The important role of vitamin D in Ca metabolism and bone health is well accepted(Reference Bischoff-Ferrari, Willett and Wong1). The discovery that most human tissues are able to produce and use active vitamin D triggered extensive research of vitamin D's biological functions beyond bone health. Experimental and epidemiological work has shown that vitamin D reduces risk and improves prognosis of cancer(Reference Holick2). Moreover, vitamin D plays important roles in the regulation of innate and adaptive immunity and seems to decrease the risk and modulate the course of various infections(Reference Chesney, Rosen and Hamstra3–Reference White6). Vitamin D itself has low biological activity, but undergoes activation by successive hydroxylations: in the liver with the formation of 25-hydroxyvitamin D (25(OH)D) and in the kidney with the production of 1,25-dihydroxyvitamin D (1,25(OH)2D)(Reference Holick7). It is becoming increasingly clear that 1,25(OH)2D also can be produced outside the kidney. This local production may have very important implications for the effects of vitamin D on health.
The body is able to synthesize vitamin D upon sufficient UV exposure. In fact, skin synthesis is the main source of vitamin D in most populations, proving a high efficiency of this reaction when the oral intake (from regular poor diet) is low(8). It is believed that a whole-body exposure at one MED (minimal erythema dose) generates 250–500μg (10 000–20 000 IU) of vitamin D, which is equivalent to the vitamin D content of 250 ml of cod-liver oil(Reference Davie, Lawson and Emberson9–Reference Stamp, Haddad and Twigg11).
Since man is to a high degree dependent on the skin production of vitamin D, factors altering either the fluence rate of UV radiation (like altitude, latitude, cloud cover, clothing habits, use of sun screens) or skin properties (like skin colour, age) are the main predictors of vitamin D status.
Romania is located at a latitude of 45°N which ensures a sufficient UV fluence rate to efficiently produce vitamin D from March to October(Reference Webb, Kline and Holick12). At the same time, Romanians have no practice of vitamin D food fortification or tradition of cod-liver oil consumption. This means that their biological pool of vitamin D has to accumulate during summer. We recruited for our study healthy medical employees (resident doctors and nurses) working in one of the main hospitals of Bucharest. Our aim was to investigate their vitamin D status and its association with risk of developing respiratory tract infections during the study period.
Materials and methods
Participants
One hundred and ten healthy volunteers were recruited from medical employees in a university hospital in Bucharest, Romania (Matei Bals National Institute for Infectious Diseases). Pregnant women were excluded from the study. The study took place between 1 December 2007 and 15 March 2008 and was approved by the Romanian Academy of Medical Science, the Matei Bals National Institute for Infectious Disease's Ethics Commission and Steering Committee. Each volunteer signed a consent form before entering the study.
Food and sun questionnaire
We used a six-page self-administered quantitative questionnaire which is a simplified version of the one used by Brustad et al. in the European Prospective Investigation into Cancer and Nutrition(Reference Brustad, Braaten and Lund13). The questionnaire contained questions about intake of food items known to contain vitamin D (about twenty-five food items and dishes). The frequency of use was given per day, week or month and the portion size was given in slices, glasses or spoons. Vitamin D intake was calculated based on standard weights. We also asked questions on supplements after checking the market for specific compounds. We also assessed the solar exposure by asking questions on holiday length/location during the summer prior to the study, skin type (assessed on a colour scale according to Fitzpatrick(Reference Fitzpatrick14)) and use of sunscreen (with protection factor). Information on gender, age and BMI (kg/m2) was also collected.
Sampling
Blood samples were collected during December 2007– January 2008. A 10 ml peripheral venous blood sample was obtained from each volunteer and centrifuged for serum separation. Serum was collected, transferred into coded tubes and stored at −80°C. The samples were shipped on ice and analysed in one batch at the Hormone Laboratory, Aker University Hospital, Oslo, Norway.
Identification of infections
Volunteers were asked to continuously report to the main physician investigator (an infectious diseases specialist) any sign and symptom of respiratory infections. Respiratory tract infection episodes (sinusitis, tonsillitis, laryngotracheitis and pneumonia) were recorded along with any use of antibiotics. We also counted the number of conjunctivitis episodes since this is a common symptom in upper respiratory infections. Some of the upper respiratory tract infections and most of the lower respiratory tract infections were confirmed by objective analysis, as sinuses and pulmonary X-ray respectively and biological samples.
Biochemical analyses
The concentration of 25(OH)D was determined by HPLC after diethyl ether extraction essentially as described by Falch et al.(Reference Falch, Oftebro and Haug15), with an inter-assay variation of 12 %.
Statistical analyses
The level of numerical variables (age, BMI, vitamin D intake, serum 25(OH)D concentration, number of infectious events) was expressed as the mean and standard deviation. The contribution of each variable in explaining the measured level of 25(OH) D was determined using multiple regression analysis and results are given as R 2 with P values. The association between 25(OH)D level and incidence of respiratory tract diseases was explored using a Spearman correlation test. Serum levels of 25(OH) D were categorized as <30 nmol/l, 30–49 nmol/l, 50–79 nmol/l and >80 nmol/l. The limit for statistical significance was set to be equal to 0·05. Statistical tests were performed with the SPSS for Windows statistical software package version 12 (SPSS Inc., Chicago, IL, USA).
Results
Out of the 110 volunteers who delivered a blood sample, 105 completed the questionnaire. Five individuals were excluded from the analysis due to lack of follow-up on infectious episodes. This left us with a group of 100 volunteers for the final analysis. The characteristics of the study population are described in Table 1.
Table 1 Descriptive characteristics of the participating population: healthy Romanian volunteers (n 105), December 2007–January 2008
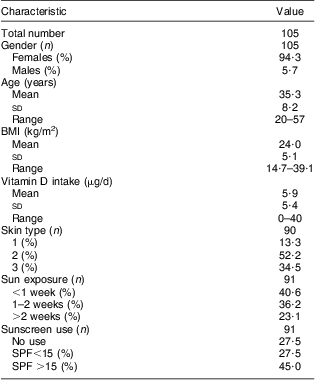
SPF, sun protection factor.
Blood samples were collected in December 2007–January 2008.
The main result of the present work is that 97 % of our volunteers had a serum level of 25(OH)D below 80 nmol/l. Eighty per cent had a level below 50 nmol/l and 35 % had 25(OH)D concentration below 30 nmol/l (Fig. 1). Men were under-represented in our study; therefore conclusions apply primarily to healthy women. We were able to include only five males and their mean 25(OH)D level was 29·8 (sd 5·5, range 23–36) nmol/l.

Fig. 1 Prevalence of low 25-hydroxyvitamin D (25(OH)D) concentration (![]() ) and mean 25(OH)D concentration (
) and mean 25(OH)D concentration (![]() ; standard deviation represented by vertical bars) according to 25(OH)D category among healthy Romanian volunteers (n 105), December 2007–January 2008.
; standard deviation represented by vertical bars) according to 25(OH)D category among healthy Romanian volunteers (n 105), December 2007–January 2008.
We analysed, independently as well as in a multivariate model, the predictor role of vitamin D intake, sun exposure, BMI, age and skin type on vitamin D status. Out of all these parameters assessed from the questionnaire, only sun exposure during the previous summer was significantly correlated with the level of 25(OH)D (P = 0·001; Table 2). We observed that a 2-week holiday predicted a winter 25(OH)D level of about 50 nmol/l, while no holiday resulted in a level of about 30 nmol/l. As expected, there seemed to be a positive correlation between serum 25(OH)D concentration and vitamin D intake and a negative one with BMI, but these associations did not reach statistical significance (P = 0·3 and 0·3, respectively). Volunteers had skin type 1, 2 or 3 with most of them belonging to type 2 (Table 1) but we found no correlation between skin type and vitamin D status in our study (data not shown).
Table 2 Results of linear regression analysis of the relationship between age, BMI, vitamin D intake and sun exposure during the summer previous to the study and serum 25(OH)D level: healthy Romanian volunteers (n 105), December 2007–January 2008
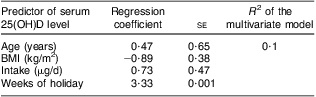
25(OH)D, 25-hydroxyvitamin D.
Another aim of our study was to assess the frequency of upper and lower respiratory tract infectious episodes and to look for a correlation between these and serum 25(OH)D level. Twenty-one per cent of our volunteers experienced one or more episodes of sinusitis. The incidence for tonsillitis was 37 %, 8 % for conjunctivitis, 28 % for laryngotracheitis and 2 % for pneumonia. Twenty per cent of the population experienced one or more fever episodes and 30 % of them were subjected to one or more antibiotic sessions during the study period. Overall, 32·4 % of volunteers did not experience any infection episode while 29·4 % experienced one episode and 37·2 % had two or more infection episodes. Table 3 presents the occurrence of none, one and two or more episodes with their corresponding 25(OH)D levels. Due to the low number of some of the clinical outcomes, we chose to look at the correlation between 25(OH) level and the occurrence of any upper respiratory tract infection, unstratified by localization. Overall we found no statistical significant correlation between serum level of 25(OH)D and occurrence of infections in our material, with a Spearman correlation coefficient of −0·12, P = 0·2.
Table 3 Mean concentration of serum 25(OH)D (nmol/l) stratified by disease status (number of episodes of upper respiratory tract infection): healthy Romanian volunteers (n 105), December 2007–January 2008
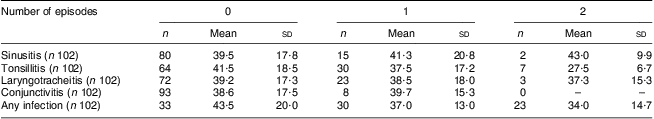
25(OH)D, 25-hydroxyvitamin D; n, total number of cases; Mean and sd, mean serum 25(OH)D concentration (nmol/l) and standard deviation.
The Spearman coefficient for the correlation between 25(OH)D level and number of infectious episodes was 0·12, P = 0·02.
Discussion
The main aim of the present study was to map the vitamin D status in a seemingly healthy Romanian population and to seek correlations between this and vitamin D intake, sun exposure during the summer previous to the study, age, BMI and risk for respiratory infections during the winter period. Romania is located at 45°N and has warm summers. Romanians residing in the capital (the place of the study) have generally no tradition for eating fish or supplementing with cod-liver oil, and food fortification is an inconsistent and almost non-existent practice. Therefore, most Romanians are dependent on cutaneous production of vitamin D to fill their physiological needs.
We report here alarmingly low 25(OH)D levels, with 97 % of our volunteers having concentrations below 80 nmol/l and almost one-third below 30 nmol/l (Fig. 1).
The understanding that vitamin D may play an important role in the prevention and cure of several chronic diseases has generated some controversy regarding the optimal level of vitamin D. Recently a panel of experts agreed that ‘the 25(OH)D level should be above 75 nmol/l for optimal health benefit’(Reference Souberbielle, Body and Lappe16), and it is known that a level of 30 nmol/l is the threshold for avoiding rickets in infants and osteomalacia in adults(Reference Zittermann17). Our results are surprising, since our population consisted of young, active and healthy medical caregivers who supposedly have higher health awareness than the average population. Among all parameters investigated, only sun exposure during the previous summer was a significant predictor of serum 25(OH)D level (Table 2). This is in agreement with several other publications showing a significant correlation between summer levels and winter levels, pointing once more to the importance of cutaneous vitamin D synthesis in predicting serum 25(OH)D levels(Reference Burgaz, Akesson and Michaelsson18–Reference Webb, Kift and Durkin20). On the other hand, our results seem to indicate that 25(OH)D has a longer half-life than previously reported(Reference Clements, Davies and Hayes21) but we cannot attempt any calculation since we have not measured summer concentrations of 25(OH)D. Figure 2 presents the seasonal variation of the UV fluence rate in Bucharest (45°N) and Oslo (60°N) and the average 25(OH)D concentration during the winter in two similar healthy populations. It is surprising that despite higher fluence during the summer in Bucharest, the residual 25(OH)D level during winter is significantly lower in Bucharest than in Oslo (38·5 nmol/l v. 65·2 nmol/l). This seems to indicate that other factors like the tradition of fish consumption and sun-seeking behaviour (common among Norwegians) play important roles in determining vitamin D status(Reference Moan, Porojnicu and Robsahm22).

Fig. 2 Seasonal variation in the UV fluence rate in Bucharest (45°N; ——) and Oslo (60°N; … ) and the average concentration of 25-hydroxyvitamin D (25(OH)D) during the winter (●, Bucharest; ▪, Oslo; standard deviation represented by vertical bars) in two similar healthy populations.
Ageing is a known factor that modifies vitamin D production(Reference Holick, Matsuoka and Wortsman23). We found no correlation between age and vitamin D status, but this may be due to the narrow age span of our population and its relatively low mean age (35·5 years). The same is true for BMI. It has been reported by several scientists that high BMI is associated with low vitamin D status (due to trapping of vitamin D in excess body fat and reduced solar exposure, especially in extremely obese patients)(Reference Lagunova, Porojnicu and Lindberg24–Reference Wortsman, Matsuoka and Chen26). Our volunteers had BMI in the range from 14·7 to 39·1 kg/m2, and we observed a slight but non-significant negative correlation with serum 25(OH)D (Table 2).
The mean vitamin D intake in our population was 5·9 μg/d. We have previously shown that intake in this range is too low to maintain a summer level of 25(OH)D(Reference Porojnicu, Bruland and Aksnes27). Our present data show, as expected, a positive but non-significant correlation between vitamin D intake and serum 25(OH)D concentration (Table 2). In a recent meta-regression analysis of the vitamin D intake–serum 25(OH)D relationship, an intake of 5 μg/d during winter would predict serum levels of about 35 nmol/l, which is in good agreement with our present data(Reference Cashman, Fitzgerald and Kiely28).
Our results seem to indicate that despite high UV fluence during the summer, Romanians’ vitamin D status during winter is far below optimal. This shows that sun exposure of just the hands/face (walking to and from work, normal outdoor daily activities in the city) is not sufficient to generate satisfactory levels of vitamin D, not even in Romania. This may be related to the fact that Romanians probably seek shade during the summer (due to high temperatures, notably in cities with intense traffic and large concrete buildings). We observed that volunteers who had a 2-week holiday at the seaside the summer previous to the study had significantly higher levels of 25(OH)D during the winter than the other volunteers. Vitamin D intake reported by our volunteers in the questionnaire had little impact on the 25(OH)D serum level (although we observed a positive but non-significant correlation).
We further recorded the occurrence of respiratory symptoms and the use of antibiotics during the period of the study. Our hypothesis was that a low vitamin D status will increase the risk of infections. The respiratory tract serves as the primary interface between the host and inhaled pathogens. Mucosa is equipped with various defence mechanisms such as: (i) the ciliary beat, cough reflex and mucus clearance; (ii) the secretion of antimicrobial peptides (like defensins and cathelicidin); and (iii) initiation of the inflammatory response and recruitment of phagocytic cells. Vitamin D regulates the production of cathelicidin and defensins(Reference Adams and Hewison29, Reference Gombart, Borregaard and Koeffler30). These are molecules produced in the epithelium of the upper and lower respiratory tracts that have antimicrobial activity, increase pro-inflammatory gene expression, are involved in reparatory mechanisms of the epithelium and induce maturation of dendritic cells.
Several clinical reports suggest that poor vitamin D status is a factor predisposing to infections of the upper and lower respiratory tracts. In Finland soldiers with low vitamin D status (<40 nmol/l) at entry into military service have a higher risk for subsequent respiratory infections(Reference Laaksi, Ruohola and Tuohimaa31). Similar results are reported also in Turkey(Reference Karatekin, Kaya and Salihoglu32) and India(Reference Wayse, Yousafzai and Mogale33), where levels below 50 nmol/l significantly increased the risk of respiratory infections in children and neonates. A recent American study reports 55 % higher odds of developing respiratory infections if 25(OH)D concentration is below 25 nmol/l compared with above 75 nmol/l(Reference Ginde, Mansbach and Camargo34). Our results showed no significant overall trend of covariance of infection occurrence and vitamin D levels. Our negative results may be due to the fact that only 4 % of the volunteers had a 25(OH)D level above 80 nmol/l and because of the relatively short duration of our study. Interpretation of the results is also weakened by the fact that disease occurrence was self-reported in some cases, without paraclinical confirmation.
To our knowledge the present study the first one that has evaluated the vitamin D status in a relatively young, active population in Romania. We acknowledge that the study has limitations: relatively low number of participants and high homogeneity (mostly females, low ranges of age, vitamin D intake, BMI) that make the analysis of associations difficult. Nevertheless, we report alarmingly low levels of 25(OH)D that should trigger more studies in larger population groups. We advocate here for information campaigns on the importance of vitamin D for health, consistent enrichment of food and encouragement of assessing vitamin D levels, at least in sick people and populations at risk.
Acknowledgements
This paper was partially supported by the Sectoral Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under contract number POSDRU/89/1.5/S/64109. The financial support of the Norwegian Cancer Association is highly appreciated. The Norwegian authors received support from the Norwegian Cancer Society and Health South-East Medical Enterprise. There are no conflicts of interest. The authors’ contributions were as follows: R.M.-C., A.L. and A.H., collection of data, interview with the patients, blood sampling; A.C.P., Z.L. and J.M., measurement of serum 25(OH)D levels, data analyses; A.D., analyses of spectral data.







