Clozapine is considered the antipsychotic agent of choice in the treatment of treatment-resistant schizophrenia, offering reduction in core positive symptoms for 50% of patients.Reference Lieberman, Saffermna, Pollack, Szymanski, Johns and Howard 1 These include hallucinations, delusions and thought disorder, as well as suicidality.Reference Kane, Honigfeld, Singer and Meltzer 2 , Reference Kane 3 However, clozapine use is associated with a risk of life-threatening agranulocytosis for 1–2% of patientsReference Alvir, Lieberman, Safferman, Schwimmer and Schaaf 4 and cardiotoxicity for 0.1–1.2% of patients.Reference Alawami, Wasywich, Cicovic and Kenedi 5 – Reference Layland, Liew and Prior 7 As such, treatment with clozapine is recommended for patients that have failed to respond to treatment with two antipsychotic medications given at appropriate doses and for a suitable duration.
As stated above, clozapine is associated with cardiotoxicity; specifically, clozapine use is often linked with the development of a sinus tachycardia and hypotension, the long-term significance of which as yet remains unclear. Of greater concern is the less common development of an acute myocarditis and/or pericarditis and a more chronic dilated cardiomyopathy.Reference Alawami, Wasywich, Cicovic and Kenedi 5 , Reference Berardis, Serroni, Campanella, Olivieri, Ferri and Carano 6 These conditions carry significant comorbidity and mortality. The vast majority of available literature examining the cardiac effects of clozapine involves studies in those with previously normal hearts; there is very little data to guide the safe use of clozapine in patients with established structural heart disease.
Hypertrophic cardiomyopathy (HCM) is the commonest inherited form of cardiovascular disease with an estimated prevalence of 1 in 500. It shows autosomal dominant transmission, and increased left ventricular wall mass is pathognomonic of the disease. Although the majority of those with phenotypic disease will have a benign course, HCM is associated with sudden cardiac death, syncope, stroke and heart failure;Reference Maron, Ommen, Semsarian, Spirito, Olivotto and Martin 8 findings of investigations, including resting electrocardiogram (ECG) and cardiac enzymes, as well as transthoracic echocardiography (TTE) are usually markedly abnormal.
This report presents our experience in initiating clozapine in a young man with treatment-resistant schizophrenia and concomitant complex cardiac disease and suggests a monitoring plan that could be used with similar patients with cardiac comorbidity.
Case presentation
The patient was a 36-year-old British male of Iranian decent with a diagnosis of treatment-resistant schizophrenia. He was single, unemployed and living with his family. He was referred for a trial of clozapine in the context of an established diagnosis of HCM (Fig. 1). At the time of admission, he presented with multimodal hallucinations, thought disorder, delusional beliefs and psychosomatic symptoms; he had no cardiovascular symptoms. On admission, he scored 40 on the Psychotic Symptom Rating Scales (PSYRATS)Reference Haddock, McCarron, Tarrier and Faragher 9 and 80 on the Depression Anxiety Stress Scale (DASS).Reference Lovibond and Lovidbond 10 He had previously trialled a number of antipsychotic agents with limited improvement, including aripiprazole, paliperidone, amisulpride and risperidone. Flupentixol and olanzapine had been moderately successful in treating his positive symptoms, but had to be stopped because of adverse motor effects. At the time of admission, he was on amisulpride 800 mg and sertraline 100 mg for low mood.
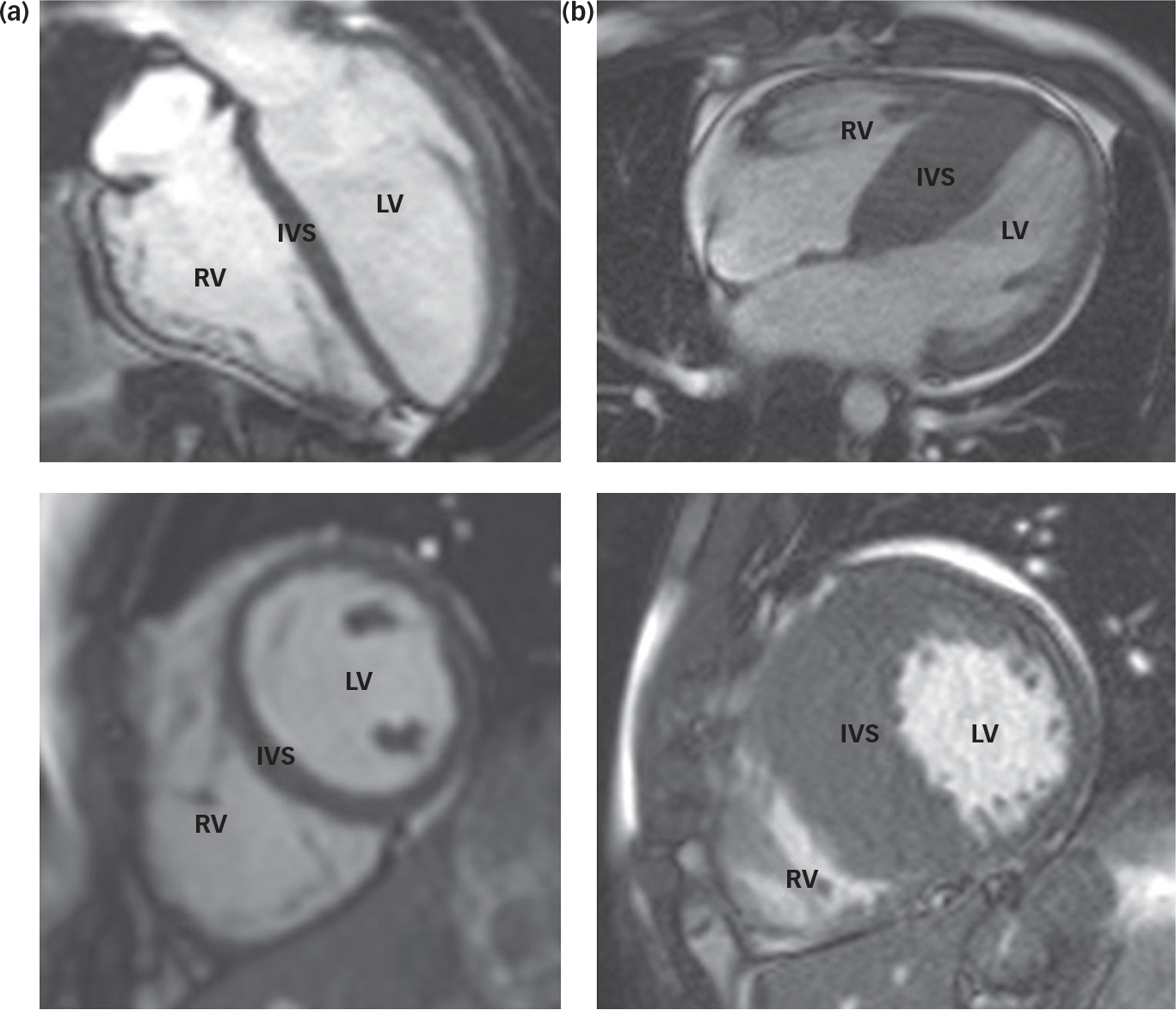
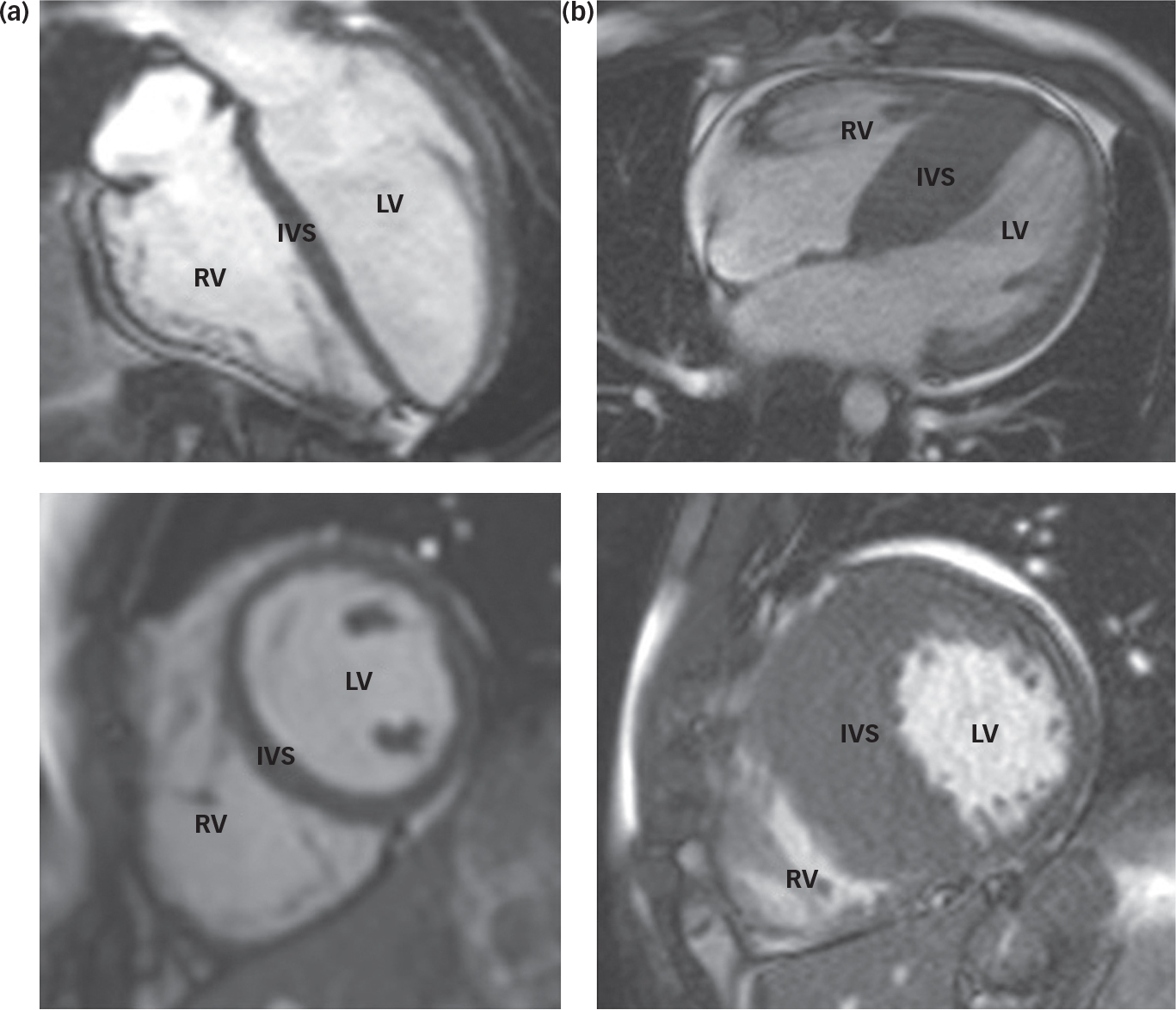
Fig. 1 Cardiac magnetic resonance images of a normal heart (a) and a heart demonstrating hypertrophic cardiomyopathy (b). Note the significant increase in muscle mass at the intraventricular septum (IVS) in the abnormal heart (b). LV – left ventricle; RV – right ventricle.
Relevant past medical history includes the diagnosis of HCM, made 3 years prior following the finding of an abnormal ECG. Further diagnostic investigations had included TTE, 24-h Holter monitoring and cardiovascular magnetic resonance imaging (CMR). During the 3-year follow-up period, the patient had been asymptomatic and was not felt to be at significant risk of sudden cardiac death (Table 1). He was taking bisoprolol 7.5 mg daily to treat hypertension – this being the agent of choice in those with HCM to help ameliorate the potential risk of arrhythmic demise and outflow obstruction associated with HCM. Other cardiovascular risk factors include a 17 pack/year smoking history and hypercholesterolaemia controlled with atorvastatin 40 mg daily.
Table 1 Major clinical risk factors for sudden cardiac death in HCM a

| Age (younger patients may be more at risk) |
|---|
| Non-sustained VT |
| LV hypertrophy >30 mm |
| Syncope |
| Family history of sudden death |
| Left atrial diameter |
| LV outflow tract obstruction |
| Blunted exercise BP response |
a From Elliot et al Reference Elliot, Anastasakis, Borger, Borggrefe, Cecchi and Charron 11
VT, ventricular tachycardia; LV, left ventricle; BP, blood pressure
The patient leads a moderately active lifestyle without limitation.
Investigations and initiation of clozapine
On arrival to our unit, the patient had a heart rate of 76 beats per minute (bpm) in sinus rhythm. A baseline ECG (Fig. 2) was carried out to monitor for any changes once clozapine was started. He was stable and asymptomatic at the time. Body mass index was high at 32 kg/mReference Kane, Honigfeld, Singer and Meltzer 2 . He was hypertensive at 145/90 mmHg. Baseline cardiac biomarkers showed a troponin of 54 ng/L (normal <16 ng/L) and creatine kinase of 224 IU/L (normal <150 IU/L).

Fig. 2 Electrocardiogram (ECG) showing sinus rhythm with a left ventricular hypertrophy pattern (a) with deep T-wave inversion (b) throughout typically seen in hypertrophic cardiomyopathy.
Cardiology advice was sought given the history of HCM and abnormal findings. The cardiology opinion concluded that the abnormalities in the cardiac biomarkers represented the patient's own normal baseline levels – given the context of his Iranian ethnicityReference Wong, Cobb, Umehara, Wolff, Haywood and Greenberg 12 and his increased cardiac muscle mass – and that it was therefore reasonable to initiate clozapine treatment. Clozapine was therefore started at 12.5 mg alongside his regular bisoprolol, which was hoped to help reduce the risk of tachycardia. Clozapine was then titrated up to 150 mg twice daily with regular monitoring. The patient was monitored through weekly troponin, creatine kinase and clozapine levels, as well as ECG every 3 weeks.
Figure 3 shows that during weeks 4–5 of clozapine up-titration, there was a spike in the troponin levels. At this point, the patient remained clinically well and was haemodynamically stable with a heart rate of 90 bpm and a blood pressure of 140/80 mmHg. Consultation with Cardiology reported that the spike represented a minor troponin leak which, in the absence of any other clinical changes, was not clinically significant. The advice was that we continue with up-titration and with continued blood testing. Figure 3 shows resolution of this event, and regular serum clozapine levels were also noted.
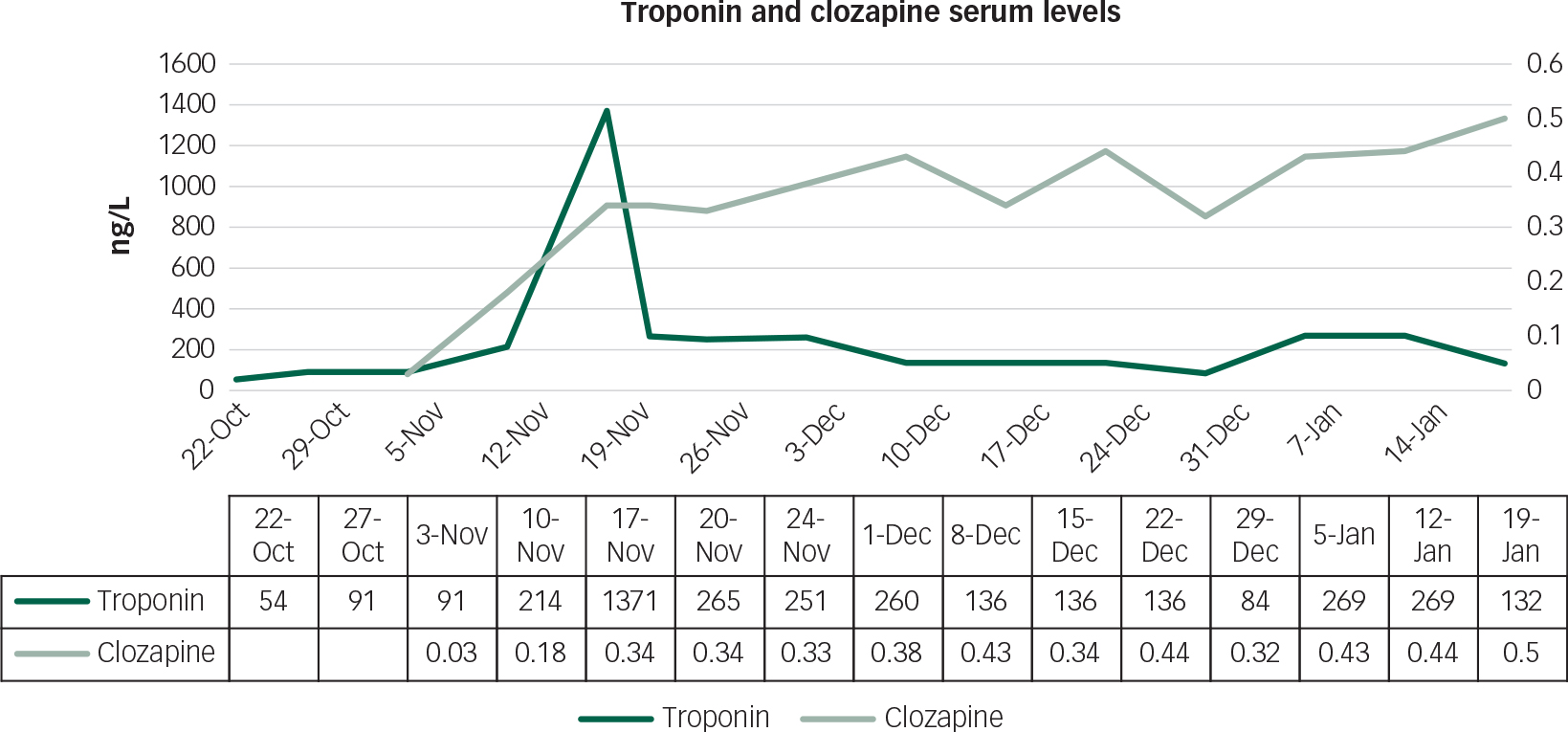
Fig. 3 Graph showing serum levels of both troponin and clozapine during starting and up-titration.
After 3 months of therapy, the patient remained well with a significant reduction in the level of his hallucinations, a cessation of his thought disorder, and decreases in the intensity of his delusional beliefs and psychosomatic symptoms. His scores on the PSYRATS reduced from 40 to 28 and from 80 to 42 on the DASS. He returned to a stable social functioning, with minor residual symptoms and increased levels of engagement and motivation. There has been no evidence of worsening of his cardiomyopathy, either clinically or with subsequent investigation. The patient has led a moderately active lifestyle, engaged well with other aspects of therapy and showed increased interest in his care.
Initiating clozapine therapy in patients with pre-existing HCM requires liaison with Cardiology for expert opinion, as well as close monitoring for any evidence of cardiac decompensation. There are currently no evidence-based guidelines for initiating clozapine in such patients; nonetheless, we show that clozapine can be initiated safely in patients with HCM with careful monitoring and titration.
In our case, despite evidence of structural cardiac disease, the patient was deemed to be at low risk for sudden cardiac death, without obstructive disease and with normal cardiac function. Clozapine was commenced at 12.5 mg and titrated up to 150 mg b.d. as per our own institution's guidelines; this included regular monitoring with ECG and blood testing. Baseline abnormalities required close liaison with our Cardiology colleague; however, the patient's course was uneventful and the treatment successful.
Despite its efficacy, cardiac side-effects of clozapine can generate concerns about its use in patients with pre-existing cardiovascular disease. Common side-effects include orthostatic hypotension, reported in up to 75% of treated patients,Reference Testani 13 in addition to a well-documented, benign tachycardia seen in up to 25% of patients.Reference Cohen, Loewenthal, Matar and Kotler 14
Clozapine is also associated with more severe forms of cardiac toxicity, most notably myocarditis,Reference Alawami, Wasywich, Cicovic and Kenedi 5 , Reference Layland, Liew and Prior 7 dilated cardiomyopathyReference Layland, Liew and Prior 7 and pericarditis.Reference Layland, Liew and Prior 7 Development of any of these conditions requires cessation of the causative agent but is often reversible with appropriate treatment. Acute myocarditis can be life-threatening however, and in some, it may not be reversible, leading to significantly increased long-term morbidity and mortality. Currently, there are no established risk factors by which to identify those who may be at increased risk of developing these significant side-effects, and as such, patients starting clozapine are subjected to regular investigations to monitor for any change. It remains unknown whether those with pre-existing cardiac disease are at increased risk.
In our patient, a mildly increased baseline troponin was felt to relate to the increased muscle mass of the left ventricle seen in HCM. Troponin is a more specific myocardial marker than creatine kinase, and the brief elevation during the course of clozapine up-titration probably represents a very mild, self-limiting myocarditis. The underlying mechanism of cardiotoxicity by clozapine remains unclear. Several different mechanisms have been proposed, and it is likely that a combination of these is causative in susceptible individuals. It remains to be determined whether the pathophysiological processes at play in HCM, itself a condition with a wide genotypic and phenotypic presentation, pose a greater short- and long-term risk for those concurrently treated with clozapine.
It is interesting to briefly mention the well-documented cardiotoxic effects of chemotherapeutic agents. Anthracyclines and HER2 antagonists are associated with the development of both acute and late onset cardiomyopathy.Reference Ewer and Ewer 15 Several different modes of action are thought to underlie these, some of which have also been described in animal models of clozapine cardiotoxicity.Reference Abdel-Wahab and Metwally 16 It may well be that a similar mechanism of action is attributable to all these agents, and as such, it is very interesting to also note that early treatment with angiotensin-converting enzyme (ACE) inhibitors (standard prognostic anti-heart failure medication) results in a protective effect against the development of cardiotoxicity in both animal models receiving clozapineReference Abdel-Wahab, Metwally, El-khawanki and Hashim 17 and humans receiving chemotherapy.Reference Cardinale, Colombo, Sandri, Lamantia, Colombo and Civelli 18 A new subspecialty has emerged, cardio-oncology, which facilitates best research, investigation and management in order to optimise the treatment and outcomes of those patients with cardiotoxic effects of chemotherapy. One pragmatic suggestion is the need for an analogous ‘cardio-psychiatric’ team involving, but not limited to, specialists in psychiatry and cardiology. Such a collaboration would provide the most comprehensive care, given the complexity of managing both pre-existing and iatrogenic cardiac disease because of antipsychotic medication. To that end, Table 2 sets out our suggested regime for the monitoring of cardiotoxicity in patients undergoing clozapine initiation and up-titration with known structural heart disease.
Table 2 Proposed monitoring regime in those with structural heart disease. Troponin elevation >2 times baseline

| Timeline | Investigations |
|---|---|
| At baseline | Temperature, CRP, troponin, ECG, TTE |
| Daily | Temperature, heart rate |
| 24-h post dose increase | CRP, troponin, ECG |
| In the event of ongoing fever or abnormal CRP/troponin | Temperature (CRP, troponin) ECG, TTE |
| 6 weeks post final dose increase | Temperature, CRP, troponin, ECG, TTE |
CRP, C-reactive protein; ECG, electrocardiogram; TTE, transthoracic echocardiography








eLetters
No eLetters have been published for this article.