INTRODUCTION
By the end of this century, the radiocarbon (14C) content of CO2 in the atmosphere is projected to decline and reach a 14C age equivalent of 2000 years before present following business-as-usual emissions of fossil fuels (Graven Reference Graven2015). This marked decline will be followed by reductions in 14C content of carbon reservoirs exchanging with the atmosphere, including biospheric, lacustrine, and oceanic pools. This decline in 14C isotopic composition is known as the Suess Effect after Hans Suess, who became the first to observe this effect in 1955 (Suess Reference Suess1955). Beginning in the 1950s, nuclear weapons testing introduced bomb-derived 14C into the atmosphere nearly doubling its content within the course of a decade (Broecker and Walton Reference Broecker and Walton1959a; Levin and Kromer Reference Levin and Kromer2004). This perturbation in atmospheric 14C has propagated through Earth surface reservoirs in communication with the atmosphere at varying rates (Graven Reference Graven2015).
In addition to carbon sourced from the atmosphere, water bodies located in areas of limestone and karst receive carbonate ions sourced from the chemical weathering of calcareous bedrock in the catchment:
Carbonate rocks are typically of ancient geological origin, giving rise to contributions of 14C-dead carbon to dissolved inorganic carbon (DIC) pools (e.g., Z. Liu et al. Reference Liu, Zhou, Cheng and Burr2017; Ishikawa et al. Reference Ishikawa, Tayasu, Yamane, Yokoyama, Sakai and Ohkouchi2015). DI14C stemming from limestone weathering depends on the reaction stoichiometry with carbonic (reaction 1) and sulfuric acid (reaction 2) giving rise to half modern and completely dead signatures, respectively. In contrast, silicate weathering delivers DIC sourced exclusively from atmospheric or soil pools, which are a source of modern carbon:
Hardwater lakes are most affected by the effect of 14C-depleted DIC stemming from limestone weathering (Keaveney and Reimer Reference Keaveney and Reimer2012) with the consequence of aquatic organisms assuming the DI14C isotopic composition of the water body from which the carbon is sourced (Broecker and Walton Reference Broecker and Walton1959b). In total, the 14C content of lake water DIC depends on the DIC inputs (river runoff, groundwater discharge, atmospheric input) and outputs (river outflow, groundwater leakage, degassing to the atmosphere, fixation by photosynthesis and calcification, and radioactive decay) (Yu et al. 2007). The resulting reservoir effect needs to be taken into account for assessing the age of aquatic materials (e.g. shells, organic matter) and for tracing pathways of carbon in food webs and in the environment (Guillemette et al. Reference Guillemette, Bianchi and Spencer2017).
In the post-industrial era, hardwater lakes will be impacted by the residual bomb-spike, as well as contributions from anthropogenic emissions of 14C-depleted CO2 that are superimposed on an already 14C-depleted reservoir due to limestone weathering. Here we present a case study from Lake Constance (Figure 1) examining changes in 14C isotopic composition of lake water DIC over time. We present results from samples collected in 2013 and combine these with historical data from 1969 and discuss how changes in atmospheric carbon contributions to DIC are modulated over time. A prediction for DI14C content is made for Lake Constance in the year 2100, which may apply similarly to other hardwater lakes and rivers.
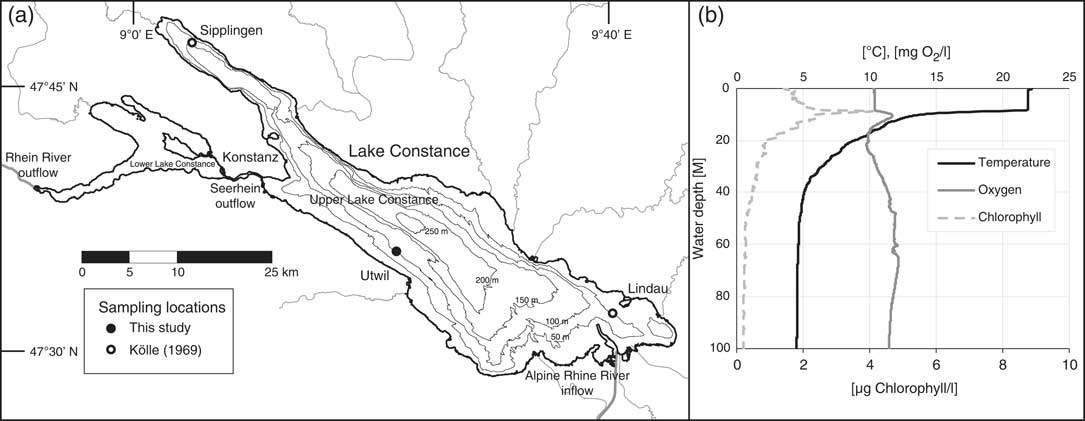
Figure 1 (a) Map of Lake Constance (IGKB 2016) showing sampling locations for this study near the township of Utwil (filled circle) and adjacent to the townships of Sipplingen and Lindau (open circles) from Kölle (Reference Kölle1969). Part (b) shows water column parameters recorded during sample collection on August 14, 2013. The upper 20 m of the water column are characterized by primary productivity with a peak in chlorophyll and oxygen concentrations between 8 and 12 m. Water temperature decreases steadily with depth until 40 m, below which it remains relatively constant.
STUDY SITE AND METHODS
Lake Constance, the second-largest perialpine lake in central Europe, formed after the Last Glacial Maximum in the wake of retreating glaciers (Wessels Reference Wessels1998). Lake Constance is composed of two basins—the Upper Lake Constance and Lower Lake Constance basins. Upper Lake Constance, the subject of this investigation, covers an area of 472 km2, holds 47.6 km3 of water with a maximum depth of 251 m as documented by the Internationale Gewässerschutzkommission für den Bodensee (IGKB 2009, 2016). The watershed encompasses an area of 11,438 km2, of which 6119 km2 is drained by the Alpine Rhine (IGKB 2009), which delivers 62% of the water flux (Gilfedder et al. Reference Gilfedder, Petri, Wessels and Biester2010). Total annual water influx is 12.02 km3, giving water a theoretical residence time of 4–5 years in the lake (Gilfedder et al. Reference Gilfedder, Petri, Wessels and Biester2010). Lake Constance is subject to annual cycles of water column stratification and mixing, which are driven by changes in temperature, leading to episodes of bottom water oxygen replenishment (Bäuerle et al. Reference Bäuerle, Ollinger and Ilmerger1998). Thermal destratification does not proceed to completion every year and is increasingly affected by rising lake temperatures (Bäuerle et al. Reference Bäuerle, Ollinger and Ilmerger1998; IGKB 2015). The widespread occurrence of limestone in the catchment makes Lake Constance a typical hardwater lake with calcite precipitation tied to seasonal primary productivity (Wessels Reference Wessels1998).
The sampling location FG (47°35′59′′N, 9°21′29′′E) located offshore of the township of Utwil is described in IGKB (2009). In brief, location FG is characterized by sediment supply predominantly of autochthonous origin and is a reference location for lake primary productivity (Fuentes et al. Reference Fuentes, Güde, Wessels and Straile2013a). A depth profile of water samples was collected on 14.08.2013 from location FG using a Hydro-Bios (Kiel, Germany) Niskin rosette sampler. Additionally, a CTD probe (conductivity, temperature, and depth) equipped with sensors for turbidity, oxygen, and chlorophyll (Sea & Sun Technologies) was used for the characterization of the water column. Data were collected at 2 Hz and was continuously lowered at about 0.5 m/s, collecting about 400 values for the 100 m profile collected at location FG. Water samples were collected in 1-L glass bottles, poisoned with 100 µL saturated HgCl2 solution, and sealed with Apiezon N-greased ground joint glass stoppers (Blattmann et al. Reference Blattmann, McIntyre, Wacker and Eglinton2013). Water samples were stored at room temperature in the dark until 14C analysis of dissolved inorganic carbon (DI14C). 40 mL of each water sample was transferred to a 60 mL glass vial sealed by Teflon-coated silicon septum cap. The headspace was purged with helium for 2–4 min at 100 mL/min. Samples were acidified with orthophosphoric acid and purged for 8 min (assessed as optimal for maximizing CO2 yields; Blattmann et al. Reference Blattmann, McIntyre, Wacker and Eglinton2013). Purged CO2 was captured, quantified, and graphitized using an AGE 3 system (Wacker et al. Reference Wacker, Němec and Bourquin2010), and then analyzed using a MICADAS accelerator mass spectrometry (AMS) system (Synal et al. Reference Synal, Stocker and Suter2007) at the Laboratory of Ion Beam Physics at ETH Zurich. A duplicate of one water sample reproduced 14C content within instrumental error (i.e., ±0.002 Fm)
Kölle (Reference Kölle1969) reports Lake Constance DI14C adjacent to the towns Sipplingen (47°47′52′′N, 9°5′44′′E) and Lindau (47°32′46′′N, 9°40′53′′E). These DI14C data were converted from their reported Bq/kgC units to fraction modern (Fm) units following Stenström et al. (Reference Stenström, Skog, Georgiadou, Genberg and Johansson2011; equation 42). For surface and deep waters, –8‰ for δ13CDIC is adopted for fractionation correcting the 14C concentration data reported by Kölle (Reference Kölle1969). Lake Constance waters below the photic zone (i.e. greater than 20 m of water depth) display steady δ13CDIC values centered around –8‰, irrespective of season (Hirschfeld Reference Hirschfeld2003). Surface water δ13CDIC is variable depending on the season, however given that samples were collected on April 17, 1969, we assume that the spring primary productivity bloom, which leads to an increase in surface water δ13CDIC (Hollander and McKenzie Reference Hollander and McKenzie1991; Hirschfeld Reference Hirschfeld2003), had not yet initiated. This assumption is supported by monthly timeseries records from Lake Constance that show that by April 15, 1969, (1) temperatures of surface waters had not warmed above their winter values of 5°C and (2) surface water phosphate concentrations remained steady, indicating low algal growth. Given these historical constraints, some uncertainty remains in fractionation correction of data reported by Kölle (Reference Kölle1969) and so the results encompass the spread from a sensitivity analysis allowing δ13CDIC values to vary in the range –8±3‰. The ±3‰ amplitude covers seasonality-induced changes in δ13CDIC observed in Lake Constance (Hirschfeld Reference Hirschfeld2003).
RESULTS AND DISCUSSION
Lake Constance DIC Fluxes
Figure 2 provides an overview of the DIC cycle of Lake Constance. Based on the lake volume, which varies between 47.2–48.2 km3 (Bäuerle et al. Reference Bäuerle, Ollinger and Ilmerger1998) and lake water DIC concentrations (IGKB 1976; Hirschfeld Reference Hirschfeld2003; this study), Lake Constance contains between 1380 and 1450 GgC in the form of DIC. The rivers deliver 350–380 GgC/yr of DIC and the Rhine releases 300–350 GgC/yr based on long-term average river fluxes (Gilfedder et al. Reference Gilfedder, Petri, Wessels and Biester2010) and riverine DIC concentrations (Hirschfeld Reference Hirschfeld2003). Based on a range of possible rainwater DIC concentrations (Górka et al. Reference Górka, Sauer, Lewicka-Szczebak and Jędrysek2011), precipitation (Gilfedder et al. Reference Gilfedder, Petri, Wessels and Biester2010) introduces 0.3–3 GgC of DIC annually. Within the lake, the sedimentation of lacustrine calcite and organic matter remove between 10–40 GgC/yr from the DIC pool (Stabel Reference Stabel1986; Straile Reference Straile1998). DIC from groundwater and water contributions from overland flow are loosely constrained. Based on the flux (Gilfedder et al. Reference Gilfedder, Petri, Wessels and Biester2010) and an assumed DIC concentration equal to that of riverine sources (Hirschfeld Reference Hirschfeld2003), an estimated flux of 10 GgC/yr into Lake Constance is proposed. On average, Lake Constance receives an average of 3.70 Tg of total sediment sourced from its terrestrial catchment annually (Gilfedder et al. Reference Gilfedder, Petri, Wessels and Biester2010). Assuming an organic carbon content of 1% of soil and plant debris origin, this would amount to 40 GgC of pedogenic origin entering Lake Constance annually. Terrestrial organic matter constitutes a source of lacustrine DIC if remineralized in the lake. This variable DIC source is discussed further in the following sections. Based on the maximum and minimum DIC input and output scenarios, the amount of carbon evading the lake along the air–water interface is constrained within the range of –20 to +130 GgC/yr (with positive values representing the net release of carbon dioxide to the atmosphere). The corresponding water surface area normalized rates of carbon dioxide release range from –0.1 gC/m2/day to 0.7 gC/m2/day (with positive values representing the net release of carbon dioxide to the atmosphere), which is within the typical range of carbon dioxide fluxes along air–water interfaces of lakes (Cole et al. Reference Cole, Caraco, Kling and Kratz1994; Huotari et al. Reference Huotari, Ojala, Peltomaa, Nordbo, Launiainen, Pumpanen, Rasilo, Hari and Vesala2011).
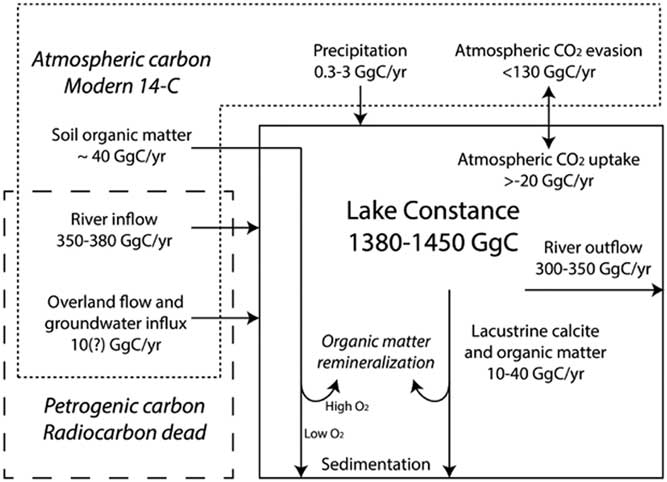
Figure 2 Model overview of DIC sources and fluxes in the Lake Constance system. The size of the DIC pool in Lake Constance varies within 1380–1450 GgC. River inflow and outflow constitute the largest DIC source and sink respectively. Further DIC inputs include overland and groundwater contributions and direct contributions from rainwater into the lake. Additional sinks of DIC include the sedimentation of calcite and organic matter formed in the lake. Furthermore, carbon dioxide evades from lake water into the atmosphere and may either be positive (net CO2 flux from the water to the atmosphere) or negative, ranging from a total net flux of –20 to+130 GgC/yr. Terrestrial organic matter is subject to partial remineralization within the lake, with organic matter remineralization efficiency depending on water column oxygen content. The different DIC sources are characterized by varying proportions of atmospheric and petrogenic carbon.
Assessing Atmospheric Carbon Contribution
At location FG, water column-suspended DIC collected at six different depths averaged 0.856±0.005 Fm, with most measured values within error of one another and no clear trend in the water column (see Table 1). Data collected by Kölle (Reference Kölle1969), converted to Fm values, show surface waters that are higher by 0.06 and 0.11 Fm units for Sipplingen and Lindau, respectively.
Table 1 14C isotopic composition of DIC from Lake Constance.

* Units in parentheses are ranges of DI14C values generated assuming –5 and –11‰ for the 13C-based fractionation correction of data reported by Kölle (Reference Kölle1969).
In a simplified scheme, we can consider that DIC originates from petrogenic (i.e., limestone-derived) and from atmospheric sources, which can be described with the binary mixing model in Equation 4:
where x represents the relative contribution of atmospheric-derived carbon. In this case, carbon derived from the weathering of ancient limestone is characterized by a 14C-dead isotopic composition as the limestone formations in the Lake Constance catchment are millions of years old (Hsü Reference Hsü1995) and thus characterized by a 14C isotopic composition of Fmpetro=0. The proportion of atmospherically derived carbon in lacustrine DIC integrates over all contributions of DIC from gas exchange along the air–water interface, degradation of soil organic matter, rainwater inputs, groundwater and overland flow inputs, and riverine inputs, which in turn integrate over atmospheric and petrogenic contributions from chemical weathering of rocks and air–water gas exchange along the transport path. Given the low DIC residence time in Lake Constance (4–5 years), loss of 14C by radioactive decay is considered negligible.
14C isotopic composition for atmospheric carbon dioxide was 1.54 and 1.02 Fm in the years 1969 and 2013, respectively (Levin and Kromer Reference Levin and Kromer2004; Graven Reference Graven2015), primarily reflecting the temporal proximity to the bomb peak in atmospheric 14C. Based on isotope mass balance calculations of the minimum and maximum measured DI14C concentrations, the contribution of atmosphere-derived carbon to Lake Constance corresponds to 83–85% in 2013. In 1969, the atmospheric proportion contributing to lake water DIC lies between 73 and 82% when, in addition to considering the extreme DI14C endpoints of Lindau (40 m) and Sipplingen surface waters, the 13C-based fractionation-based sensitivity analysis is applied. Given these two constraints, it suggests a modest but significant increase in atmospheric carbon contributions to Lake Constance DIC over the four decades between the two sample collection times.
Mechanisms for Changes in DI14C over Time
There are various factors that may lead to changes in the 14C isotopic composition of lake water DIC with time. The alkalinity of the water and the bedrock of the catchment exert first order control on the DI14C value (Keaveney and Reimer Reference Keaveney and Reimer2012). Depending on the stoichiometry of the bedrock weathering reaction, the carbon may entirely be sourced from the atmosphere (Equation 3), from equal proportions of atmospheric and ancient bedrock sources (Equation 1), or entirely from ancient carbonate (Equation 2). Changes in weathering behavior of bedrock could thus lead to changes in DI14C. Another source of change may come from the intensity of gas exchange between air and water. Greater exchange would lead to DI14C approaching an atmospheric 14C content. This may in turn also be related to lake water circulation controlling water mass exposure to the air–water interface. The replacement and vertical mixing of deep waters is expected to change with future changes in climate and precipitation (Fink et al. Reference Fink, Wessels and Wüest2016). Similar to vertical mixing, the export of organic remains from photosynthetic organisms into deep waters and their subsequent degradation offers another pathway by which surface water DIC can contribute to deep water DIC (Schwalb et al. Reference Schwalb, Dean, Güde, Hanisch, Sobek and Wessels2013). A further source of carbon that may influence DI14C within the lake is that resulting from the degradation of allochthonous organic matter introduced to the lake by riverine or atmospheric transport. Allochthonous organic matter and products formed from its degradation are utilized by aquatic microorganisms and thus this carbon can propagate through the lacustrine food web before finding its way into the lacustrine DIC reservoir (Fuentes et al. Reference Fuentes, Güde and Straile2013b). Oxidation of allochthonous organic matter and intermediate degradation products such as methane (Bussmann et al. Reference Bussmann, Damm, Schlüter and Wessels2013) will contribute more directly to lake water DIC. Soil organic matter, constituting an important component of allochthonous organic carbon supplied to Lake Constance by rivers (Fuentes et al. Reference Fuentes, Güde, Wessels and Straile2013a), is characterized by relatively modern 14C isotopic compositions (van der Voort et al. Reference van der Voort, Hagedorn, McIntyre, Zell, Walthert, Schleppi, Feng and Eglinton2016). Increased degradation of soil organic matter within Lake Constance sediments may thus to lead to an increase in atmospheric carbon contribution to water column DIC, and vice versa.
The most striking difference between Lake Constance in 2013 and 1969 is its nutrient state, as echoed by changes in lake ecology (Wessels et al. Reference Wessels, Mohaupt, Kümmerlin and Lenhard1999). Due to anthropogenic activity, Lake Constance successively increased its nutrient state transitioning from oligotrophic to mesotrophic in 1939, and with advanced eutrophication beginning in the mid 1950s. Eutrophication continued until 1979/1980 when phosphorous concentrations reached their peak (Wessels et al. Reference Wessels, Mohaupt, Kümmerlin and Lenhard1999). During this time of intensified lacustrine primary productivity, lacustrine and terrestrial organic matter sourced from the catchment was subject to enhanced preservation in the lake sediments, particularly along the margins, due to reduced water column oxygen conditions which even reached an anoxic, sulfidic state (Müller Reference Müller1966). In the newfound oligotrophic state of the lake with oxic bottom waters, terrestrial organic carbon provides an important substrate for microbes, which provides a source of carbon for benthic organisms (Sobek et al. Reference Sobek, Durisch-Kaiser, Zurbrügg, Wongfun, Wessels, Pasche and Wehrli2009; Fuentes et al. Reference Fuentes, Güde and Straile2013b), allowing soil carbon to act as a vector for transferring atmospheric carbon into lake water DIC. Based on the historically documented changes in the carbon cycle of Lake Constance, we hypothesize that the inhibition of soil organic matter degradation led to a decreased transformation of 14C-enriched soil carbon into DIC, which in turn indirectly results in a net reduction in the atmospheric carbon contribution to the DIC pool. The amount of soil organic carbon entering Lake Constance corresponds to about 3% of the size of the total Lake Constance DIC pool (see Figure 2). With a 4- to 5-year residence time for DIC in the lake, integrated over the course of a few years, diminished soil organic carbon degradation and the corresponding decreased contribution to the aquatic DIC pool can account for the lower atmospheric carbon contributions to the DIC pool in the late 1960s. Thus, changes in the remineralization behavior of allochthonous soil organic carbon due to eutrophication may influence lacustrine DI14C inventories.
Other superimposed effects can introduce additional influence on DI14C. The utilization of DIC by photosynthetic organisms can lead to higher uptake of atmospheric CO2 leading to higher DI14C signatures (Kempe Reference Kempe1982; Li et al. Reference Li, Qiang, Jin, Liu, Zhou and Zhang2017). In the case of Lake Constance, 14C data reveals that atmospheric carbon contributions in the eutrophic waters of 1969 were lower than today. This is contrary to what would be expected in a freshwater system with elevated primary productivity, and suggests that—despite eutrophication—direct assimilation of atmospheric CO2 into lake water DIC was significantly outweighed by the effects of reduced remineralization of soil organic carbon. Other mechanisms for changing DI14C cannot be wholly excluded based on the available data. Given however the pervasive changes introduced by eutrophication in various aspects of the Lake Constance carbon cycle and ecosystem (Müller 1966, Reference Müller1997; Güde et al. Reference Güde, Rossknecht and Wagner1998; Wessels et al. Reference Wessels, Mohaupt, Kümmerlin and Lenhard1999), and little reason to assume large changes in the other mechanisms, this hypothesis provides a realistic explanation aligned with historically documented changes.
Projections for Past and Future DI14C
Based on the above line of reasoning, we propose an estimate of DI14C for a pre-eutrophication, pre-industrial, and pre-bomb Lake Constance. In pre-industrial Lake Constance, prior to the earliest signs of eutrophication in 1870–1880 (Müller Reference Müller1997), the oligotrophic state of today likely best characterizes the DI14C lake as it once may have been. We thus project the pre-anthropogenic Lake Constance DIC atmospheric contribution to be around 83–85%, corresponding to an Fm value of 0.83–0.85 for lake water DI14C. Besides eutrophication, another difference between Lake Constance today and in the 19th century was the change in circulation induced by the channelization of the Rhine (Wasmund Reference Wasmund1928), which may have had additional effects on lake-atmosphere CO2 exchange. Furthermore, changes in land use, deforestation, fertilizer usage, and erosion (Güde et al. Reference Güde, Rossknecht and Wagner1998) introduce additional uncertainty and may lead to error in our projection.
Based on the estimates of 83–85% atmospheric carbon contributing to Lake Constance DIC, we expect that in the year 2100, following the predictions by Graven (Reference Graven2015) with a “business-as-usual” fossil fuel emission scenario (RCP 8.5, Fmatmosphere=0.73), a DI14C signature of 0.61–0.62 Fm is predicted. However, concurrent to depletion in atmospheric 14C, lake water temperatures are experiencing an observable increase, and this increase is projected to continue (IGKB 2015, O’Reilly et al. Reference O’Reilly, Sharma, Gray, Hampton, Read, Rowley, Schneider, Lenters, McIntyre, Kraemer, Weyhenmeyer, Straile, Dong, Adrian, Allan, Anneville, Arvola, Austin, Bailey, Baron, Brookes, de Eyto, Dokulil, Hamilton, Havens, Hetherington, Higgins, Hook, Izmest’eva, Joehnk, Kangur, Kasprzak, Kumagai, Kuusisto, Leshkevich, Livingstone, MacIntyre, May, Melack, Mueller-Navarra, Naumenko, Noges, Noges, North, Plisnier, Rigosi, Rimmer, Rogora, Rudstam, Rusak, Salmaso, Samal, Schindler, Schladow, Schmid, Schmidt, Silow, Soylu, Teubner, Verburg, Voutilainen, Watkinson, Williamson and Zhang2015). The resulting increase in water column stratification will decrease exchange between atmospheric and deep water DIC pools, but, more significantly, also lead to depletion in bottom water oxygen concentrations. This may create a situation similar to the 1960s, which saw as little as 74% atmospheric contributions. If this scenario were to be realized then, DI14C may reach 0.54 Fm by the end of the century. The projection range for Lake Constance DI14C, 0.54–0.62 Fm, for the year 2100 corresponds to a reservoir age of 3800–5000 Libby 14C years before present. Globally, DI14C from other hardwater lakes and rivers will likely follow a similar trend. Based on these projections, assuming linear changes, and taking the 85% and 74% atmospheric contribution scenarios, the difference to 2013 measured values would exceed 0.02 Fm units within 8 and 6 years, respectively, rendering it possible to track such changes well beyond margins of analytical uncertainty within the course of a single decade. Larger lakes with relatively little DIC sourced from carbonate weathering and influence from terrestrial organic carbon, e.g., Lake Superior (Zigah et al. Reference Zigah, Minor and Werne2012), will likely show a DI14C trajectory more tightly coupled to atmospheric 14C content both in terms of absolute 14C/12C ratio and rate of change. Similarly, lakes situated in endorheic basins may exhibit DI14C values mirroring atmospheric values due to extensive air-water gas exchange, as found for several Chinese lakes (T. Liu et al. Reference Liu, Zhou, Cheng and Burr2017). Lakes exhibiting limited mixing characteristics might show lagged and muted responses, e.g., meromictic lakes such as Lac Pavin, France (Albéric et al. Reference Albéric, Jézéquel, Bergonzini, Chapron, Viollier, Massault and Michard2013). Additionally, lake geometry (surface area to volume ratio), and changes thereof, control gas exchange with the atmosphere and can lead to additional changes in the reservoir effect over time periods of millennia to centuries (e.g., Schleinsee, Germany; Geyh et al. 1998).
Deviations from these DI14C projections from Lake Constance exposed by future measurements, will shed light on processes we have not considered or underestimated here, or further carbon cycle perturbations. Deviations from these forecasts may reflect additional changes in lake hydrology, chemical weathering of rocks in the catchment or other changes in DIC source. In coming decades, Lake Constance and lakes globally are likely to undergo significant hydrological change due to modification of precipitation patterns, runoff, vertical mixing, and other processes linked to climate change (Battin et al. Reference Battin, Luyssaert, Kaplan, Aufdenkampe, Richter and Tranvik2009; Tranvik et al. 2009; Fink et al. Reference Fink, Wessels and Wüest2016). With ongoing rising water temperatures observed for Lake Constance (IGKB 2015) and for lakes globally (O’Reilly et al. Reference O’Reilly, Sharma, Gray, Hampton, Read, Rowley, Schneider, Lenters, McIntyre, Kraemer, Weyhenmeyer, Straile, Dong, Adrian, Allan, Anneville, Arvola, Austin, Bailey, Baron, Brookes, de Eyto, Dokulil, Hamilton, Havens, Hetherington, Higgins, Hook, Izmest’eva, Joehnk, Kangur, Kasprzak, Kumagai, Kuusisto, Leshkevich, Livingstone, MacIntyre, May, Melack, Mueller-Navarra, Naumenko, Noges, Noges, North, Plisnier, Rigosi, Rimmer, Rogora, Rudstam, Rusak, Salmaso, Samal, Schindler, Schladow, Schmid, Schmidt, Silow, Soylu, Teubner, Verburg, Voutilainen, Watkinson, Williamson and Zhang2015), it is becoming increasingly important to understand how these changes will affect lakes as carbon sinks, ecosystems, and as a water resource for humans.
SUMMARY AND OUTLOOK
Future work on Lake Constance and other water bodies will need to consider the impact of changing DI14C to assign end-member 14C values to aquatic primary productivity for the purpose of both natural isotope tracing and 14C dating studies. Bomb-derived carbon as well as the continued release of fossil fuel-derived CO2 are set to continue the isotopic perturbation of carbon reservoirs including the hydrosphere (Graven Reference Graven2015). Changes in DIC sources include supply of terrestrial organic carbon to lakes and its degradation behavior, with the latter coupled to lake water oxygen content. Additional changes to DIC source may stem from changes in carbonate and silicate rock weathering as well as changes in air-water gas exchange. Coeval changes in climate and anthropogenic impacts will lead to a variety of superimposed effects modulating DI14C in terrestrial water bodies. Time-series monitoring of 14C in lake water DIC and other carbon reservoirs provide important constraints on the origin and trajectories of change in carbon cycle processes.
ACKNOWLEDGMENTS
Daniel Montluçon is thanked for his technical support in the laboratory. We thank Lukas Wacker, Martin Suter, and Mantana Maurer for their support in the Laboratory of Ion Beam Physics at ETH Zurich. For collecting samples in Lake Constance, we thank the crew of R/V Kormoran and Klaus Weih (ISF Langenargen) for acquisition and processing of water column data.





