ).
Determination of nitric oxide, TNF-α and IL-6 production
NO production was estimated by measuring nitrites and nitrates by a modified Griess method (R&D Systems). The intra- and inter-assay CV were about 1·5 and 4 %, respectively, and sensitivity was 0·78 μmol/l.
TNF-α (R&D Systems) and IL-6 (PromoKine; PromoCell GmbH) produced by peritoneal macrophages were assayed using ELISA kits. For the TNF-α assay, the intra- and inter-assay CV were about 3 and 9 %, respectively, and sensitivity was 5 pg/ml. For the IL-6 assay, the intra- and inter-assay CV were < 5 and < 10 %, respectively, and sensitivity was 12 pg/ml. NO, TNF-α and IL-6 concentrations were measured in the same supernatants.
Statistical analysis
The required number of experiments was deduced from our previous studies( Reference Breuillard, Belabed and Bonhomme 1 , Reference Blanc, Moinard and Béziel 2 , Reference Bonhomme, Belabed and Blanc 10 , Reference Breuillard, Darquy and Curis 11 ). Data are presented as means with their standard errors. Statistical analysis was performed using StatView software (SAS Institute, Inc.). For the determination of weight and plasma glucose and insulin concentrations, comparisons were made using a t test followed by a post hoc Student's test. To assess the simultaneous influence of strain and citrulline on macrophage function, a two-factor ANOVA was used. Other comparisons were made by ANOVA followed by Fisher's protected least significant difference tests and linear regression analysis. A P value < 0·05 was considered significant.
Results
Characteristics of Zucker diabetic fatty rats
As expected, diabetic obese rats presented significantly higher body weight (P =0·001), glycaemia (P <0·001) and plasma insulin concentration (P <0·01) than lean control rats, and tended to have a higher plasma insulin:glucose ratio (P =0·086) (Table 1).
Table 1 Main metabolic characteristics of Zucker diabetic fatty rats* (Mean values with their standard errors)
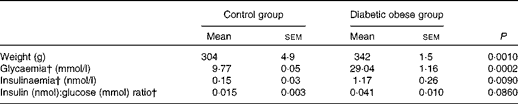
* Results were obtained from the t test followed by post hoc Student's test.
† Data for glycaemia, insulinaemia and insulin:glucose ratio were log-transformed for statistical analysis.
Determination of nitric oxide, TNF-α and IL-6 production
Peritoneal macrophages effectively produced NO (Fig. 1(A)) in an arginine-free medium in the presence of citrulline; however, NO production did not vary with increasing citrulline concentrations. Data for each group (lean or diabetic obese rats) were therefore pooled for all citrulline concentrations in the medium. Mean NO production was significantly lower in the cells from the diabetic obese group (56·3 (sem 5·5) nmol/2 × 106 cells per 6 h) than in those from the control group (69·7 (sem 9·1) nmol/2 × 106 cells per 6 h) (P =0·032).

Fig. 1 Effects of citrulline concentration on (A) nitric oxide, (B) TNF-α and (C) IL-6 production by macrophages from lean control (![]() ) or diabetic obese (
) or diabetic obese (![]() ) Zucker diabetic fatty (ZDF) rats. Macrophages from the lean control or diabetic obese ZDF rats (n 5 rats per group) were incubated in arginine-free Dulbecco's modified Eagle's medium with increasing citrulline concentrations (0·1–2 mmol/l) for 6 h. Each incubation was performed in duplicate under each condition. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA+Fisher's protected least significant difference test).
) Zucker diabetic fatty (ZDF) rats. Macrophages from the lean control or diabetic obese ZDF rats (n 5 rats per group) were incubated in arginine-free Dulbecco's modified Eagle's medium with increasing citrulline concentrations (0·1–2 mmol/l) for 6 h. Each incubation was performed in duplicate under each condition. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA+Fisher's protected least significant difference test).
TNF-α production (Fig. 1(B)) was markedly higher in the macrophages obtained from the diabetic obese group (P =0·0004; two-way ANOVA) and decreased with increasing citrulline concentrations (r 0·21, P =0·0004), whereas TNF-α production in the macrophages of the control group remained low at all citrulline concentrations.
IL-6 production (Fig. 1(C)) increased with increasing citrulline concentrations (P <0·0001; two-way ANOVA) in the two groups, and positive relationships were observed (control group: r 0·442, P =0·009; diabetic obese group: r 0·64, P <0·0001). IL-6 production became significantly higher in the macrophages from the diabetic obese group than in those from the control group at citrulline concentrations of 1 mmol/l (P= 0·0435) and 2 mmol/l (P= 0·0023).
In both diabetic obese (r 0·60, P =0·0002) and control (r 0·64, P <0·0001) groups, a significant inverse relationship was found between IL-6 and TNF-α production.
Discussion
The present study first shows the alterations in macrophage function associated with diabetes and obesity, confirming the elevated TNF-α production described previously( Reference Breuillard, Belabed and Bonhomme 1 ). In agreement with the results reported in the literature( Reference Baydoun, Bogle and Pearson 12 , Reference Bryk, Ochoa and Correia 13 ), our data demonstrate the significant NO production by peritoneal macrophages from both lean and diabetic obese rats in an arginine-free medium in the presence of increasing citrulline concentrations. Our data also reveal a significant regulatory effect of citrulline on cytokine production, as reported in our previous results demonstrating the regulatory properties of arginine in these cells( Reference Breuillard, Belabed and Bonhomme 1 ).
Although macrophages effectively produced NO in an arginine-free citrulline-supplemented medium, NO production did not increase with increasing citrulline concentrations, which is at variance with the dose–effect relationship observed with arginine in the same model( Reference Breuillard, Belabed and Bonhomme 1 ). However, taking into account the differences in experimental conditions, NO production level was on a par with that observed with arginine supplementation( Reference Breuillard, Belabed and Bonhomme 1 ). As citrulline transport in macrophages is saturable at a citrulline concentration of 1 mmol/l( Reference Baydoun, Bogle and Pearson 12 ), use of citrulline in macrophages under basal conditions may have already reached a plateau at a citrulline concentration of 0·1 mmol/l.
In line with our previous study, macrophages from diabetic obese rats produced higher levels of inflammatory cytokines – an illustration of the chronic low-grade inflammatory state observed under conditions of diabetes and obesity. Interestingly, citrulline affected TNF-α released by peritoneal macrophages, which is consistent with our previous results on arginine supplementation: TNF-α production decreased with increased citrulline availability in T2D rats( Reference Breuillard, Belabed and Bonhomme 1 ). This could be due to the recycling of citrulline into arginine, which is known to have anti-inflammatory effects( Reference Breuillard, Belabed and Bonhomme 1 ). However, the fact that NO production did not increase further at a citrulline concentration above 0·1 mmol/l challenges this hypothesis. Alternatively, this action of citrulline could be related to a direct antioxidant effect of the amino acid( Reference Bonnefont-Rousselot, Le Plenier and Cynober 17 ).
A major finding of the present study was the influence of citrulline concentration on IL-6 production: increased citrulline availability increased the release of IL-6 by peritoneal macrophages in both diabetic obese and control rats. In the context of the metabolic syndrome, IL-6 has long been considered detrimental due to the elevated plasma IL-6 levels in obese subjects( Reference Browning, Krebs and Magee 18 ). In contrast, it appears to be beneficial in terms of insulin sensitivity and glucose homeostasis. Indeed, it has been shown that deletion of the IL-6 gene in mice fed a high-fat diet led to insulin resistance( Reference Matthews, Allen and Risis 19 ). Conversely, overexpression of human IL-6 in high-fat diet-fed or genetically obese (ob/ob) mice has resulted in lower body and adipose tissue weights and in decreased plasma concentrations of glucose and insulin( Reference Sadagurski, Norquay and Farhang 20 ). This effect on insulin sensitivity has been confirmed in human subjects. IL-6 infusion in T2D patients has been shown to decrease plasma insulin concentration without affecting glycaemia, indicating an improvement in insulin sensitivity( Reference Petersen, Carey and Sacchetti 21 ). Moreover, IL-6 infusion in patients with T2D up-regulated proteins normally associated with enhanced insulin sensitivity in adipose tissue( Reference Carey, Petersen and Bruce 22 ).
Interestingly, it is likely that IL-6-induced improvement in insulin resistance is associated with decreased inflammation, as IL-6 infusion has been shown to decrease TNF-α production and stimulate anti-inflammatory cytokine production in human subjects( Reference Steensberg, Fischer and Keller 23 , Reference Starkie, Ostrowski and Jauffred 24 ). Conversely, in the macrophages of mice fed a high-fat diet, IL-6 receptor deletion led to a switch of macrophage polarisation from an inflammatory (M2) to a pro-inflammatory phenotype (M1) and increased plasma pro-inflammatory cytokine response to endotoxin compared with wild-type mice( Reference Mauer, Chaurasia and Goldau 25 ). Thus, it suggests that IL-6 could improve insulin sensitivity by decreasing the T2D-associated low-grade pro-inflammatory state. This is consistent with the negative correlation found between TNF-α and IL-6 production in the present study. Notably, among the putative mechanisms of this effect, the production of these cytokines is controlled by different transcription factors, e.g. CCAAT/enhancer-binding protein β for IL-6( Reference Bradley, Zhou and Smale 26 ) or NF-κB for TNF-α( Reference Anstead, Chandrasekar and Zhang 27 ). Moreover, mitogen-activated protein/extracellular signal-regulated kinase kinase 3 has been considered to be essential for IL-6 production by lipopolysaccharide-activated macrophages, unlike TNF-α( Reference Kim, Duramad and Qin 28 ). The effect of citrulline availability on these transcription factors warrants further research.
Another important finding is that in addition to the citrulline-induced decrease in TNF-α production, which is similar to that observed with arginine( Reference Breuillard, Belabed and Bonhomme 1 ), IL-6 production is increased by increasing citrulline concentrations, an effect not observed with arginine in T2D rats( Reference Breuillard, Belabed and Bonhomme 1 ). This suggests that the effects of citrulline availability on IL-6 production are not entirely due to its conversion into arginine, and that these two amino acids may act through partly different mechanisms. A possible explanation is that part of the regulatory effects of arginine requires NO production, and we observed that NO production was not modified by citrulline. Although the precise mechanism involved remains unknown, the regulatory properties of arginine on immune function have been shown to be dependent on the control of mitogen-activated protein kinase activation and thus cytokine production via mitogen-activated protein kinase kinase kinase tumour-promoting locus 2( Reference Mieulet, Yan and Choisy 29 ). It could be interesting to evaluate the influence of extracellular citrulline on this transduction pathway. Moreover, as in vivo citrulline administration leads to a simultaneous increase in plasma arginine and citrulline concentrations, it could be interesting to evaluate whether the effects of these two amino acids are indeed additive in terms of the regulation of inflammatory status.
In conclusion, the present study shows that citrulline is able to induce NO production and improve cytokine release in macrophages from diabetic obese rats. Citrulline may thus help restore macrophage function under conditions of diabetes and obesity. Therefore, it would be interesting to investigate the supplementation of citrulline in subjects with T2D, both for its direct effects on immune cells and, as a precursor of arginine, for arginine-induced improvement in insulin sensitivity( Reference Piatti, Monti and Valsecchi 30 ).
Acknowledgements
The authors thank Servane Le Plénier, Céline Dumez and Josephine Kohler for their technical support, and Dr Emmanuel Curis for valuable advice on the statistical analyses.
The present study work was funded by the French Ministry of Research and Technology (EA 4466).
The authors' contributions are as follows: S. B., R. C. and L. C. conceived and designed the study; C. B. and S. B. carried out the studies; C. B. and J.-P. D. B. analysed and interpreted the data, performed the statistical analysis, and wrote the manuscript. All authors contributed to the writing of the manuscript, and read and approved the final version.
C. B., R. C., L. C. and J.-P. D. B. are shareholders of Citrage. S. B. has no conflict of interest to declare.




