Orthostatic intolerance is defined as the development of symptoms related to cerebral hypoperfusion or sympathetic activation when standing that are relieved by recumbency. Reference Low, Sandroni, Joyner and Shen1 Symptoms of orthostatic intolerance can include both cardiac symptoms (such as palpitations, light-headedness, chest discomfort, and dyspnoea) and non-cardiac symptoms (such as exercise intolerance, fatigue, diminished concentration, nausea, and blurred or tunnelled vision). Reference Low, Opfer-Gehrking and Textor2,Reference Raj3 In paediatrics, postural tachycardia syndrome is defined as the presence of orthostatic intolerance symptoms for at least 3 months accompanied by a heart rate increase of at least 40 beats/minute within 10 minutes of assuming an upright posture and in the absence of orthostatic hypotension (a decrease in blood pressure >20/10 mmHg) Reference Raj3–Reference Stewart, Boris and Chelimsky5 . The prevalence of orthostatic intolerance and postural tachycardia syndrome has been difficult to establish and varies between age groups, with one study finding a prevalence of 6.8% in children between ages 7–18. Reference Lin, Han and Li6 Studies have consistently shown a predilection in females, with a female to male ratio of at least 3:1. Reference Low, Sandroni, Joyner and Shen1,Reference Singer, Sletten and Opfer-Gehrking4,Reference Chen, Du, Jin and Huang7,Reference Bernadzikowski and Boris8
Patients with postural tachycardia syndrome and orthostatic intolerance sometimes undergo evaluation by cardiology. Referral patterns may vary based on practice location; at our institution, most patients with dysautonomia symptoms are referred to cardiology for initial screening. In addition to history and physical exam, the cardiac evaluation routinely includes an electrocardiogram , and in patients with significant palpitations, a 24-hour ambulatory rhythm monitor (Holter monitor) may be performed. Reference Raj3 The heart rate alterations in these patients are usually attributed to excessive sinus tachycardia, and thus therapies, such as beta-blocker medications and volume expansion (fluids and salt supplementation), are aimed at blunting this inappropriate heart rate response via different mechanisms. Reference Low, Sandroni, Joyner and Shen1,Reference Raj3
Anecdotally, it was our experience that some children with postural tachycardia syndrome/orthostatic intolerance have true cardiac arrhythmias such as supraventricular tachycardia, and a subset of these patients have benefited from arrhythmia ablation. However, there is very sparse literature examining the prevalence of concomitant cardiac arrhythmias in the postural tachycardia syndrome/orthostatic intolerance population, and this was the impetus for our current study. The objective of our study was to determine the prevalence of cardiac arrhythmias in patients with postural tachycardia syndrome or orthostatic intolerance who were treated at our tertiary health system.
Materials and methods
This study was approved by the Children’s National Hospital Office for the Protection of Human Subjects and Institutional Review Board. All data were collected retrospectively. As described above, the Holter monitor is a 24-hour ambulatory rhythm monitor which is used to detect arrhythmias. The Zio® patch is a relatively newer device which provides extended cardiac rhythm monitoring for up to 2 weeks. Reference Fung, Jarvelin and Doshi9
Using diagnosis codes and clinic notes, we identified all patients who were seen at the Children’s National Hospital Electrophysiology Clinic from January 2001 through December 2020 and diagnosed with postural tachycardia syndrome or orthostatic intolerance. Typically, the diagnosis of postural tachycardia syndrome/orthostatic intolerance was made based on clinical history, a physical exam demonstrating no evidence of structural heart disease, a normal electrocardiogram, and either a bedside orthostasis test or formal tilt-table testing. Patients with dysautonomia symptoms who did not meet the requisite heart rate criteria for postural tachycardia syndrome (i.e., had heart rate increase less than 40 beats/min) were diagnosed with orthostatic intolerance. Patients with significant palpitation or chest pain symptoms which were felt to not be fully explained by dysautonomia underwent further evaluation with ambulatory rhythm monitoring. For the purpose of comparison, we also identified all patients at the same clinic who were diagnosed with syncope. We divided our population into three groups: patients with postural tachycardia syndrome/orthostatic intolerance; patients with syncope; and patients with both diagnoses, as one possible manifestation of dysautonomia is syncope. We reviewed all available Holter and Zio® data from these patients and classified each Holter or Zio® as “arrhythmia” or “normal.” Holter and Zio® monitors performed in patients with a known prior arrhythmia diagnosis were excluded.
We defined “arrhythmia” as any of the following abnormalities seen on Holter or Zio® monitoring: supraventricular tachycardiac, defined as runs of three or more consecutive ectopic supraventricular beats; frequent premature ventricular contractions greater than 30 in 1 hour; ventricular couplets; non-sustained ventricular tachycardia , defined as runs of three or more consecutive ectopic ventricular beats; type 2, second-degree heart block; third-degree heart block; and Wolff-Parkinson-White syndrome. The following findings were not considered “arrhythmia” in our study: frequent isolated supraventricular beats; supraventricular couplets; rare to occasional isolated premature ventricular contractions; first-degree heart block; type 1 (Wenckebach), second-degree heart block; and sinus pauses.
Demographic summary statistics were described using mean ± standard deviation. We determined the proportion of Holter and Zio® monitors that identified an arrhythmia out of the total number of monitors that met inclusion criteria. Using the Z-test for binomial proportions with two-tailed hypothesis [www.socscistatistics.com], we compared our postural tachycardia syndrome/orthostatic intolerance group versus our syncope group. Additionally, within each group (postural tachycardia syndrome/orthostatic intolerance or syncope), we used the chi-squared test [www.socscistatistics.com] to compare the frequency of arrhythmia detected using a Holter versus a Zio® monitor.
Results
Of the 829 patients with a diagnosis or postural tachycardia syndrome or orthostatic intolerance, 202 patients (24%) had at least one Holter or Zio® study performed. Of the 331 patients with a diagnosis of syncope, 101 (30%) had at least one Holter or Zio® study performed. Table 1 summarises the baseline demographic data of patients who had at least one Holter or Zio® result that met inclusion criteria. A total of 436 Holter monitors met inclusion criteria (266 from patients with postural tachycardia syndrome/orthostatic intolerance, 140 from patients with syncope, and 30 from patients with both diagnoses). A total of 88 Zio® monitors met inclusion criteria (66 from patients with postural tachycardia syndrome/orthostatic intolerance, 17 from patients with syncope, and 5 from patients with both diagnoses). As the proportion of patients with both postural tachycardia syndrome/orthostatic intolerance and syncope diagnoses was relatively small, we did not include those patients in our primary analysis of arrhythmia frequency. On secondary analysis, grouping of these patients with either the postural tachycardia syndrome/orthostatic intolerance group or the syncope group did not significantly alter the results.
Table 1. Baseline demographic data of postural tachycardia syndrome/orthostatic intolerance patients, syncope patients, and patients with both diagnoses. The mean age and gender distribution of patients with both diagnoses more closely resembles that of the POTS/OI group than the syncope group. Some patients underwent more than one Holter or Zio monitor, so the total number of monitors is greater than the number of patients
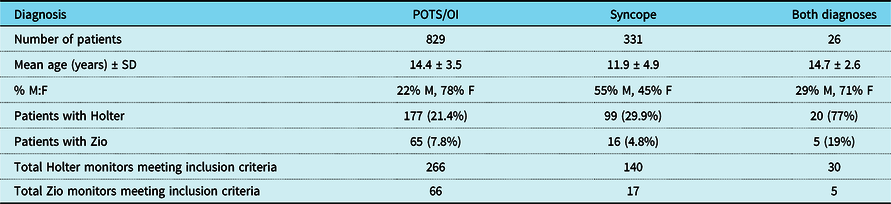
OI = orthostatic intolerance; POTS = postural tachycardia syndrome; SD = standard deviation.
In the postural tachycardia syndrome/orthostatic intolerance group, arrhythmia was identified on 50 of the 332 ambulatory rhythm monitors (36/266 Holters and 14/66 Zios®), giving an overall diagnostic yield of 15%. In the syncope group, arrhythmia was identified on 25 of the 157 ambulatory rhythm monitors (21/140 Holters and 4/17 Zios®), giving an overall diagnostic yield of 16%. There was no significant difference between the postural tachycardia syndrome/orthostatic intolerance group and the syncope group in arrhythmia frequency as detected by Holter monitors (p-value 0.69) or Zio® monitors (p-value = 0.83). Table 2 summarises the Holter and Zio® patch findings and further divides the arrhythmia findings into specific subtypes of arrhythmias.
Table 2. Incidence of various arrhythmias detected by 24-hour Holter monitoring and Zio® patch monitoring. “Any arrhythmia” refers to the number of monitors that identified at least one type of arrhythmia listed in the table. Yield (%) was determined by calculating the proportion of total monitors that identified at least one arrhythmia. There was no significant difference in the diagnostic yield of Holter or Zio® between the three groups
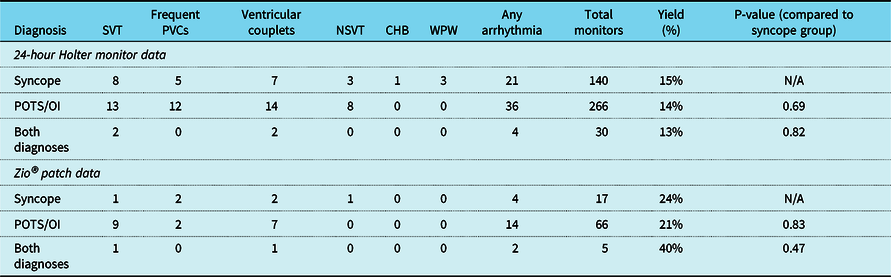
CHB = complete heart block; NSVT = non-sustained ventricular tachycardia; OI = orthostatic intolerance; POTS = postural tachycardia syndrome; PVC = premature ventricular contraction; SVT = supraventricular tachycardia; WPW = Wolff-Parkinson-White syndrome.
On both Holter and Zio® monitoring, supraventricular tachycardia and ventricular couplets were the most common arrhythmias seen in the postural tachycardia syndrome/orthostatic intolerance population. Supraventricular tachycardia was identified in 6.6% (22/332) of ambulatory rhythm monitors in the postural tachycardia syndrome/orthostatic intolerance group. Ventricular couplets were identified in 6.3% (21/332) of ambulatory rhythm monitors in the postural tachycardia syndrome/orthostatic intolerance group. As was the case when comparing overall rates of arrhythmia, these findings were not statistically significant from the syncope group in our study (p-value = 0.70 for supraventricular tachycardia, p-value = 0.79 for ventricular couplets). None of the postural tachycardia syndrome/orthostatic intolerance patients were found to have third-degree heart block or Wolff-Parkinson-White syndrome. These abnormalities were identified at low rates in the syncope group, in which 0.6% (1/157) of ambulatory monitors identified third-degree heart block and 1.9% (3/157) of monitors identified Wolff-Parkinson-White syndrome.
In the postural tachycardia syndrome/orthostatic intolerance group, 9.3% (31/332) of ambulatory rhythm monitors detected supraventricular couplets, 3.3% (11/332) detected first-degree heart block, and 6.3% (21/332) detected type 1, second-degree heart block. At the outset of the study, these findings were not defined as abnormal and were therefore not included in data analysis.
Finally, the diagnostic yield of Zio® monitoring was not statistically different than the yield of Holter monitoring in either the postural tachycardia syndrome/orthostatic intolerance group or the syncope group (p-value = 0.12 for the postural tachycardia syndrome/orthostatic intolerance group, p-value = 0.36 for the syncope group).
Discussion
This study demonstrates that a significant proportion of postural tachycardia syndrome/orthostatic intolerance patients who were evaluated in our Electrophysiology Clinic were also found to have concomitant arrhythmias on Holter and/or Zio® monitoring. There have been several prior studies on Holter and Zio® monitoring in various populations, including healthy ambulatory children, children with congenital heart disease, and children undergoing primary cardiac evaluation for symptoms such as chest pain, syncope, and palpitations. Reference Scott, Williams and Fiddler10–Reference Ayabakan, Ozer, Celiker and Ozme20 To the best of our knowledge, our study is the first to investigate the prevalence of cardiac arrhythmias specifically in children with postural tachycardia syndrome and orthostatic intolerance.
Review of previous literature suggests that the frequency of detecting arrhythmia with Holter monitoring is very low in healthy children. Direct comparisons of overall arrhythmia rates between different studies can be challenging because the definition of “arrhythmia” is quite heterogeneous across various studies; it is therefore more practical to compare subtypes of arrhythmia. For example, studies by Nagashima et al and Massin et al used the same definition of supraventricular tachycardia (three or more consecutive ectopic supraventricular beats) and non-sustained ventricular tachycardia (three or more consecutive ectopic ventricular beats) as was used in our current study. Furthermore, both studies included adolescent patients that closely matched the age group of postural tachycardia syndrome/orthostatic intolerance patients in our study. The Holter monitor study by Nagashima et al was performed in 360 healthy school children, including a subgroup of 97 children who were ages 13–15. The Holter monitor study by Massin et al was performed in 264 healthy ambulatory children and 112 hospitalised children with no cardiac symptoms; their study included a subgroup of 83 children who were ages 12–16. In both studies, none of the ambulatory rhythm monitors detected supraventricular tachycardia or non-sustained ventricular tachycardia.
Conversely, previous studies on Holter and Zio® monitoring in symptomatic patients undergoing cardiac evaluation showed higher arrhythmia detection rates. For example, a study by Pradhan et al found the detection of supraventricular or ventricular tachycardia – using the same definitions as described above – to be 9% by Holter and 10% by Zio. Reference Pradhan, Robinson, Shivapour and Snyder17 A recent study by Bolourchi et al found an arrhythmia detection rate of 12.5% by Holter and 15% by Zio®, though their study also included congenital cardiac patients and had slightly more strict definitions of supraventricular tachycardia and non-sustained ventricular tachycardia (requiring at least 4 consecutive ectopic beats). Reference Bolourchi, Silver, Muwanga, Mendez and Liberman19 In our postural tachycardia syndrome/orthostatic intolerance population, supraventricular or ventricular tachycardia were detected on 21/266 (7.9%) of Holter monitors and 9/66 (14%) of Zio® monitors. These findings are more similar to studies in symptomatic patients undergoing cardiac evaluation than studies in healthy children.
Previous studies in children have suggested that the longer 2-week monitoring provided by the Zio® patch does not necessarily improve arrhythmia detection rate when compared to the 24-hour Holter. Reference Pradhan, Robinson, Shivapour and Snyder17,Reference Bolourchi, Silver, Muwanga, Mendez and Liberman19 This is in contrast to adult studies, in which the arrhythmia detection rate of the Zio® patch has been consistently shown to be superior to traditional 24-hour monitors. Reference Yenikomshian, Jarvis and Patton21 The findings in our study are consistent with previous paediatric studies, as the arrhythmia detection rate was not significantly different between Zio® and Holter monitoring in our postural tachycardia syndrome/orthostatic intolerance or syncope population.
Currently, there is very limited literature describing the overlap of postural tachycardia syndrome/orthostatic intolerance with cardiac arrhythmias. One recent study in adults analysed 64 patients (mean age 43 years) with a diagnosis of postural tachycardia syndrome who were found to have concomitant supraventricular tachycardia during ambulatory rhythm monitoring and subsequently underwent supraventricular tachycardia ablation. Reference Nesheiwat, Towheed and Eid22 In that study, all patients experienced improvement in symptoms, with palpitations and light-headedness being the two symptoms that improved the most after ablation. Reference Yenikomshian, Jarvis and Patton21
The results from our study suggest that paediatric postural tachycardia syndrome/orthostatic intolerance patients have a higher frequency of cardiac arrhythmias than the general paediatric population, and evaluation with either Holter or Zio® ambulatory rhythm monitoring can be particularly helpful in identifying arrhythmias. As there exists some overlap in the clinical characteristics of postural tachycardia syndrome/orthostatic intolerance and arrhythmia, identifying an underlying arrhythmia can be challenging but important in dysautonomia patients. Treatment of these patients’ arrhythmias, either through medical therapy or an ablation procedure, has the potential to significantly improve their quality of life and may even address some of their postural tachycardia syndrome/orthostatic intolerance symptoms.
One limitation of our study was that not every postural tachycardia syndrome/orthostatic intolerance patient who was evaluated at our electrophysiology clinic underwent ambulatory rhythm monitoring. Only 24% of the 829 patients diagnosed with postural tachycardia syndrome or orthostatic intolerance underwent Holter or Zio® evaluation. Patients underwent ambulatory monitoring based on self-reported severity of their symptoms of palpitations and chest pain. By nature of only being able to analyse a subset of all postural tachycardia syndrome/orthostatic intolerance patients seen at our clinic, our study may have overestimated the true incidence of cardiac arrhythmias in this population. For this reason, we also analysed patients seen in the same clinic who were diagnosed with syncope, and 30% of the 331 syncope patients underwent Holter or Zio® evaluation. In essence, our syncope group was our “control” group, against which we compared our postural tachycardia syndrome/orthostatic intolerance group. As described above, comparison of Holter and Zio® data between these two groups showed a similar frequency of arrhythmia.
In addition to selection bias, our study was limited by the fact that it was retrospective and performed at a single centre. Future multi-centre prospective studies in this patient population would address some of the aforementioned limitations. More research is needed to evaluate the impact of treatment, both medication and interventional ablation, for postural tachycardia syndrome/orthostatic intolerance patients who are concomitantly diagnosed with arrhythmia.
Conclusion
In this retrospective descriptive study, a substantial proportion of paediatric postural tachycardia syndrome/orthostatic intolerance patients who underwent Holter or Zio® monitoring were found to have cardiac arrhythmias, at a frequency significantly higher than what is reported in healthy children. In the appropriate clinical context, physicians caring for postural tachycardia syndrome/orthostatic intolerance patients should consider ambulatory rhythm monitoring to evaluate for arrhythmias beyond sinus tachycardia.
Acknowledgements
We would like to thank Dr. James Bost, PhD, for providing us with statistical analysis guidance during this study.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.





