LEARNING OBJECTIVES
After reading this article you will be able to:
• identify the neurophysiology of bright light therapy
• discuss the recommended protocols of bright light therapy in older adults with dementia
• describe the clinical and research problems related to the use of bright light therapy in this population.
In the early 1990s, research showed that bright light therapy improved depressive symptoms in individuals with seasonal affective disorder (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009). Since that time, many studies have been conducted and the efficacy of bright light exposure in treating seasonal affective disorder has become accepted (Pail Reference Pail, Huf and Pjrek2011). Subsequently, bright light therapy has been studied for other conditions that are thought to be associated with circadian rhythm disturbances, such as adult attention-deficit hyperactivity disorder, bulimia, jet lag, non-seasonal depression, obsessive–compulsive disorder, premenstrual syndrome, shift work sleep disorder and other sleep disorders (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009; Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011; Landry Reference Landry and Liu-Ambrose2014). Bright light therapy has also been examined and shown to be effective as a treatment for depression and agitation in older adults with dementia (van Hoof Reference van Hoof, Westerlaken and Aarts2012; Hanford Reference Hanford and Figueiro2013; Onega Reference Onega, Pierce and Epperly2016).
In this article we outline the neurophysiology of bright light exposure and discuss what is known about the use of bright light therapy to treat depression and agitation in older adults with dementia.
Neurophysiology of bright light therapy
Mechanism of action of bright light treatment
As a source of neural stimulation, through its action on the retina, the wavelength of visible light ranges from 380 nm (violet in colour) to 760 nm (red in colour). When used therapeutically, bright white light comprises a mix of all wavelengths across this portion of the electromagnetic spectrum. It is also called ‘full-spectrum’ light. The intensity of light hitting the retina can vary widely at different times of day and in different indoor and outdoor settings. For example, the intensity of light typically ranges from a high of approximately 100 000 lux during the brightest part of a sunny day outdoors, to 7000–12 000 lux outdoors at dawn (Shirani Reference Shirani and St Louis2009) to 200–1000 lux indoors (Shirani Reference Shirani and St Louis2009; Landry Reference Landry and Liu-Ambrose2014).
The exact mechanism of bright light therapy in treating depression and agitation in older adults with dementia is unknown. What is known is that bright light enters the eye and is received and transduced by the photopigment melanopsin in the ganglion cells of the retina (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011; Ekström Reference Ekström and Beaven2014). Neural signals are transmitted via the retinohypothalamic tract (RHT) to the suprachiasmatic nucleus (SCN), which is located in the anterior hypothalamus. The SCN controls circadian rhythms, activity, sleep and emotional patterns. Retinal light also causes release of excitatory neurotransmitters, such as glutamine (produced by RHT neurons), which stimulate the SCN (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011; Vadnie Reference Vadnie and McClung2017; Hastings Reference Hastings, Maywood and Brancaccio2018). The SCN controls the production of melatonin in the pineal gland. Melatonin, a derivative of the neurotransmitter serotonin, induces sleep and is produced in greater amounts during the darker seasons and at night (Landry Reference Landry and Liu-Ambrose2014; Vadnie Reference Vadnie and McClung2017).
Serotonin is produced in the raphe nucleus of the hypothalamus and has been demonstrated to influence mood, appetite, sleep, memory and learning (Yang Reference Yang and Schmitt2001; Vadnie Reference Vadnie and McClung2017). Serotonin has been linked with seasonal affective disorder through higher levels of serotonin reuptake (resulting in decreased synaptic levels) during the autumn and winter months (Vadnie Reference Vadnie and McClung2017). In an experimental study, aan het Rot et al (Reference aan het Rot, Benkelfat and Boivin2008) showed that lower levels of mood induced by depleting a precursor of serotonin (tryptophan) were largely blocked by exposure to bright light. (See Table 1 for an overview of two concurrent neurophysiological processes involved in bright light therapy.) Although much more work is needed to fully understand the physiological action of bright light therapy, it is clear that bright light entering the eyes has a direct effect on the SCN as it regulates circadian rhythms, and that it is linked to increased serotonin levels in the synaptic cleft (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011). Fig. 1 gives a detailed depiction of the neurophysiology of bright light therapy.
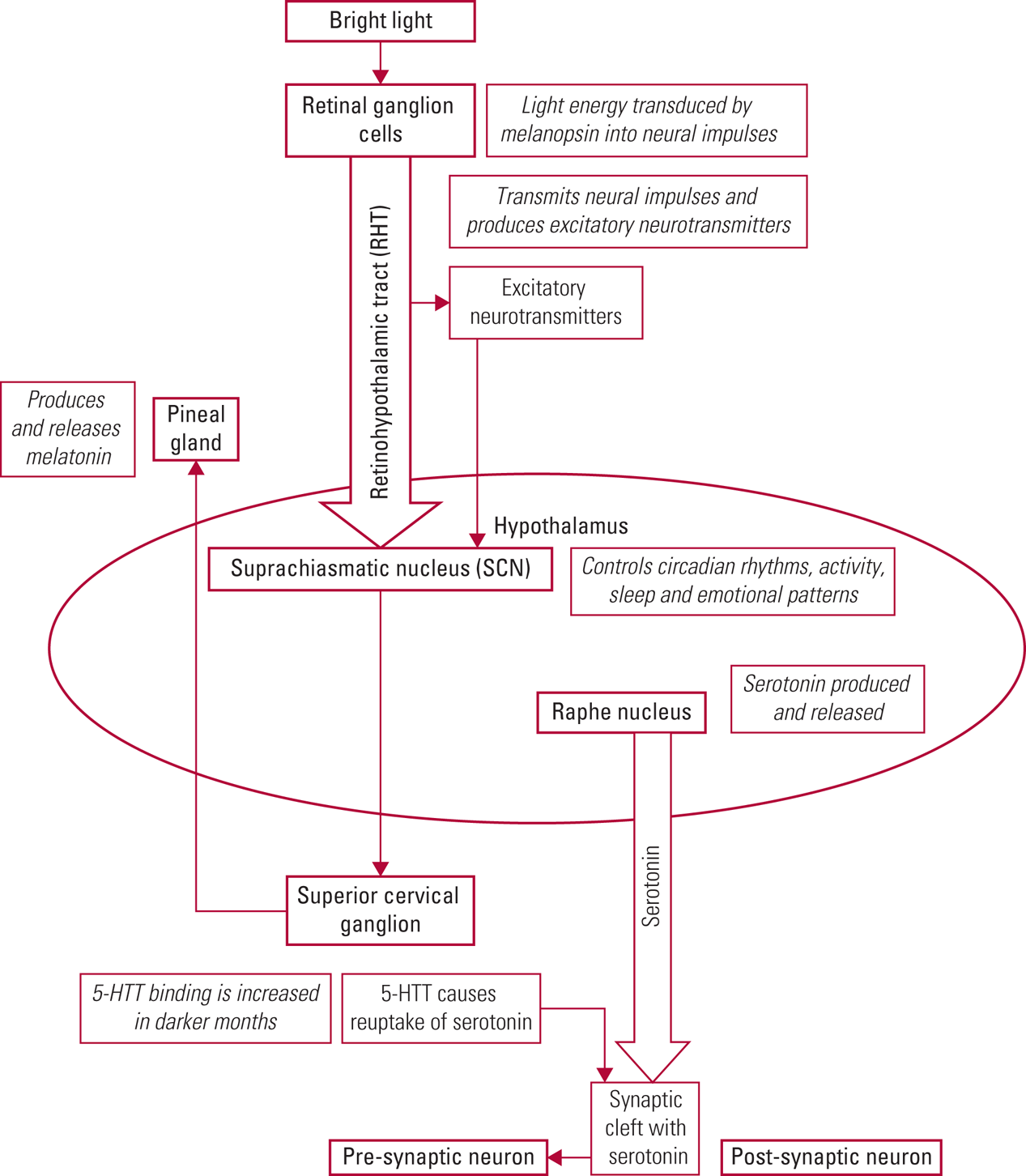
FIG 1 Detailed depiction of the neurophysiology of bright light therapy.
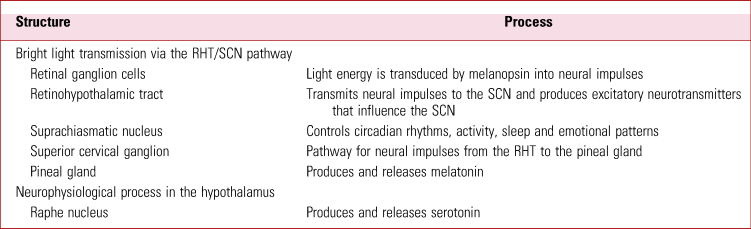
TABLE 1 Two concurrent processes involved in the neurophysiology of bright light therapy

RHT, retinohypothalamic tract; SCN, suprachiasmatic nucleus.
Bright light therapy to treat depression
Seasonal affective disorder
In seasonal affective disorder, research has identified different symptom profiles that may be most improved by bright light therapy. Specifically, individuals exhibiting fatigue, hypersomnia and increased appetite are more likely to improve with bright light therapy than those with symptoms of melancholia, suicidality, guilt, anxiety and insomnia (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009).
Non-seasonal affective disorder
The way in which bright light therapy improves non-seasonal affective disorder is not understood; however, theories draw from the body of knowledge of circadian rhythms and seasonal affective disorder, which have been studied more extensively (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009; Vadnie Reference Vadnie and McClung2017). Bright light treatment appears to be effective when used as a first-line treatment or as an adjunctive therapy combined with pharmacological intervention (Golden Reference Golden, Gaynes and Ekstrom2005; Crowley Reference Crowley and Youngstedt2012; Ekström Reference Ekström and Beaven2014). Bright light exposure improves daytime alertness, reduces fatigue and improves sleep, thus countering symptoms of depression (Crowley Reference Crowley and Youngstedt2012; Ekström Reference Ekström and Beaven2014). One possible mechanism to explain the relationship between bright light therapy and depression is that bright light therapy causes changes in the production of serotonin and melatonin, which in turn alters plasma levels of serotonin and melatonin, resulting in reduced levels of depression (Shirani Reference Shirani and St Louis2009).
Depression and agitation in individuals with dementia
Dementia may have a circadian rhythm component, as manifested by ‘sundowning’ (changes in behaviour at dusk) (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009; Hanford Reference Hanford and Figueiro2013). Although the normal ageing process results in decreased cell volume and numbers in the SCN, in individuals with Alzheimer's dementia this effect is amplified (Yang Reference Yang and Schmitt2001; Hanford Reference Hanford and Figueiro2013; Landry Reference Landry and Liu-Ambrose2014). Bright light therapy may improve depression and agitation in older adults with dementia by directly influencing the circadian aspects inherent in the disease process or by improving the sleep–wake cycle, decreasing daytime napping, increasing restful night-time sleep and increasing plasma levels of serotonin (Shirani Reference Shirani and St Louis2009; Hanford Reference Hanford and Figueiro2013; Landry Reference Landry and Liu-Ambrose2014).
Ultimately, bright light therapy is intended to mirror the effects of sunlight. On bright, sunny days, individuals feel energetic, active and engaged. On cloudy or rainy days, people may feel like sleeping in, napping and relaxing (Hanford Reference Hanford and Figueiro2013; Traynor Reference Traynor, Fernandez and Caldwell2013). Sunny and cloudy days are both normal parts of nature, but a problem occurs when older adults are chronically fatigued, have low energy, nap in the day, stay awake at night and experience other symptoms of depression that diminish their quality of life. When they are depressed and have decreased restful sleep at night, they may feel more agitated and irritable in the day (Hanford Reference Hanford and Figueiro2013). The goal of bright light therapy in treating depression and agitation in older adults is to improve quality of life.
Bright light evidence/research
Methodological considerations
Empirical evidence should guide clinicians' decision-making in selecting interventions to treat depression and agitation in older adults with dementia. Random assignment of an adequate number of participants to a treatment or a control group is necessary to accurately evaluate the effectiveness of bright light treatment (Golden Reference Golden, Gaynes and Ekstrom2005; Shirani Reference Shirani and St Louis2009; Crowley Reference Crowley and Youngstedt2012). Factors that can influence the quality of research include a variety of research design parameters, particularly the type of control condition used and the wavelength of light selected, but also variables such as the light intensity, duration of light administration, procedures implemented, sample size and randomisation method (Chang Reference Chang, Liu and Chen2018).
Control conditions
When researching bright light therapy in older adults with dementia, often participants are assigned to a treatment or a control group. Most commonly, the control group receives placebo light, which is less than 300 lux of light and is generally a different colour, such as red. Consequently, participants with mild dementia may be able to differentiate treatment and control conditions, which could adversely influence study results (Golden Reference Golden, Gaynes and Ekstrom2005; Pail Reference Pail, Huf and Pjrek2011).
In response, some researchers have used a low-dose negative-ion generator, instead of a placebo light, with the control group. Negative ions are generated when extra electrons (with negative charges) are added to the shells of atoms and molecules that comprise the air a person breathes. High-dose negative-ion exposure may influence the serotonin system, thereby reducing depression, whereas low-dose negative-ion exposure has no effect on depression (Perez Reference Perez, Alexander and Bailey2013). An advantage of using a negative-ion generator control condition is that, similar to a bright light treatment condition, participants are required to sit near the negative-ion generator (Golden Reference Golden, Gaynes and Ekstrom2005; Pail Reference Pail, Huf and Pjrek2011). A negative-ion generator produces no light; a placebo light is either low level or red. Therefore, each control condition is noticeably different from bright light, but participants might be more likely to believe that the negative-ion generator is a ‘real’ treatment.
Wavelength
Five subtypes of photosensitive retinal ganglion cells receive and absorb light (categorised as M1 to M5). Each subtype works with rods and cones in the eye to encode a specific spectrum of light. The M1 subtype, which is associated with short-wavelength blue light, is thought to have a strong effect on the SCN. The blue light used in some variants of bright light therapy targets specifically the M1 ganglion cells (West Reference West, Jennum and Simonsen2017).
Bright light therapy comprised of a mixed spectrum of wavelengths is commonly used clinically and in research; however, several studies have found that low-intensity short-wavelength blue light (460 nm) reduces depression (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011; Hanford Reference Hanford and Figueiro2013; Landry Reference Landry and Liu-Ambrose2014). These studies have not compared low-intensity blue light with high-intensity white light; therefore, the efficacy of low-intensity blue light has not been fully established (Pail Reference Pail, Huf and Pjrek2011; Landry Reference Landry and Liu-Ambrose2014). Blue light is thought to increase neural processes, resulting in melatonin suppression and improved mood and alertness (Ekström Reference Ekström and Beaven2014). Other light spectra (green, red and yellow) have not been adequately researched (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009); however, ultraviolet wavelengths are avoided as, similar to a sunburn of the skin, they cause photochemical injury to the sclera, cornea, lens and retina (Sliney Reference Sliney1983; Reichow Reference Reichow, Citek and Edlich2006).
Research design limitations
Design limitations that have hindered bright light research include small sample sizes, use of low-light intensity for inadequate amounts of time, lack of randomisation to treatment or control groups, and lack of control with regard to light administration and data collection procedures (Golden Reference Golden, Gaynes and Ekstrom2005; Landry Reference Landry and Liu-Ambrose2014; Chang Reference Chang, Liu and Chen2018). Comparison of bright light therapy with pharmacological treatment would provide information about its benefits as an adjunctive or alternative approach for treatment of depression in older adults with dementia (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009); however, the research has yet to be conducted.
An important and vexing problem for researchers is the best method of light delivery. Generally, in both clinical and research settings, light boxes have been used to administer bright light treatment to older adults with dementia. Often, specific details regarding the light box, such as the brand, model, number, light source and width of light beam, are not provided in research reports. In addition to light boxes, other methods, such as ceiling-mounted lights, have been examined, with positive results, including improved sleep and decreased agitation (van Hoof Reference van Hoof, Westerlaken and Aarts2012). Further research is required to determine the optimal light intensity, frequency, timing and duration of treatment associated with each method for delivering bright light to older persons with dementia (Box 1).
BOX 1 Bright light research considerations for older adults with dementia
• Random assignment to a treatment or a control group
• Type of control condition used
• Wavelength of light selected
• Adequate sample size
• Duration of light administration
• Experimental procedures implemented
• Light box or other delivery method
• Type and severity of dementia
Findings of bright light research in older adults
Research has yielded inconsistent results regarding the effectiveness of bright light therapy in older adults with dementia (van Hoof Reference van Hoof, Westerlaken and Aarts2012; Hanford Reference Hanford and Figueiro2013; Forbes Reference Forbes, Blake and Thiessen2014; Onega Reference Onega, Pierce and Epperly2016; Chang Reference Chang, Liu and Chen2018). In addition to methodological considerations, the type and severity of dementia may also contribute to variations in results (Forbes Reference Forbes, Blake and Thiessen2014).
Chang et al (Reference Chang, Liu and Chen2018) conducted a systematic review and meta-analysis to determine the effectiveness of bright light therapy in treating depression in older adults. The cognitive status of participants was not reported. Depression was measured using the Beck Depression Inventory, Geriatric Depression Scale, Hamilton Rating Scale for Depression, and Kurz-Skala Stimmung/Aktivierung (KUSTA) rating scale. Eight experimental studies between 2001 and 2017 with 395 older adults found that bright light treatment was significantly more effective than control treatments (effect size d = 0.422). Specifically, bright (white) light significantly improved depression in older adults (d = 0.460); results were similar when blue light was used (d = 0.464).
Working with a colleague, we examined the effect of bright light therapy on depression and agitation in 60 older adults with dementia who lived in long-term care facilities (Onega Reference Onega, Pierce and Epperly2016). Thirty participants in a bright light condition received 10 000 lux of light 10 times a week for 8 weeks, and 30 participants in a low light-intensity control condition received 250 lux of light 10 times a week for 8 weeks. The Mini-Mental State Examination was used to determine dementia severity. Three instruments were used to measure depression: the Depressive Symptom Assessment in Older Adults, the 17-item Dementia Mood Assessment Scale and the Cornell Scale for Depression in Dementia. Four scales were used to measure agitation: the Cohen–Mansfield Agitation Inventory-Frequency, the Cohen–Mansfield Agitation Inventory-Disruptiveness, the Pittsburgh Agitation Scale and the Brief Agitation Rating Scale. Scores for these dependent measures were obtained both before (T 1) and after the 8-week intervention period (T 2). Significant bright light condition × time of testing interactions were observed for all seven measures of depression and agitation (mean value for partial η2 = 0.372). Simple effects tests examining the effect of time of testing (T 1 versus T 2) for participants in the bright light condition showed that significant improvements were observed for all seven measures of depression (mean partial η2 = 0.794) and agitation (mean partial η2 = 0.433). For participants in the low light intensity control condition, no significant changes were observed in measures of depression (mean partial η2 = 0.012), while scores on measures of agitation in this group became significantly worse over the eight-week period (mean partial η2 = 0.209).
Forbes et al (Reference Forbes, Blake and Thiessen2014) conducted a systematic review and meta-analysis of 11 experimental studies with 398 older adults with dementia to determine the effectiveness of bright light therapy in improving activities of daily living, cognition, behavioural problems, psychiatric conditions and sleep problems. The studies used the Mini-Mental State Examination to determine dementia severity. They variously used the Cornell Scale for Depression in Dementia and the Geriatric Depression Scale to measure depression; and the Agitated Behavior Rating Scale, the Agitation and Aggression Subscale from the Neuropsychiatric Inventory-Nursing Home Version, and the Cohen–Mansfield Agitation Inventory to evaluate agitation. Three experimental studies between 2005 and 2009 evaluated the effect of bright light treatment on depression and four between 2003 and 2009 evaluated the effect on agitation. Meta-analyses showed that bright light therapy had no significant effect on depression or agitation.
Protocol for using bright light therapy in older adults with dementia
In general terms, the goal of bright light therapy is to provide exposure to bright light at a level of intensity and schedule of administration that combines the greatest levels of therapeutic benefit with the lowest levels of cost in terms of time and negative effects. Although research to date has not established a definitive set of ‘best practices’ that define a single, ideal protocol for bright light therapy in persons with dementia, in this section we highlight those components of bright light therapy that have received significant levels of empirical support.
A number of bright light protocols have been employed to treat depression and agitation in older adults with dementia (e.g., Golden Reference Golden, Gaynes and Ekstrom2005; van Hoof Reference van Hoof, Westerlaken and Aarts2012). These protocols typically deliver bright light using a rectangular light box with fluorescent bulbs covered by a diffusion screen (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009; Shirani Reference Shirani and St Louis2009; Chang Reference Chang, Liu and Chen2018) to distribute light evenly and to protect users from harmful ultraviolet light wavelengths (Shirani Reference Shirani and St Louis2009). The distance between the light box and the person receiving light should be between 30–90 cm, depending on the size of the light box (Shirani Reference Shirani and St Louis2009), and the box should be placed at or above eye level. Because light can reach the eye at an angle of 30–60°, individuals do not need to look directly at the bright light. A protocol that we recommend is outlined in Box 2.
BOX 2 Bright light therapy protocol to reduce depression and agitation in older adults with dementia
1 Administer 10 000 lux of full-spectrum (white) bright light from a fluorescent light box.
2 Place the light at or above eye level and position the individual 30–90 cm from the light box at an angle of 60° or less.
3 Provide light therapy for 30 min in the morning. Note that evening administration is preferable for some individuals, and some older adults require treatment twice a day.
Dosing is commonly 10 000 lux of bright white light for a duration of 30 min every morning (Golden Reference Golden, Gaynes and Ekstrom2005; Paino Reference Paino, Fonseca-Pedrero and Bousoño2009; Shirani Reference Shirani and St Louis2009; Forbes Reference Forbes, Blake and Thiessen2014; Chang Reference Chang, Liu and Chen2018). In older adults with dementia living in long-term care facilities, this schedule has been shown to be effective when administered 5 days a week with at least 80% adherence (Onega Reference Onega, Pierce and Epperly2016).
Although morning administration is most common, for some older adults with dementia evening administration is more effective (Pail Reference Pail, Huf and Pjrek2011; Hanford Reference Hanford and Figueiro2013; Chang Reference Chang, Liu and Chen2018). In these individuals, evening dosing improves night-time sleep and reduces night-time agitation (Shirani Reference Shirani and St Louis2009; Hanford Reference Hanford and Figueiro2013).
If improvement does not occur in 1–2 weeks after beginning once-a-day therapy, morning and afternoon/evening doses are recommended (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011).
The length of time needed for exposure to be effective increases as the lux of the high-intensity white light decreases. The recommended exposure time is 30 min for 10 000 lux, 60 min for 5000 lux and 90 min for 2500 lux (Golden Reference Golden, Gaynes and Ekstrom2005; Pail Reference Pail, Huf and Pjrek2011; Forbes Reference Forbes, Blake and Thiessen2014; Chang Reference Chang, Liu and Chen2018). The onset of effect is thought to be 3–7 days after beginning bright light therapy. The effect disappears shortly after therapy is discontinued (Pail Reference Pail, Huf and Pjrek2011).
Problems related to the use of bright light therapy in older adults with dementia
Adherence
As with pharmacological treatment, adherence to the prescribed bright light treatment regimen is necessary to receive beneficial effects (Pail Reference Pail, Huf and Pjrek2011). Individuals with dementia may have difficulty adhering to a treatment protocol for a variety of reasons, including wandering and difficulty keeping their eyes open for 30 min (Shirani Reference Shirani and St Louis2009; Onega Reference Onega, Pierce and Epperly2016). Consistent administration (same time of day, location and environment) of therapy may facilitate adherence (Chang Reference Chang, Liu and Chen2018).
Adverse effects of bright light therapy
Bright light therapy has fewer adverse effects than pharmacological treatment (Crowley Reference Crowley and Youngstedt2012). Even with prolonged bright light treatment, adverse ocular effects have not been identified (Golden Reference Golden, Gaynes and Ekstrom2005; Shirani Reference Shirani and St Louis2009; Forbes Reference Forbes, Blake and Thiessen2014; Chang Reference Chang, Liu and Chen2018). Although generally well-tolerated, mild adverse effects of bright light therapy include headaches, eye irritation, glare, irritability, restlessness, overactivity, hypomania, fatigue, weakness, sleep disturbance with evening exposure, and nausea. These effects are temporary and resolve spontaneously or by: (a) reducing the intensity of light exposure, its duration or both (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009; Pail Reference Pail, Huf and Pjrek2011); (b) increasing the distance from the light source; or (c) moving bright light administration to later in the day (Shirani Reference Shirani and St Louis2009). Chang et al (Reference Chang, Liu and Chen2018) found that, in the eight experimental studies included in their systematic review and meta-analysis, none of the 395 older adult participants experienced adverse effects from bright light treatment.
After prolonged bright light exposure in rodents, retinal degeneration was identified; however, retinal damage has not been identified in humans (Golden Reference Golden, Gaynes and Ekstrom2005; Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011). If an older adult has existing retinal damage or is taking photosensitive medication, an ophthalmologist should be consulted before light therapy is begun (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011).
It is postulated that, as a result of individuals' increasing energy levels, suicidal thoughts and behaviour may occur early in bright light treatment, as with medications for depression. Some studies have shown improvement in people with bipolar depression, yet bright light therapy could precipitate manic/hypomanic states (Golden Reference Golden, Gaynes and Ekstrom2005; Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011).
Clinical implications for bright light therapy in older adults with dementia
Bright light treatment is a safe, low-cost treatment (Pail Reference Pail, Huf and Pjrek2011; Crowley Reference Crowley and Youngstedt2012) that is underused in treating depression and agitation in older adults with dementia (Paino Reference Paino, Fonseca-Pedrero and Bousoño2009). In the USA, regardless of disease process or age, the cost of bright light treatment is generally not paid for by health insurance (Golden Reference Golden, Gaynes and Ekstrom2005; Pail Reference Pail, Huf and Pjrek2011), and many individuals are uninsured or underinsured anyway. Unfortunately, as is often the case, those who could benefit most from this treatment are the least able to afford it (Oldham Reference Oldham and Ciraulo2014). However, for individuals who lack insurance and financial resources, bright light therapy is less expensive than pharmacological options. Clinicians in the USA should consider offering bright light treatment to individuals for whom cost is not a concern and seek mechanisms, such as equipment loan programmes, to provide this treatment to individuals for whom the cost is prohibitive (Pail Reference Pail, Huf and Pjrek2011; Oldham Reference Oldham and Ciraulo2014). In many US counties with national health insurance, cost and payment are not barriers to use of bright light therapy (Oldham Reference Oldham and Ciraulo2014).
Research has primarily evaluated the effectiveness of bright light treatment in in-patient settings using light boxes; however, administrators and clinicians should work with engineers, architects and designers to create living environments that replicate natural outdoor light (Pail Reference Pail, Huf and Pjrek2011; van Hoof Reference van Hoof, Westerlaken and Aarts2012; Ekström Reference Ekström and Beaven2014). Long-term care facilities, adult day services and other environments that care for older adults with dementia could have lighting similar to that of a sunny day, beginning around 07.00 h, increasing in intensity and brightness by 14.00 h and decreasing by 19.00 h, with low-level evening lighting from 19.00 h to 07.00 h. The same lighting technology currently employed in light boxes could be used, but the delivery system could be refined to make the light emitted more natural and consistent with daily light patterns (Shirani Reference Shirani and St Louis2009; Pail Reference Pail, Huf and Pjrek2011; Hanford Reference Hanford and Figueiro2013; West Reference West, Jennum and Simonsen2017). Additionally, community-based research to evaluate the effect of bright light therapy on depression and agitation in older adults using innovative bright light delivery systems or natural light will provide evidence-based guidelines for treatment (Forbes Reference Forbes, Blake and Thiessen2014; Chang Reference Chang, Liu and Chen2018).
Overall summary
Bright light therapy is safe and may be beneficial in treating depression and agitation in older individuals with dementia. The neurophysiological basis of bright light therapy's effect is that light entering the eyes is received and encoded by melanopsin in retinal ganglion cells of the retina. Neural signals are transmitted by the retinohypothalamic tract (SCN) to the suprachiasmatic nucleus (SCN), which controls circadian rhythm, activity, sleep and emotional patterns. The recommended protocol for administering light therapy is 10 000 lux of bright white light for 30 min every morning, 5 days a week. The light box should be placed at or above eye level and located 30–90 cm from the light recipient. Adherence is challenging as older adults with dementia may wander and have difficulty keeping their eyes open.
Controlled research studies with large samples will provide further evidence regarding the clinical utility of bright light therapy in this population. Particularly, research determining whether bright light treats different symptom clusters of depression and agitation depending on dementia severity would be valuable. Clinicians should strive to overcome fiscal and administrative barriers to ensure access to bright light therapy for older adults with dementia. Bright light exposure is a safe, non-pharmacological treatment that is currently underutilised. Clinicians may find bright light therapy beneficial as a primary or adjunctive treatment in reducing depression and agitation in older adults with dementia.
Acknowledgements
The authors would like to acknowledge J. Elyse Krupp, MPH, and David C. Wood for their assistance with and review of this manuscript.
MCQs
Select the single best option for each question stem
1 As regards the neurophysiology of bright light therapy:
a the photopigment melanopsin is located in the pineal gland
b the retinohypothalamic tract controls the production of serotonin in the pineal gland
c retinal light causes a release of excitatory neurotransmitters, such as glutamine, which stimulates the suprachiasmatic nucleus
d serotonin is a derivative of melatonin and is produced along the retinohypothalmanic tract
e reuptake of serotonin by the serotonin transporter 5-HTT results in increased plasma levels.
2 Circadian rhythms, activity, sleep and emotional patterns are controlled by:
a serotonergic neurons from the raphe nuclei of the brainstem
b the pineal gland
c the retinohypothalamic tract
d the suprachiasmatic nucleus
e the photopigment melanopsin in the ganglion cells.
3 An older adult with dementia will be receiving bright light therapy to reduce depression and agitation. The bright light protocol is most likely to include:
a looking directly at the bright light
b use of bright light boxes emitting 10 000 lux of high-intensity white light
c light exposure for 1 h three times a day
d low-intensity long-wavelength red light in the morning
e sitting at a distance of 150–250 cm from the light box
4 There is some evidence that bright light treatment may cause mild:
a restlessness
b diarrhoea
c somnolence
d delirium
e increased blood pressure.
5 The quality of research on bright light therapy is not adversely affect by:
a use of red light at <300 lux in the treatment group
b use of long-wavelength light
c cost of treatment
d small sample size
e varied method of light administration.
MCQ answers
1 c 2 d 3 b 4 a 5 c




eLetters
No eLetters have been published for this article.