Fishmeal offers a balanced amino acid profile, great digestibility and excellent palatability, making it an incredibly high-quality protein source for animal feed. The expansion of the global aquaculture industry has led to a shortage of fishmeal supply. It has been criticised for using high-quality marine proteins that are directly consumable by humans(Reference Zhou, Chen and Ji1). Fishmeal substitution has become an unavoidable issue in the feed industry, while plant proteins, which are inexpensive, stable in origin and pollution-free, have become a mainstream research direction for fishmeal substitution(Reference Gatlin, Barrows and Brown2). Among them, soybean meal has been widely used in aquafeeds because of its balanced nutritional composition. However, using soybean meal in aquafeeds in large quantities can lead to growth inhibition and intestinal damage due to essential amino acid deficiency and anti-nutritional factors(Reference Miao, Zhao and Zhu3). One-third or higher fishmeal replacement levels by soybean meal can significantly decrease the growth performance, feed efficiency, intestinal villi height and muscular thickness of Pacific white shrimp (Litopenaeus vannamei)(Reference Yao, Zhang and Li4–Reference Liu, Yang and Yan6). Hence, improving intestinal barrier integrity and immunology may be an effective approach to increasing the utilisation of soybean meal and other plant protein sources in aquafeeds.
Soybean-derived bioactive peptides (SBP) are short peptide mixtures consisting of 2 to 10 amino acids produced from soybean proteins after protease hydrolysis, with a molecular weight mainly distributed below 1000 and a protein content of about 85 %. SBP is quickly absorbed and used by the intestine since they are short-chain peptides(Reference Kim, Yang and Kim7). A variety of these short bioactive peptides have been shown to be effective in improving growth and digestive performance(Reference Song, Li and Wang8) as well as immunomodulation(Reference Singh, Vij and Hati9), antioxidants(Reference Sanjukta, Rai and Muhammed10) and other physiological functions in mammals and fish. Fermented soybean meal contains a lot of SBP(Reference Wang, Cui and Liu11), and partial replacement of fishmeal with fermented soybean meal can improve the growth performance and crude protein content of Chinese mitten crab (Eriocheir sinensis)(Reference Xu, Liu and Zhang12). Antibacterial peptides contained in SBP have strong antibacterial activity against Vibrio alginolyticus and Vibrio parahaemolyticus (Reference Cheng, Lin and Shiu13). The effects of SBP are connected to the preservation, augmentation or repair of the intestinal barrier function, which controls the inflow of microbes from the intestinal lumen while selectively allowing the absorption of water, nutrients and ions(Reference Martinez-Augustin, Rivero-Gutierrez and Mascaraque14). The research has reported that SBP or fermented soybean meal could improve digestive enzyme activities, distal intestinal morphology and intestinal microbial flora in fish(Reference Duan, Zhang and Huang15–Reference Zhao, Song and Xie17). Nevertheless, few studies focus on the intestinal regulation of SBP in crustaceans.
Chinese mitten crab is an important economically farmed species in China, known for its delicious taste and rich nutrition(Reference Wang, He and Wang18). However, there is a strong dependence on fishmeal in the cultural process. The amount of fishmeal in the general compound feed is normally around 30 %. Therefore, it is crucial to cut back on fishmeal consumption. However, farmed crabs may experience growth suppression and poor digestibility when more plant proteins are used to replace fishmeal in the feed(Reference Jiang, Chen and Qin19,Reference Liu, Wen and Man20) . The addition of bioactive substances under low fishmeal conditions (LF) has become a popular research direction for fishmeal substitution(Reference Hu, Yang and Li21–Reference Serradell, Torrecillas and Makol23), while related studies in Chinese mitten crab have been few.
In view of this, the present study aimed to evaluate the effects of LF diet on the growth, digestion and intestinal health of E. sinensis, and the amelioration of SBP supplementation in a LF diet. The results will shed more light on the molecular mechanism of physiological response induced by protein sources and also provide a reference for the development and application of LF diet.
Materials and methods
Experimental diets
Six isonitrogenous (40 % crude protein) and isoenergetic (18 MJ/kg gross energy) diets were formulated in this study. The formula and approximate components of the control diet (CT) and the LF diet are shown in Table 1, in which fishmeal, soybean meal, rapeseed meal, peanut meal and blood meal served as protein sources; fish oil and soybean oil served as lipid sources; α-starch served as the carbohydrate source. Fishmeal content in LF was reduced from 32 % to 22 % with the inclusion of soybean meal. 1 %, 2 %, 4 % and 6 % SBP were supplemented in LF, respectively. The SBP (Tian le tai) was produced by enzymolysis with soybean protease that was obtained from Jiangsu FIELD Technology Co., Ltd. Its main components are shown in Table 2, and the relative molecular weight distribution of peptides detected according to GB/T 22 492–2008 is presented in Table 3.
Table 1. The ingredients and proximate composition of experimental diets (%, air dry basis)

* Obtained from Jiangsu Fuyuda Food Products Co., Ltd., Yangzhou, China.
† Obtained from Foshan Guonong Starch Co., Ltd., China.
‡ Obtained from Wuxi Hanove Animal Health Products Co., Ltd., Wuxi, China.
Table 2. Main components index requirements of soybean-derived bioactive peptides (SBP)

Values were obtained from commercial specifications of Jiangsu FIELD Technology Co., Ltd.
Table 3. Relative molecular weight distribution of peptides in SBP

All experimental diets were prepared in the laboratory. Various raw materials were crushed and sieved through a 60 (unit) mesh; the ingredients were thoroughly mixed and blended to ensure homogeneity. Lipid sources and 25 % water were added to the mixture. SBP, in paste form, was added to the feed along with 25 % water based on the feed weight. A twin-screw extruder (Guangzhou Huagong Optical Mechanical and Electrical Technology Co., Ltd., Guangzhou, China) was used to produce wet granular feed with a particle size of 2·5 mm. After drying in a hot air oven, the feeds were provided to crabs.
Animal ethics
The care and use of animals followed Animal Research Institute Committee guidelines of Nanjing Agriculture University, China. This study has been approved by the Committee of the Animal Research Institute of Nanjing Agricultural University, China (permit number: SYXK (Su) 2011–0036).
Crabs and the feeding trial
Chinese mitten crabs were obtained from a commercial farm in Nanjing (Jiangsu, China). After 2 weeks of acclimation, 180 male and 180 female crabs of similar size (1·61 ± 0·05 g) were selected, from which 10 male and 10 female crabs were randomly selected and stocked into 18 outdoor concrete ponds (1 m × 1 m × 1 m), each with plastic tubes for crabs to hide. Six experimental diets were randomly allotted to crabs with triplicate tanks. Crabs were fed thrice daily (07.00, 13.00 and 19.00) for 8 weeks, and the residual feed was weighed one hour after each feeding to calculate the total feed intake. The weight of the crabs was measured every 2 weeks, and the daily feed amount was adjusted accordingly.
During the feeding trial, 1/3 water was changed every 2 d, water temperature ranged from 25 to 27°C, pH fluctuated between 7·4 and 8·0, and dissolved oxygen was maintained between 4–5 mg/l, and nitrite, ammonia nitrogen and sulphide were kept < 0·01, 0·05 and 0·01 mg/l, respectively.
Sample collection
At the end of the feeding experiment, all cultured crabs in each concrete pond were weighed to assess their growth performance. Two male and two female crabs were randomly selected from each replica. Haemolymph was extracted from the base of the second pereiopod, mixed with an anticoagulant (1:1) composed of glucose (14·7 g/l), sodium citrate (13·2 g/l) and citric acid (4·8 g/l). The mixture was then centrifuged at 4°C for 15 min at 3000 rpm. The supernatant was collected and stored at −20°C. The intestine was also collected and stored at −80°C for subsequent experiments. The hindgut was preserved in 4 % paraformaldehyde for histopathological and apoptosis measurement.
Growth parameter
The body weight of the collected samples was measured, and the weight gain rate and specific growth rate (SGR) were calculated as follows:
Weight gain rate (%) = [(Wt−W0)/W0] × 100
Specific growth rate (SGR, %/d) = [(lnWt−lnW0)/t] × 100
Feed conversion ratio = F/(Wt1−W)
Relative feed intake (%/d) = 100 × F’ × 2/[(W0 + Wt) × t]
where Wt is the final crab average body weight (g), W0 is the initial crab average body weight (g), t is the breeding time (days), Wt1 is the final total body weight, W is the initial total body weight, F is total amount of the feed consumed, and F’ is crab average feed consumed.
Proximate assays
Diets and ingredients were analysed for proximate composition. Crude protein (nitrogen × 6·25) was determined by the Kjeldahl method using an Auto Kjeldahl System (2300; FOSS Tector, Hoganas, Sweden); crude lipid by diethyl ether extraction using an Auto Soxtec System (2050; FOSS Tector); ash by combustion at 550°C for 6 h; crude fibre by fritted glass crucible method using an automatic analyser (ANKOM A2000i; Macedon, New York, USA); and gross energy by an adiabatic bomb calorimeter (PARR 1281, USA). Acid-soluble protein was determined according to Greene and Babbitt(Reference Greene and Babbitt24). Stachyose and raffinose contents were measured using Foss XDS–NIR Rapid Content Analyzer (FOSS Analytical, Slangerupgade, Denmark) through near-infrared reflectance(Reference Matei, Woyann and Meneguzzi25) Tripartite analyses were conducted for each sample.
Digestive enzyme assays
Hepatopancreatic tissue stored at −20°C was carefully weighed and homogenised in ice-cold buffer (dilution 1:10) using a motor-driven tissue cell disruptor in a 2 ml centrifugal tube. The homogenisation buffer consisted of 0·02 M Tris/0·01 M phosphates at pH 7·0. The resulting homogenate was centrifuged at 2500 rpm for 10 min at 4°C, and the supernatant was collected as the enzyme source. The activities of α-amylase (AMS), trypsin (TPS), lipase (LPS) and the protein concentration were measured using kits (ref. no. C016, A080 and A054, A045) provided by Jiancheng Bioengineering Institute (Nanjing, China).
Antioxidant index assays
Intestinal tissues stored at −20°C were taken from each group and were prepared in the same way as hepatopancreas supernatant. The protein concentration in the tissue supernatant was determined using kits (ref. no. A045) produced by Jiancheng Bioengineering Institute (Nanjing, China) to calculate the specific activity of the enzymes. The anion on Coomassie brilliant blue dye combines with protein −NH3 + to make the solution blue, and the protein content can be calculated by measuring the absorbance.
Superoxide dismutase, anti-superoxide anion, catalase, peroxidise activities, malondialdehyde (MDA) content and total antioxidant capacity levels were measured using kits (ref. no. A001, A052, A007, A084, A003, A015) provided by Jiancheng Bioengineering Institute (Nanjing, China). The Water-soluble tetrazolium salt (WST-1) method was employed to determine superoxide dismutase activity, where the electron transfer substance and a chromogenic agent resulted in a purplish-red reaction system; anti-superoxide anion activity was calculated based on the absorbance difference using vitamin C as the standard; the addition of ammonium molybdate quickly stops the reaction of catalase decomposing H2O2, and the remaining H2O2 reacts with ammonium molybdate to produce a yellowish complex. The change at 405 nm was measured to calculate catalase activity; peroxidase activity, which catalyses H2O2, was determined by measuring the absorbance change at 420 nm; MDA content was assessed by condensing it with thiobarbituric acid to form a red product with a maximum absorption peak at 532 nm, allowing for its quantification. The total antioxidant capacity level was measured using colorimetry, wherein antioxidant substances reduce Fe3+ to Fe2+, and Fe2+ forms solid complexes with phenanthroline substances.
Gene expression assays
Intestinal tissues stored at −80°C were taken from each group. Total RNA was isolated by RNAiso Plus (Takara, Japan), and RNA concentration was measured by spectrophotometer (DN-1000, Thermo, USA). The RNA concentrations were homogenised to 500 ng/μl. Reverse transcription was performed with HiScript III RT SuperMix for quantitative PCR (+ gDNA wiper) premix (Vazyme, China), and the reverse-transcribed cDNA was stored at −80°C for standby. The target genes were selected as peritrophin 2 (PM2), IL enhancer binding factor (ILF2), anti-lipopolysaccharide factor (ALF1), anti-lipopolysaccharide factor 2 (ALF2), anti-lipopolysaccharide factor 3 (ALF3), peritrophin-44-like protein (PT) and crustin I (Crustin1), and the primers were according to Wang et al. (Reference Wang, Wang and Huang26) and Han et al. (Reference Han, Wang and Guo27) (Table 4). Real-time quantitative PCR was performed using the CFX96 RT-PCR system (Bio-Rad, CA) to analyse the relative expression of the above target gene mRNAs in the crab intestines. The RT-quantitative PCR reaction system was referenced by Sun et al. (Reference Sun, Shan and Liu28). In this study, beta-actin (β-actin) was used as the internal reference gene, and the 2−ΔΔCT method was used to calculate the relative expression of genes. A standard curve was made for each primer pair before the formal test to ensure that the amplification efficiency of the target gene was the same as that of the internal reference gene and was around 100 %.
Table 4. Primer pair sequences of the genes used for real-time quantitative PCR

Histopathological and transferase-mediated dUTP nick end labelling assays
Hindgut tissues stored at 4°C were placed in an embedding box and rinsed with distilled water for half an hour. Haematoxylin-eosin staining was performed sequentially by dehydration, wax immersion, embedding and sectioning regarding Lai et al. (Reference Lai, Nie and Zhang29). The terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling method was used to determine intestinal apoptosis. The specific procedure for intestinal transferase-mediated dUTP nick end labelling analysis was according to Abbasi-Oshaghi et al. (Reference Abbasi-Oshaghi, Mirzaei and Pourjafar30). Quantification was performed using Image Pro Plus 4·1 software.
Statistical analysis
SPSS 20·0 software was applied for data analysis, and all the data were presented as the mean of replicates with se m(n 3). An independent-samples t test was used to assess whether there was a significant difference between CT and LF. Orthogonal polynomial contrasts were used to assess the significance of linear, quadratic or cubic models in describing the response in the dependent variable to SBP level. Statistical significance was set as P < 0·05. Quadratic regression analysis was used to analyse the optimal dietary SBP level based on SGR. The Pearson correlation coefficient was employed to examine the relationship between different indicators (https://www.chiplot.online/correlation_heatmap.html).
Results
Growth performance
As shown in Table 5, the final weight, weight gain rate and SGR of crabs in LF group were significantly lower than those in CT group (P < 0·05) while feed conversion ratio was significantly higher (P < 0·05). The orthogonal polynomial contrasts showed that the final weight, weight gain rate and SGR of crabs were all quadratically increased (P < 0·05) with increasing dietary SBP levels in LF diet while feed conversion ratio quadratically decreased. Relative feed intake did not vary significantly among these groups (P > 0·05). Using the SGR as an indicator, the most fitted curve to the model is quadratic with the equation y = –0·0202x2 + 0·1258x + 3·6404; R2 = 0·4381 (P = 0·010). Based on the model, the estimated optimal addition of SBP in the LF diet for Chinese mitten crab is 3·1 % (Fig. 1).
Table 5. Effects of different diets on the growth performance of Eriocheir sinensis

Independent-samples t test was used between CT and LF.
* Indicated P < 0·05; polynomial orthogonal contrasts were used among LF groups, if statistical significance (P < 0·05) detected, the model that fits best with the data was selected; WGR, weight gain rate; SGR, specific growth rate; FCR, feed conversion ratio; RFI, relative feed intake; Ns, P > 0·05; Ln, Linier; Qd, Quadratic; Cu, Cubic; n 3.

Fig. 1. The optimum level of dietary soybean-derived bioactive peptides supplementation in the low fishmeal diet based on quadratic regression analysis on the specific growth rate of Eriocheir sinensis.
Hepatopancreas digestive enzyme activities
As shown in Table 6, no significant differences in AMS, TPS and LPS activities were observed between LF and CT groups (P > 0·05). The orthogonal polynomial contrasts showed that the activities of AMS and TPS were both quadratically increased (P < 0·05) while LPS was not significantly correlated (P > 0·05) with increasing dietary SBP levels in LF diet.
Table 6. Effect of soybean-derived bioactive peptides on the digestive enzyme activities of Eriocheir sinensis

Independent-samples t test was used between CT and LF.
Polynomial orthogonal contrasts were used among LF groups, if statistical significance (P < 0·05) detected, the model that fits best with the data was selected; AMS, α-Amylase; TPS, Trypsin; LPS, Lipase; Ns, P > 0·05; Ln, Linier; Qd, Quadratic; Cu, Cubic; n 3.
Intestinal antioxidant indexes
As shown in Table 7, the MDA content in LF group was significantly higher than that in CT group (P < 0·05). The orthogonal polynomial contrasts showed that the content of MDA was linearly decreased, while the activities of anti-superoxide anion and total antioxidant capacity were both cubically increased (P < 0·05) with increasing dietary SBP levels in LF diet.
Table 7. Effect of soybean-derived bioactive peptides on the antioxidant indexes of Eriocheir sinensis

Independent-samples t test was used between CT and LF.
* Indicated P < 0·05; polynomial orthogonal contrasts were used among LF groups, if statistical significance (P < 0·05) detected, the model that fits best with the data was selected; MAD, Malondialdehyde; SOD, Superoxide dismutase; CAT, Catalase; ASA, Anti-superoxide anion; T-AOC, Total antioxidant capacity; POD, Peroxidase; Ns, P > 0·05; Ln, Linier; Qd, Quadratic; Cu, Cubic; n 3.
Histopathology and transferase-mediated dUTP nick end labelling observation
As shown in Fig. 2, compared with CT group, the peritrophic membrane (PM) was separated, the epithelial cells were damaged, and the muscularis was thinner in LF group. With the gradual increase of SBP addition, these adverse effects were gradually improved. In 6 % SBP added group, the morphology of PM was normal, and the thickness of muscularis was similar to that of CT group. As shown in Table 8, the muscular thickness and fold height in LF group were significantly decreased (P < 0·05) than that in CT group. The orthogonal polynomial contrasts showed that the muscular thickness was linearly increased (P < 0·05) with increasing dietary SBP levels in LF diet. The results of transferase-mediated dUTP nick end labelling and DAPI staining of intestinal tissues are shown in Fig. 3 clearly, and the apoptotic signal was significantly induced (P < 0·05) in LF group, while it was significantly decreased (P < 0·05) in 2 % SBP added group.

Fig. 2. Photomicrographs of H&E-stained hindgut sections of E. sinensis; (100×). Yellow arrow: muscularis; Blue arrow: peritrophic membrane; White arrow: epithelial cell.
Table 8. Effect of soybean-derived bioactive peptides on muscular thickness and fold height of hindgut sections of Eriocheir sinensis

Independent-samples t test was used between CT and LF.
* Indicated P < 0·05; polynomial orthogonal contrasts were used among LF groups, if statistical significance (P < 0·05) detected, the model that fits best with the data was selected; Ns, P > 0·05; Ln, Linier; Qd, Quadratic; Cu, Cubic; n 3.

Fig. 3. Effect of soybean-derived bioactive peptides on intestinal apoptosis of Eriocheir sinensis. Under fluorescence microscope (a), the apoptotic cells fluoresced in green, while blue colour represents the nuclei. The values (b) are expressed as the mean ± se (n 3). The absence of the same lowercase letter indicates a significant difference (P < 0·05).
Intestinal gene expression
As shown in Table 9, LF group showed the lowest expression of PM2, PT and Crustin1, as well as the highest expression of ALF1, ALF2, ALF3 and ILF2. The expression of ALF2 in LF group was significantly higher (P < 0·05) than that in CT group. The orthogonal polynomial contrasts showed that the increasing dietary SBP levels in LF diet quadratically increased (P < 0·05) the expression of PT and Crustin1. The expression of ALF1 linearly and ALF3 and ILF2 quadratically decreased (P < 0·05) with increasing dietary SBP levels in LF diet.
Table 9. Effect of soybean-derived bioactive peptides on the intestinal gene expression of Eriocheir sinensis

Independent-samples t test was used between CT and LF.
* Indicated P < 0·05; polynomial orthogonal contrasts were used among LF groups, if statistical significance (P < 0·05) detected, the model that fits best with the data was selected; PT: peritrophin-44-like protein; PM2, peritrophin 2; ILF2: IL enhancer binding factor; ALF1: anti-lipopolysaccharir; ALF2: anti-lipopolysaccharir 2; ALF3: anti-lipopolysaccharir 3; Crustin1: crustin I. Ns, P > 0·05; Ln, Linier; Qd, Quadratic; Cu, Cubic; n 3.
Correlation and cluster analysis of indicators
As shown in Fig. 4, the correlation heat map showed that TPS, PM2 and fold height had significant positive correlations with growth performance, feed conversion ratio displayed a negative relationship with growth performance (P < 0·05). Furthermore, there were positive correlations observed between PM2 and TPS, PT and AMS, as well as MDA and ALF1, ALF3 and ILF2 (P < 0·05). Conversely, MDA exhibited negative correlations with muscular thickness and fold height (P < 0·05).

Fig. 4. Pearson’s correlation coefficients for 24 indicators of Eriocheir sinensis. The colour assigned to a tile in the heatmap grid indicates the strength of association between two indicators. Red signifies a positive correlation, while blue signifies a negative correlation., * indicates P < 0·05, ** indicates P < 0·01.
Discussion
This study investigated the effects of adding SBP to a LF diet on growth, digestion and intestinal health of Chinese mitten crab. The results showed that the LF diet reduced the growth performance of crabs, which may be due to the high level of soybean meal substitution(Reference Collins, Overland and Skrede31). In contrast, the increasing addition level of SBP to LF diets quadratically improved the growth performance of crabs and reached the level of normal diets. Relevant studies have also shown that SBP can improve animal growth performance(Reference Zhao, Song and Xie17,Reference Zheng, Park and Kim32) . However, in this study, more than 6 % SBP to the diet of Chinese mitten crabs will have no obvious effect on promoting growth. Drawing on the study by Song et al. (Reference Song, Li and Wang8), we speculated that excessive ingestion of SBP could precipitate this phenomenon. Excessive SBP consumption could overload intestinal transporters, augmenting luminal peptide influx and free amino acid concentrations. Simultaneously, rapid peptide influx could boost amino acid oxidation and endogenous excretion, resulting in more nitrogen excretion than intake. This could engender a negative nitrogen balance that hampered cell growth.
Growth performance is closely related to digestion and absorption ability(Reference Zhang, Wang and Su33). In crustaceans, the hepatopancreas is an important digestive organ that secretes various digestive enzymes(Reference Long, Sun and Wade34). In this study, the increasing addition level of SBP to LF diets quadratically increased the AMS and TPS activities of crabs. In the study of Pelteobagrus fulvidraco, Zhao et al. (Reference Zhao, Song and Xie17) replaced fishmeal with soybean peptide, and they found that AMS and protease activities in 20 % soybean peptide substitution group were higher than in the control group. But with the increase in fishmeal substitution with soya peptides, there were no significant differences in the levels of AMS, LPS and protease activities between 50 % soybean peptide substitution group and the control group. This could be because the soybean peptide was easier to digest and did not trigger the digestive enzyme activities. These results suggested that low levels of added SBP can increase protease and AMS activities, while high levels almost completely eliminate this enhancement.
The digestive and absorption capacity is closely related to the intestinal health condition(Reference Zhang, Duan and Jiang35). MDA is one of the stable products of lipid peroxidation, which can indirectly reflect the extent of lipid peroxidation leading to cell membrane damage and consequently cell death(Reference Domijan, Ralic and Brkanac36). In this study, feeding LF diet led to a significant increase in MDA content, which is consistent with the results of Jiang et al. (Reference Jiang, Xu and Feng37) while the increasing addition level of SBP linearly reduced the degree of cellular oxidative damage and cubically increased the anti-superoxide anion activity and total antioxidant capacity level. This should be an effective activation of Nrf2/antioxidant response element mediated activity by SBP(Reference Yi, Din and Zhao38). Research has discovered that the synthesised antioxidant peptides provide protection to human intestinal Caco-2 cells against oxidative damage induced by H2O2. This protection is achieved by reducing intracellular ROS (Reactive oxygen species) generation and lipid peroxidation (Reference Zhang, Tong and Li39). Additionally, the continuous renewal of intestinal epithelial cells plays a crucial role in the recovery process following various damages. Bioactive peptides from bovine colostrum have been demonstrated to enhance in human epithelial T84 cells (Reference Morgan, Riley and Sheehy40). Intestinal tissue sections showed impairment in intestinal epithelial cells in the LF diet group, while it was recovered with the addition of SBP, which suggested that SBP might also promote the proliferation of epithelial cells. However, feeding LF diet significantly induced intestinal apoptosis, which may be caused by substances such as soya globulin in soybean meal (Reference Han, Xu and Qi41,Reference Jiang, Hu and Zhang42) . The addition of SBP effectively inhibited the apoptotic signal. Therefore, SBP was highly likely to maintain the cell population by inhibiting epithelial cell apoptosis, while the possibility of SBP directly stimulating the proliferation of intestinal epithelial cells cannot be excluded. The specific mechanisms of action need to be further investigated and verified through in vitro cell culture experiments. The correlation heatmap demonstrated a significant link between intestinal fold height and growth performance. A decrease in fold height was observed with the LF diet, which was partially alleviated by the addition of SBP. The increasing addition level of SBP also cubically increased the thickness of the intestinal muscularis; this was a rare occurrence among multiple indicators where SBP demonstrated favourable outcomes even at a 6 % addition level, presumably because SBP is short peptides, which can be directly absorbed and utilised by the intestine and promote intestinal development (Reference Zhao, Zhang and He43).
This study also found that LF diets disrupt intestinal PM structure (Reference Han, Wang and Guo27). The PM is a membranous structure on the surface of the intestinal lumen in arthropods, similar to the goblet cells in vertebrates (Reference Cai, Wu and Ye44). It is rich in PM factors that protect the intestine from mechanical damage and microbial invasion (Reference Park, Kim and Jin45). PM2 is a PM protein, and PT is a PM-liked factor of the Chinese mitten crab, which played important roles in innate immune defence processes (Reference Huang, Ma and Wang46,Reference Wang, Cai and Shui47) . The results showed that adding SBP to the LF diet increased the relative expression of PM2 and PT, while the intestinal histology also showed that it could effectively reduce the intestinal PM shedding of crabs caused by the LF diet. In addition, the expression of ILF2 gene is related to immune response and the key transcription factor of IL-2 (Reference Yang, Wang and Huang48). In this study, we found that the LF diet up-regulated the expression of ILF2, while the increasing addition level of SBP quadratically reduced the expression level to normal levels, which indicated that the immune response was stimulated by the LF diet and disappeared after the addition of SBP. ALFs are important antimicrobial peptides in crustaceans and are widely involved in antimicrobial and antiviral responses (Reference Wu, Tian and Zhou49). It was found that ALF1 and ALF2 can decrease the immune response of the organism (Reference Wang, Jiang and Feng50); ALF3 showed strong antibacterial activity against Gram-negative R bacteria, and the expression of ALF3 in blood cells was significantly up-regulated after several hours of attacking the Chinese mitten crab with pathogenic bacteria (Reference Wang, Zhang and Wang51). In this study, we found that all ALFs were up-regulated to varying degrees by LF diets, and the up-regulation was NS with the addition of SBP. The correlation heatmap revealed a strong correlation between ALF1 and ALF3 with ILF2. Considering the expression of ILF2, it was speculated that the LF diet probably stimulated the immune response of the crab and promoted the expression of ALFs. After the addition of SBP, the immune response disappeared and the expression of ALFs returned to normal. Numerous bioactive peptides originating from diverse food sources have been found in mammals to possess anti-inflammatory properties by enhancing the expression of proinflammatory cytokines while reducing the expression of anti-inflammatory cytokines, thereby influencing immune regulation (Reference Chakrabarti, Jahandideh and Wu52–Reference Zhou, Ma and Xu54). Soybean peptides have also been shown to possess such beneficial effects, as they can alleviate LPS-induced intestinal inflammation by decreasing the expression of IL-1β, IL-6 and TNF-α, while increasing the expression levels of IL-10 (Reference Wen, Bi and Zhou55). It is known that the intestinal structure of crustaceans is significantly different from that of vertebrates. For example, the invertebrate gut has a unique PM, which is mainly secreted by midgut cells. Because the PM is close to the inner wall of the midgut and wraps the food, it has the functions of protecting the epithelial cells of the midgut and helping the digestion and absorption of food. The expression of PM-related genes such as PT and PM2 reflect the function of the PM to some extent. In addition, the expression of ILF2, ALF1, ALF2, ALF3 and other genes can reflect the immune function of the intestine. However, the proteins encoded by the above genes are unique to invertebrates or have low homology with vertebrates. In our experiments, SBP also exhibited a significant regulatory effect on the intestinal structure and the expression of PT, PM2, ILF2, ALF1, ALF2 and ALF3 in Chinese mitten crabs. Therefore, the experiments we conducted can help us further understand the role of active peptides on crustaceans.
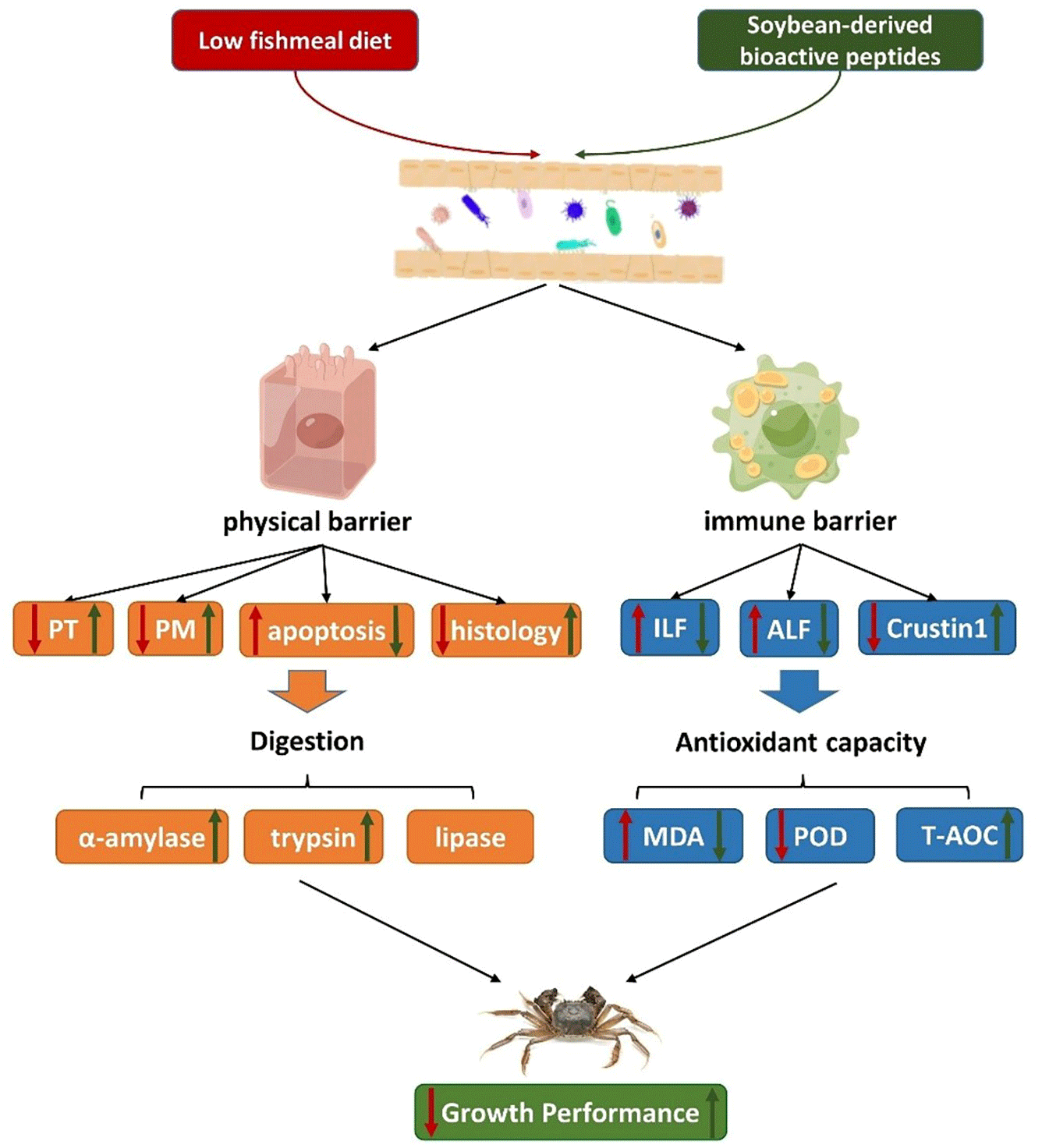
The correlation analysis revealed several significant relationships. There was a positive correlation between PM2 and TPS, as well as between PT and AMS, indicating that a well-developed PM structure can enhance digestive enzyme activity. MDA showed positive correlations with ALF1, ALF3 and ILF2, suggesting that oxidative damage in the intestine can trigger immune regulation in Chinese mitten crabs. MDA also exhibited negative correlations with muscular thickness and fold height, indicating that oxidative damage can disrupt the physical barrier of the crab’s intestine. On the other hand, TPS, PM2 and fold height showed positive correlations with growth performance, indicating that improved digestive enzyme activity and a well-maintained physical barrier in the intestine contribute to better growth in Chinese mitten crabs. Based on the information above, we have organised the impact of SBP on Chinese mitten crabs into a pathway diagram. Optimal level of SBP enhanced the relative expression levels of PM2 and PT, maintaining the integrity of the PM structure. Additionally, it reduces cell apoptosis and promotes the growth of intestinal cells. The improved physical barrier further enhanced the activities of digestive enzymes. Furthermore, through immune regulation, SBP supplementation decreased the relative expression of ILFs and ALFs, while increasing the relative expression of Crustin1. This leads to a reduction in MDA levels and then a decrease in oxidative damage. Through these pathways, SBP ultimately promotes the growth of Chinese mitten crabs.
Additionally, the growth-promoting effect of high doses of SBP was not obvious in the present study. Compared with the appropriate dose group, high doses of SBP increased the feed coefficient, reduced the activity of intestinal digestive enzymes, but the difference in antioxidant performance was NS. Therefore, we speculated that high doses of SBP may affect the absorption of nutrients by inhibiting the activity of intestinal digestive enzymes in Chinese mitten crabs, thereby reducing growth performance. The relevant molecular mechanisms require further study.
According to previous studies, soya protein peptides were commonly used as protein sources to replace fishmeal in aquafeed, which are produced by hydrolysis or microbial fermentation. The substitution of fish meal with soya protein peptides was shown to positively impact the intestinal health, immunity, digestive capacity and liver lipid metabolism of fish and shrimp, with no significant effect on growth performance(Reference Zhao, Song and Xie17,Reference Lin and Chen56,Reference Xuquan, Weilan and Ruixue57) . The optimal inclusion level of soya protein peptides as a protein source in aquafeed ranges from 10 % to 15 % (air dry base). These advantages might be due to that soya protein peptides do not include any anti-nutritional components and are abundant in amino acids, low-molecular-weight short peptides and unique nutritional elements. Furthermore, this food item possesses qualities that facilitate efficient digestion and absorption, while also containing bioactive compounds that stimulate the metabolic process of adipose tissue(Reference Lin, Tan and Ray58). Nevertheless, our experiments demonstrated that dietary 2–4 % SBP (30–35 % DM) can also enhance the growth performance and digestive ability of Chinese mitten crabs, as well as repair intestinal barrier damage induced by LF diets. Therefore, we believed that soya peptides not only possess notable attributes as a highly assimilable protein source, but are recognised to have functional properties that facilitate the restoration of the intestinal barrier and confer antioxidant benefits, even when administered in minute quantities. Our research may provide the possibility for the application of SBP as feed additives in aquafeed.
Conclusion
In summary, feeding LF diets under the present experimental conditions reduced the growth performance of Chinese mitten crab, caused oxidative damage to the intestine and damaged the intestinal immunological and physical barriers. The adverse effects of LF diets on the growth and intestinal health of Chinese mitten crab could be significantly improved by supplementing with SBP. In this study, the appropriate dietary level of SBP in LF diets for Chinese mitten crab was obtained as 2–4 %. The growth performance and digestive enzyme activities of Chinese mitten crab were significantly increased, and the antioxidant capacity and intestinal morphology were improved at the appropriate level. However, as the addition amount increases, the positive effects brought about by optimal level SBP were gradually eliminated. The mechanism of action may be that SBP promoted the intestinal barrier function and then improved the digestive, absorption and antioxidant capacity of the intestine to realise the growth performance.
Acknowledgements
The authors thank Prof. Wenbin Liu and Prof. Dingdong Zhang (Nanjing Agricultural University, China) for providing the convenience of the breeding site, and Dr. Weibo Jiang (Nanjing Agricultural University, China) for his help in the crab breeding process.
This work is supported by China Postdoctoral Science Foundation (2020M671601), Central Public-interest Scientific Institution Basal Research Fund, CAFS (2021XT703) and Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD63).
K. J.: Formal analysis and writing-original draft; C. S.: Conceptualisation, writing-review and funding acquisition; Q. Z., G. X. and H. T.: Writing-review & editing; W. J., M. L. and X. Z.: Feeding trial, sampling and parameters measurement; B. L.: Funding acquisition, supervision and conceptualisation; H. H.: Feed production and feeding trial.
The authors confirm that there is no conflict of interest.
The data for this work will be made available when demanded and on request.