Introduction
A significant amount of antibiotics is prescribed for illnesses caused by respiratory viruses including influenza [Reference Havers1], with many of those prescriptions being inappropriate [Reference Fleming-Dutra2–Reference Silverman4]. Yet, there is limited information on the overall volume of antibiotic prescribing triggered by influenza infections in different age groups. Such information could help guide vaccination efforts and efforts at reducing unnecessary antibiotic prescribing for viral infections without bacterial complications with the aim of reducing antibiotic prescribing and the propagation of antimicrobial resistance [Reference Klugman and Black5]. For example, results of a randomised trial of influenza vaccination in children aged 6–36 months suggest that vaccination is associated with a substantial reduction in influenza-related medical utilisation, including decreases in antibiotic prescribing for PCR-confirmed influenza episodes [Reference Dbaibo6]; however, it is uncertain how much influenza vaccination would affect the overall volume of antibiotic prescribing in different age groups. A statistical analysis of data on antibiotic prescriptions to Scottish children aged under 5 years between 2009 and 2017 estimated that 2.4% of those prescriptions are influenza-associated [Reference Fitzpatrick7]. A French study covering a 7-year period found that during the cold seasons, outpatient visits related to influenza-like illness (ILI) contributed between 5% and 10% of all outpatient antibiotic prescriptions in children aged under 5 years. However, the estimates in [Reference Cheysson8] refer to antibiotic prescriptions during cold seasons and not the entire year (with proportions of antibiotic prescribing that are influenza-associated being quite lower for the entire year compared to cold seasons), and many ILI episodes have aetiology other than influenza. Moreover, the average weekly rate of antibiotic prescribing to children aged under 5 years during the entire year in a study [Reference Cheysson8] (3268 prescriptions per week) is quite higher than the antibiotic prescribing rates to younger children in the US, which further suggests higher rates of antibiotic prescribing for respiratory infections including influenza to younger French children compared to younger children in the US. A study of 14 987 outpatients with an acute respiratory infection (ARI) during two influenza seasons in the US found that antibiotic prescribing to persons with laboratory-confirmed influenza accounted for 17% of all antibiotic prescribing for non-pneumonia ARI [Reference Havers1]. However, the contribution of influenza to the overall volume of antibiotic prescribing cannot be estimated from that study. Moreover, the study [Reference Havers1] only refers to ARI episodes during the 4.5-month period in the 2013–2014 influenza season (driven by a novel A/H1N1 variant [Reference Linderman9]) and the 5-month period in the 2014–2015 influenza season (a major influenza season driven by a novel A/H3N2 variant [Reference Skowronski10]) – thus, the average relative contribution of influenza to the volume of outpatient ARI prescribing during the entire year is expected to be much lower.
Individuals prescribed antibiotics for respiratory and other diagnoses are rarely tested for influenza, and the volume of influenza-associated antibiotic prescribing cannot be estimated directly from population-level clinical data. Using a previously developed statistical method [Reference Goldstein11, Reference Quandelacy12], we here estimate influenza-associated prescribing (prescribing stemming from influenza infections and their complications) by the proportion of all antibiotic prescribing that can be explained statistically by weekly variation in influenza incidence, and we evaluated this proportion for different diagnoses and different age groups of children and adults in Kaiser Permanente Northern California (KPNC) during 2010–2018. Our estimates are relevant for estimating some of the quantities mentioned above, namely (i) the effect of influenza vaccination on antibiotic prescribing, and the mitigation of antibiotic resistance [Reference Klugman and Black5]; (ii) the role of influenza aetiology in unnecessary/inappropriate antibiotic prescribing, particularly for respiratory illness [Reference Fleming-Dutra2–Reference Silverman4].
Methods
Proprietary data on antibiotic prescriptions (fills), related diagnoses, and influenza tests were extracted from the KPNC's Virtual Data Warehouse and Electronic Health Records [Reference Ross13].
Study population and setting
KPNC is an integrated health care system with a membership of approximately 4 million in 2018, including approximately 3 million members between 4 and 64 years of age. Members receive nearly all their KPNC-related medical care at KPNC facilities (unless referred to outside specialists), which include 46 medical clinics and 21 hospitals [Reference Gordon and Lin14, Reference Selby and Strom15]. KPNC members comprise more than 30% of the population in Northern California and are representative of the population's racial, ethnic, and socioeconomic distribution, although KPNC somewhat underrepresents those at the very lowest incomes [Reference Gordon and Lin14, Reference Gordon16].
Antibiotics and diagnoses
We extracted all antibiotic fills, and the diagnoses associated with those prescriptions, for KPNC members between September 2010 and August 2018. Antibiotic fills were those prescriptions that were actually picked up by, or delivered to, the patient. The diagnoses associated with the antibiotic prescriptions were classified into three categories: (1) all diagnoses; (2) ear infections; (3) respiratory diagnoses without an indication of a bacterial infection (such as a diagnosis of streptococcal pharyngitis) – see Tables S1 and S2 in the Supplementary Material for more details on diagnoses in categories (2), (3). The reason for considering respiratory diagnoses without an indication of a bacterial infection is that antibiotics are prescribed frequently and often inappropriately for such illness episodes [Reference Fleming-Dutra2–Reference Silverman4], and evaluation of the role of influenza aetiology for such antibiotic prescribing should further help to support the case for avoiding unnecessary prescribing for respiratory illness without a bacterial indication. Further details on the motivation behind the examination of diagnosis categories (1)–(3) are provided in the Discussion. Using these data, we calculated weekly rates of antibiotic prescriptions (fills) for the different categories of diagnoses in different age groups. The denominator for the rates of antibiotic prescribing was the KPNC weekly member-time for each of the respective age groups.
Influenza incidence
Weekly rates of respiratory tests positive for influenza A and for influenza B per 100 000 Kaiser Permanente members in different age groups were used as incidence proxies for influenza A and B in different age groups. The California Department of Health data for Public Health labs [17] for the San Francisco Bay Area and Northern California were used to estimate the (weekly) relative share of influenza A/H1N1 and A/H3N2 among influenza A specimens. Those weekly relative shares of influenza A/H3N2 and A/H1N1 among influenza A specimens were multiplied by the incidence proxies for influenza A described above to define incidence proxies for influenza A/H3N2 and A/H1N1 in different age groups.
Statistical inference
To estimate the volume of influenza-associated antibiotic prescribing for different diagnoses and age groups, we performed separate inference for antibiotic prescriptions for each of the three above categories of diagnoses in each of the 23 selected age groups (Results). Adapting previously developed methodology [Reference Goldstein11, Reference Quandelacy12], weekly rates of antibiotic prescribing for different diagnosis categories in different age groups were regressed linearly on the age-specific incidence proxies for the major influenza subtypes (A/H3N2, A/H1N1 and B), periodic weekly rates of antibiotic prescribing (baseline seasonality) that are not associated with influenza circulation modelled by trigonometric (sine and cosine) functions with annual periodicity and temporal trend terms (quadratic polynomials in week). In the regression model, we put a lag of up to one week between influenza incidence proxies and the rates of associated antibiotic prescriptions (eq. 1) under the assumption that there is the time between illness episodes and fills at Kaiser Permanente pharmacies for prescriptions for complications stemming from influenza infections.
During our study period, the circulating influenza A/H3N2 strains were replaced by a genetically different lineage during the 2014–2015 season that rendered previously used vaccines ineffective [Reference Skowronski10]. We, therefore, split the incidence proxy for influenza A/H3N2 into two separate proxies for the periods before and after the start of the 2014–2015 season. Similarly, starting from the 2013–2014 season, influenza A/H1N1 circulation was driven by novel strains [Reference Linderman9]. Accordingly, we split the incidence proxy for influenza A/H1N1 into two proxies for the periods before and after the start of the 2013–2014 season.
With the incidence proxies for the major influenza subtypes defined above and split into different time periods, the model equation is as follows: let R(t) be the weekly rates of prescribing of antibiotics for a given category of diagnoses (including all prescribing) per 100 000 persons in a given age group. We linearly regress

Here, for the covariates related to influenza incidence proxies, A(H3N2)10−14(t, s) is the incidence proxy for influenza A/H3N2 during the 2010–2014 period (so the proxy is set to zero for weeks beginning after 1 September 2014) lagged up to 1 week, so that A(H3N2)10−14(t,s) = s ⋅ A(H3N2)10−14(t) + (1 − s) ⋅ A(H3N2)10−14(t − 1), with the number 0 ≤ s ≤ 1 (used in the linear interpolation for the incidence proxies on two consecutive weeks) chosen to minimise the Akaike information criterion (AIC) score of the linear regression model in eq. 1. The covariates Baseline Seasonality(t) and Trend(t) are described in the 1st paragraph of this section.
Using this regression framework, we estimate the average annual rates of influenza-associated antibiotic prescribing for the studied categories of diagnosis in different age groups of individuals during 2010–2011 through the 2017–2018 influenza seasons. Influenza-attributable prescribing is estimated by the difference between the predicted value of the dependent variable (fills) with the observed levels of influenza incidence proxies to the predicted value when those proxies are set to zero, with confidence intervals that account for uncertainty in the regression coefficients. Confidence bounds for the model estimates are bootstrapped to account for potential residual auto-correlation as described in [Reference Goldstein11]. We also estimate the proportion of the overall antibiotic prescribing for those diagnoses in different age groups that are influenza-associated, with proportions of antibiotic prescribing that are influenza-associated being more representative nationally than the prescribing rates themselves.
Results
Children
Overall antibiotic prescribing
Rates of annual overall antibiotic prescribing per 1000 children during the study period ranged from 375 in children aged 10–14 years to 974 in children aged 1 year (12–23 months), Table 1. The estimated annual rates of influenza-associated antibiotic prescribing per 1000 children ranged from 9.1 (5–13) in aged 10–14 years to 18.1 (8.9–27.4) in aged 1 year. The proportion of influenza-associated antibiotic prescribing among all antibiotic prescribing was higher in children aged 5–17 years compared to children aged under 5 years, ranging from 1.4% [95% CI (0.7–2.1)] in aged <1 year to 2.7% (1.9–3.4) in aged 15–17 years.
Table 1. Overall and influenza-associated annual antibiotic prescribing rates per 1000 in different age groups of children, 2010–2018

Antibiotic prescribing for ear infection and respiratory diagnoses without a bacterial indication
Rates of antibiotic prescribing for ear infections generally declined with age, ranging from 24.2 per 1000 children aged 15–17 years to 430.6 per 1000 children aged 1 year (Table 2). Rates of antibiotic prescribing for respiratory diagnoses without a bacterial indication ranged from 57.8 per 1000 children aged <1 year to 132.9 per 1000 children aged 4 years (Table 2). The proportion of antibiotic prescribing for ear infections that was influenza-associated ranged from 3.1% (1.9–4.2) for children aged under 1 year to 9.2% (6.2–12.3) for children aged 15–17 years (Table 2). Rates of influenza-associated antibiotic prescribing for ear infections in children aged under 10 years were quite high (highest in children aged 1 year), with influenza-associated antibiotic prescribing for ear infections accounting for most of the overall influenza-associated antibiotic prescribing in those children (Table 2 vs. Table 1). The proportion of antibiotic prescribing for respiratory diagnoses without a bacterial indication that was influenza-associated ranged from 0.8% (0–2.3) for children aged 4 years to 4% (1.5–6.5) for children aged 15–17 years (Table 2), with rates of influenza-associated antibiotic prescribing for respiratory diagnoses without a bacterial indication being higher in children aged over 10 years vs. children aged under 10 years (Table 2).
Table 2. Overall and influenza-associated annual antibiotic prescribing rates for ear infections and for respiratory diagnoses without a bacterial indication per 1000 in different age groups of children, 2010–2018

Adults aged 18–59 years
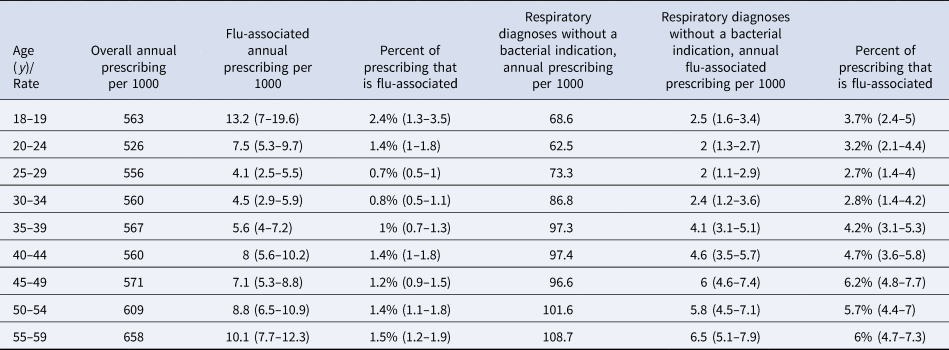
Rates of annual overall antibiotic prescribing per 1000 younger/middle-aged adults during the study period ranged from 526 in aged 20–24 years to 658 in aged 55–59 years (Table 3). The proportion of overall antibiotic prescribing that was influenza-associated ranged from 0.8% (0.5–1.2) in those aged 30–34 years to 2.4% (1.3–3.5) in those aged 18–19 years. (Table 3). Rates of influenza-associated antibiotic prescribing were highest in adults aged 18–19 years, followed by those aged 55–59 years. Between 49% and 84% of influenza-associated antibiotic prescribing in age groups of adults over 25 years was for respiratory diagnoses without a bacterial indication. The proportion of antibiotic prescribing for respiratory diagnoses without a bacterial indication that was influenza-associated ranged from 2.7% (1.4–4) in those aged 25–29 years to 6.2% (4.8–7.7) in those aged 45–49 years (Table 3).
Table 3. Overall and influenza-associated annual antibiotic prescribing rates per 1000 in different age groups of younger/middle-aged adults, as well as prescribing for respiratory diagnoses without a bacterial indication, 2010–2018
Adults aged over 60 years
Rates of annual overall antibiotic prescribing per 1000 older adults during the study period increased with age, from 724 in those aged 60–64 years to 1208 in those aged over 85 years (Table 4). The proportion of overall antibiotic prescribing that was influenza-associated was relatively stable across age groups of older adults, ranging from 1.1% (0.8–1.3) in those aged 75–79 years to 1.6% (1.2–1.9) in those aged 60–64 years (Table 4). Rates of influenza-associated antibiotic prescribing were highest in the oldest adults, reaching 18.5 (13.7–22.9) annual prescriptions per 1000 adults aged over 85 years. Between 45% and 62% of influenza-associated antibiotic prescribing in age groups of adults over 60 years was for respiratory diagnoses without a bacterial indication. The proportion of antibiotic prescribing for respiratory diagnoses without a bacterial indication that was influenza-associated ranged from 4.7% (3.8–5.7) in those aged 65–69 years to 6.6% (5.4–7.8) in those aged over 85 (Table 4).
Table 4. Overall and influenza-associated annual antibiotic prescribing rates per 1000 in different age groups of older adults, as well as prescribing for respiratory diagnoses without a bacterial indication, 2010–2018

Discussion
Influenza vaccination coverage in different age groups in the US has increased gradually with time [18]. Further increases in vaccination coverage have the potential to further mitigate influenza epidemics and influenza-related outcomes, including antibiotic prescribing. However, our understanding of the contribution of influenza to antibiotic prescribing in different age groups is limited. In this study, we evaluated the contribution of influenza to antibiotic prescribing for different indications in different age groups of children and adults in KPNC between 2010 and 2018. This contribution is related to both the effect of influenza vaccination on overall antibiotic prescribing, and the role of influenza aetiology in unnecessary/inappropriate antibiotic prescribing, particularly for respiratory illness [Reference Fleming-Dutra2–Reference Silverman4]. We found that for children aged under 18 years, the relative contribution of influenza to the overall volume of antibiotic prescribing was higher in children aged 5–17 years compared to children aged under 5 years, ranging from 1.4% [95% CI (0.7–2.1)] in aged <1 year to 2.7% (1.9–3.4) in aged 15–17 years. For adults aged over 20 years, the proportion of influenza-associated antibiotic prescribing among all antibiotic prescribing was lower than for children, ranging from 0.7% (0.5–1) for aged 25–29 years to 1.6% (1.2–1.9) for aged 60–64 years. Overall, our findings support the notion that the benefit of influenza vaccination for reducing the volume of antibiotic prescribing is expected to be modest overall, with that benefit being greatest in school-age children. Most of the influenza-associated antibiotic prescribing in children aged under 10 years was for ear infections, while in age groups of adults over 25 years, 45–84% of influenza-associated antibiotic prescribing was for respiratory diagnoses without a bacterial indication. The estimates of the contribution of influenza to antibiotic prescribing for respiratory diagnoses without a bacterial indication in persons aged over 25 years are important not only in terms of reducing influenza-associated antibiotic prescribing through vaccination but also in terms of reducing antibiotic prescribing for respiratory illness not involving evidence of a bacterial infection, with antibiotics being prescribed frequently and often inappropriately for such illness diagnoses [Reference Fleming-Dutra2–Reference Silverman4]. We also note in that regard that the estimated relative contribution of influenza to antibiotic prescribing for respiratory illness not involving evidence of a bacterial infection generally increased with age (Tables 2–4), which suggests that the likelihood of influenza-associated antibiotic prescribing for respiratory illness not involving evidence of a bacterial infection is increasing with age, and is highest for older adults. Finally, our estimates of the rates of influenza-associated antibiotic prescribing for ear infections in Table 2, which are quite high for children aged under 10 years provide further support for the benefit of wider influenza vaccination coverage for children for reducing antibiotic prescribing for ear infections in children and reducing the volume of illness related to ear infections, particularly acute otitis media (AOM) in younger children.
Our estimates of the relative contribution of influenza to the overall volume of antibiotic prescribing in children aged under 5 years (around 1.8%) are somewhat lower than the corresponding estimate (2.4%) in Scotland [Reference Fitzpatrick7]. Those differences might be potentially related to differences in the practices of antibiotic prescribing in the KPNC healthcare compared to the UK, with the latter practices in the UK changing with time [Reference Chae19]. We note that we estimated that around 2.6% of antibiotic prescribing to school-age children (aged 5–17 years) in the Kaiser Permanente population was influenza-associated. Influenza infection was detected in 4.4% of AOM episodes (25/566) in children in [Reference Patel20], and 5.3% of AOM episodes in children (24/456) in [Reference Heikkinen21], both studies extending throughout the year (not only influenza season). These findings are in good agreement with our estimates of the contribution of influenza to antibiotic prescribing for ear infections in children (Table 2).
Compared to estimates in children, e.g. studies [Reference Fitzpatrick7, Reference Patel20, Reference Heikkinen21], less is known about the relative contribution of influenza to antibiotic prescribing in adults, though this contribution is expected to be lower than the relative contribution of influenza to antibiotic prescribing in children because (i) influenza generally circulates more actively in children compared to adults (e.g. [Reference Monto22]); (ii) the relative share of antibiotic prescribing for respiratory causes and ear infections among all antibiotic prescribing is higher in children than in adults – compare Tables 1 and 2 vs. Tables 3 and 4 (with very limited antibiotic prescribing for ear infections in adults). This is consistent with our finding that the proportion of overall antibiotic prescribing that is influenza-associated is generally lower in adults than in children (Table 1 vs. Tables 3 and 4).
Our inference method has limitations, primarily in not being able to account for year-to-year variability in antibiotic prescribing associated with circulating viruses other than influenza, including the respiratory syncytial virus. Further work is needed to assess the contribution of different respiratory viruses to antibiotic prescribing, including the contribution of influenza to antibiotic prescribing in adults. Prescribing patterns at Kaiser Permanente may not be broadly generalisable because KPNC frequently tests for influenza; for example, physicians elsewhere may prescribe antibiotics for visits stemming from influenza infections (particularly undetected influenza infections) more frequently. Around 22% of all antibiotic prescriptions in the KPNC dataset are missing a diagnosis. This has no effect on the estimates of the contribution of influenza to overall antibiotic prescribing. It should also not bias the estimates of the proportion of antibiotic prescribing for respiratory illness without a bacterial indication and for ear infections that is influenza-associated (unless the likelihood of missing those diagnoses is different for influenza-associated cases vs. cases that were not influenza-associated). Some antibiotic prescriptions issued to KPNC members may not be recorded in the KPNC database. However, KPNC has a closed pharmacy system that ensures virtually complete capture of prescription drug dispensing. Patients with KPNC prescription drug benefits must purchase their medications at one of ~120 walk-in pharmacies or, since 1999, via mail order [Reference Parker23]. While it is possible to transfer a prescription to an out-of-plan pharmacy, a recent study in a similar patient population (Kaiser Permanente Colorado) found that the prevalence of out-of-plan pharmacy prescription transfers was still fairly low in 2011 (about 5%) even after the widespread introduction of bargain generic prescription programs beginning in 2006 [Reference Parker23, Reference Delate24]. Moreover, even if a fraction of antibiotic prescriptions issued to individuals who are KPNC members (including prescriptions by doctors who are not part of the KPNC healthcare) is not included in the dataset that we've used, this shouldn't bias the estimates of the proportion of antibiotic prescriptions that are influenza-associated unless influenza-associated antibiotic prescriptions have a different likelihood of being un-recorded in the KPNC database compared to antibiotic prescriptions that are not influenza-associated. Finally, for respiratory diagnoses without a bacterial indication, some of those illness episodes may actually involve (undiagnosed) bacterial infections, and the contribution of influenza aetiology to antibiotic prescribing is expected to be higher for respiratory diagnoses for which undetected bacterial infection is less likely. Thus, the estimates for respiratory diagnoses without a bacterial indication in our paper are meant to be conservative, namely that the relative contribution of influenza to antibiotic prescribing for respiratory illness with an unlikely bacterial infection may be (somewhat) higher than the corresponding estimates for respiratory diagnoses without a bacterial indication in our paper.
Our results provide estimates of the relative contribution of influenza to antibiotic prescribing for different diagnoses in different age groups which are compatible with related findings in the literature. Our results suggest an overall modest benefit of increasing influenza vaccination coverage for reducing antibiotic prescribing, with that benefit being greatest for school-age children, as well as the benefit of reducing unnecessary antibiotic prescribing for respiratory diagnoses with no bacterial indication in persons aged over 25 years, both of which may further contribute to the mitigation of antimicrobial resistance.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268822000371.
Financial support
This work was supported by the Wellcome Trust, Award # 219759/Z/19/Z (EG, BHF, NPK, ML, GTR), and by Award Number U54GM088558 from the National Institute of General Medical Sciences (EG, ML).
Conflict of interest
Dr Lipsitch reports grants from NIH/NCI, during the conduct of the study; grants from Pfizer, personal fees from Merck, personal fees from Bristol-Meyers Squibb, personal fees from Sanofi Pasteur, grants from NIH (US), grants from National Institute for Health Research (UK), grants from CDC (US), grants from Open Philanthropy Project, grants from Wellcome Trust, outside the submitted work. Dr Ray has received research support on grants through his institution in the past 3 years from Pfizer and the Industry PMR Consortium, a consortium of companies working together to conduct opioid analgesic-related postmarketing studies required by the Food and Drug Administration. Dr Klein reports research support from Sanofi Pasteur, Protein Science (now Sanofi Pasteur), GlaxoSmithKline, Pfizer and Merck. Other authors report no conflicts of interest.
Data availability statement
Data on the circulation of influenza subtypes in the San Francisco Bay Area/Northern California between 2010 and 2018 are publicly available from the California Department of Public Health, and can be accessed at https://data.chhs.ca.gov/dataset/influenza-surveillance.







