ApoE is a polymorphic multifunctional protein with three common isoforms in man (E2, E3 and E4). ApoE3 is the wild type and most common isoform, while apoE4 carriers account for about 25 % of the Caucasian population(Reference Eichner, Dunn, Perveen, Thompson, Stewart and Stroehla1). Presence of the apoE4 allele is associated with a 40–50 % higher risk of CVD(Reference Song, Stampfer and Liu2) and apoE4 is the major known genetic risk factor for maturity-onset Alzheimer's disease(Reference Mahley, Weisgraber and Huang3). Although traditionally apoE has been classified as a mediator of lipoprotein metabolism and the increased CVD risk was attributed to higher LDL-cholesterol in apoE4 carriers(Reference Davignon, Gregg and Sing4, Reference Wilson, Myers, Larson, Ordovas, Wolf and Schaefer5), accumulating experimental evidence indicates that apoE is multifunctional and exerts many lipoprotein-independent activities in a wide range of tissues, including liver, macrophages and the brain. Among the putative functions, apoE is thought to act as an antioxidant, with in vitro assays indicating that it may do so in an isoform-dependent manner (apoE4 < apoE3 < apoE2)(Reference Miyata and Smith6). We have previously reported in a cell-culture model that macrophages expressing apoE4 demonstrate increased oxidative stress(Reference Jofre-Monseny, de Pascual-Teresa, Plonka, Huebbe, Boesch-Saadatmandi, Minihane and Rimbach7) and a pro-inflammatory response following stimulation(Reference Jofre-Monseny, Loboda, Wagner, Huebbe, Boesch-Saadatmandi, Jozkowicz, Minihane, Dulak and Rimbach8). Even though the differential antioxidant properties of apoE isoforms in vitro are convincing, there are limited in vivo data which show direct effects of apoE isoforms on markers of oxidative status. Nevertheless, it was observed that apoE4 carriers are more vulnerable to damage induced by tobacco smoking than non-apoE4 carriers(Reference Humphries, Talmud, Hawe, Bolla, Day and Miller9, Reference Talmud, Stephens, Hawe, Demissie, Cupples, Hurel, Humphries and Ordovas10) and that in individuals with cholesterol levels>5·6 mmol/l, apoE4 carriers presented 30 % higher plasma F2-isoprostane concentrations(Reference Dietrich, Hua, Block, Olano, Packer, Morrow, Hudes, Abdukeyum, Rimbach and Minihane11).
The impact of apoE genotype on the responsiveness to dietary fat manipulation (reduced total fat, reduced saturated fat, or reduced cholesterol) in human subjects has been extensively studied, with the greatest reduction in plasma cholesterol levels following reduced total and saturated fat and dietary cholesterol evident in the E4 subgroup(Reference Minihane, Jofre-Monseny, Olano-Martin and Rimbach12). Despite accumulating evidence that apoE4 carriers may be a subpopulation who would possibly benefit from dietary antioxidant supplementation(Reference Peroutka and Dreon13), to date, apoE genotype–antioxidant associations have not been systematically investigated in an intervention study.
Vitamin E consists of a group of isoprenoid compounds of plant origin, with α-tocopherol (α-Toc) being the most biologically active form(Reference Rimbach, Minihane, Majewicz, Fischer, Pallauf, Virgli and Weinberg14). There is experimental evidence supporting atheroprotective properties of vitamin E, both by acting as a free radical chain-breaking antioxidant, and thereby inhibiting LDL oxidation and isoprostane production(Reference Esterbauer, Schmidt and Hayn15–Reference Pratico, Tangirala, Rader, Rokach and FitzGerald17), but also by modulating cellular signalling in processes such as cytokine release by macrophages, NADPH oxidase activity and platelet aggregation(Reference Bulger, Helton, Clinton, Roque, Garcia and Maier18–Reference Rosenblat and Aviram20). All of these functions have been observed to be altered in the apoE4 genotype(Reference Miyata and Smith6–Reference Jofre-Monseny, Loboda, Wagner, Huebbe, Boesch-Saadatmandi, Jozkowicz, Minihane, Dulak and Rimbach8, Reference Dietrich, Hua, Block, Olano, Packer, Morrow, Hudes, Abdukeyum, Rimbach and Minihane11, Reference Riddell, Graham and Owen21). However, the results from randomised clinical trials, epidemiological investigations and animal studies on the use of vitamin E to prevent atherosclerosis are at times conflicting, and a recent meta-analysis reported increased mortality in clinical trial participants randomised to high-dose vitamin E, indicating that high dosage should be avoided(Reference Miller, Pastor-Barriuso, Dalal, Riemersma, Appel and Guallar22). It has been proposed that a possible explanation for the lack of effects of vitamin E in randomised clinical trials could be the inclusion of patients without biochemical evidence of increased oxidative stress(Reference Robinson, de Serna, Gutierrez and Schade23). We hypothesise that the apoE4 subgroup could possibly benefit from vitamin E supplementation, given that the alterations associated with the apoE4 genotype are potential targets of this vitamin.
In the present study, we examine the interactions of apoE genotype and dietary α-Toc on enzymic and non-enzymic markers of oxidative status and inflammation in vivo using targeted replacement mice expressing either the human apoE3 or apoE4 gene.
Materials and methods
Mice and diets
Mice, 6–8-week-old female homozygous apoE3 and apoE4 targeted replacement mice ‘humanised’ for the apoE gene, were purchased from Taconic Europe (Ry, Denmark). The apoE3 and apoE4 models were created by targeting the murine apoE gene for replacement with the human apoE3 and E4 allele in E14TG2a embryonic stem cells and injecting the targeted cells into blastocysts. Resultant chimeras were backcrossed to C57BL/6 for seven generations. The mice were then backcrossed once more and embryo transfer derived. The colony is maintained through incrossing of homozygotes, thus the animals used are genetically almost identical. Mice of both genotypes were assigned into one of two possible dietary groups (n 6), and were kept in macrolon cages at 21–25°C, with a 12 h day–night cycle. The diets and water were provided ad libitum for 12 weeks, food intake was measured daily and live weight was recorded weekly. Mice were kept according to the German Regulation of Animal Welfare with permission of the responsible authority. The basic diet (Table 1) was purchased from Ssniff Special Diets (Soest, Germany). Diets were supplemented with all-rac-α-tocopheryl acetate (0 or 200 mg/kg diet). Independent analysis for α-Toc content determined that the diets contained 3·4 mg/kg ( − α-Toc diet) and 235 mg/kg (+α-Toc diet) (SGS; Laboratory Services, Hamburg, Germany).
Table 1 Composition of the basic diet purchased from Ssniff Special Diets (Soest, Germany)
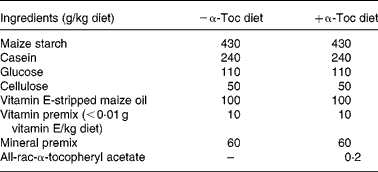
α-Toc, α-tocopherol.
Sample collection and tissue preparation
At the end of the 12-week dietary intervention period, mice were anaesthetised and killed by cervical dislocation. The blood was collected in heparinised tubes, and the plasma was separated by centrifugation (8000 g; 4 min; 4°C) and stored at − 80°C. Following removal of the plasma, the leucocyte layer was removed, and the erythrocytes were lysed in four times their volume of ice-cold HPLC-grade water and stored at − 80°C. Liver, lung, heart and musculus quadriceps femoris were removed and stored immediately at − 80°C. For the determination of thiobarbituric acid-reactive substances (TBARS), glutathione (GSH), antioxidative enzymes and vitamin E, total tissues were homogenised in 5 volumes of cold PBS solution and representative samples were taken for analysis. Samples were centrifuged (3500 g; 10 min; 4°C) and the supernatant fractions were used for analysis. The total protein content of tissue homogenates, plasma and erythrocyte lysates was measured with a bicinchoninic acid (BCA) assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Lipid levels and apolipoprotein E concentrations
Plasma total cholesterol as well as apoE concentrations were determined on an ILAB 600 automatic analyser (Instrumentation Laboratories UK Ltd, Warrington, Cheshire, UK), using commercially available spectrophotometric kits (Instrumentation Laboratories Ltd) and turbimetric immunoassay kits (Apolipoprotein E-HAWako; Alpha Laboratories Ltd, Eastleigh, Hants, UK).
α-Tocopherol concentration
To 750 μl of tissue homogenate, 1200 μl ethanol (1 % ascorbic acid) and 1500 μl hexane were added, and vortexed. After phase separation by centrifugation, the upper layer was collected and dried under N2 and the samples were re-suspended in methanol. For HPLC analysis, the mobile phase (methanol–water, 98:2, v/v) was isocratically delivered at a flow rate of 1·2 ml/min. α-Toc was quantified by an external standard curve (Calbiochem, Schwalbach, Germany) using a Jasco HPLC system (Jasco Corporation, Tokyo, Japan) with a Waters Spherisorb ODS-2 3 μm column (100 × 4·6 mm). The fluorescence detector was set to an excitation wavelength of 290 nm and emission wavelength of 325 nm.
Antioxidant enzyme activities and glutathione concentrations
Superoxide dismutase (SOD) activity was quantified spectrophotometrically by monitoring inhibition of pyrogallol auto-oxidation at 420 nm, 25°C and pH 8·2(Reference Marklund and Marklund24); 1 unit SOD was defined as the amount of enzyme that produced 50 % inhibition(Reference Marklund and Marklund24). Catalase (CAT) activity was determined by using the peroxidative function of the enzyme, and by measuring the formaldehyde production with 4-amino-3-hydrazino-5-1,2,4-triazole (Purpald) as chromogen. A standard curve of formaldehyde was used for quantification, and 1 unit of CAT was defined as the amount of enzyme producing 1 nmol formaldehyde per min at 25°C(Reference Johansson and Borg25). Se-dependent glutathione peroxidase (GPx) and glutathione reductase (GR) activities were measured by monitoring the disappearance rate of NADPH at 340 nm(Reference Cohen and Duvel26, Reference Lawrence and Burk27). For Se-dependent GPx, H2O2 was used as a substrate. For both enzymes, 1 unit was defined as the amount of enzyme which would consume 1 μmol NADPH/min. Total GSH concentration in tissues was determined by an enzymic recycling method(Reference Griffith28), following protein precipitation of tissue homogenates with sulfosalicylic acid. Glutathione disulfide was used to generate a standard curve, with absorption at 412 nm followed for 3 min. All determinations were carried out on a spectrophotometer (DU® 800; Beckman Coulter, Krefeld, Germany) except for CAT, which was analysed on an Infinite F200 plate reader (Tecan, Crailsheim, Germany). The tissue enzyme activities and GSH concentrations were expressed in relation to the amount of protein in the homogenates.
Thiobarbituric acid-reactive substances
Lipid peroxidation was assayed fluorometrically using the Infinite F200 plate reader (Tecan, Crailsheim, Germany) by determining TBARS in liver homogenates after protein precipitation and extraction in 1-butanol as described previously(Reference Rimbach and Pallauf29).
F2-isoprostanes
A commercial competitive immunoassay kit was used for the quantification of plasma 8-iso-PG F2α (Assay Designs, Ann Arbor, MI, USA) according to the manufacturer's instructions. The optical density of the samples was measured on the Infinite F200 plate reader (Tecan). The cross-reactivity for a number of related eicosanoid compounds was determined by the supplier of the ELISA kit and is rather low (PGF1α = 4·6 %; PGF2α = 1·85 %; PGE1 = 0·19 %; TXB2 = 0·023 %; PBG1 = 0·02 %).
Splenocyte mitogenic response
To assess the potential differential effects of apoE genotype and dietary α-Toc supplementation on lymphocyte proliferation, mitogenic proliferation assays in splenocytes were carried out. The whole spleen was harvested from each mouse and dissociated mechanically by cutting it into small pieces and pressing it through a 70 μm cell strainer (BD Biosciences, San Jose, CA, USA). After erythrocyte lysis with ammonium chloride potassium (ACK) lysing buffer, cells were washed twice with Roswell Park Memorial Institute (RPMI) buffer. A cell suspension of 1 × 106 cells in RPMI containing antibiotics, 10 % fetal bovine serum and 50 μm-2-mercaptoethanol was prepared and 180 μl were added per well in ninety-six-well plates. For splenic T-lymphocytes, concanavalin A (Con A) was employed, whereas splenic B-lymphocytes were stimulated using bacterial lipopolysaccharide (LPS)(Reference Champney, Prado, Youngblood, Appel and McMurray30). Cells were stimulated by adding 20 μl of medium containing LPS or Con A to obtain final concentrations of 0·1, 0·25, 0·5, 0·75 and 1 μg LPS/ml and 0·25, 0·5, 1 and 2·5 μg Con A/ml. After 72 h incubation, cell proliferation was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test. A quantity of 20 μl of MTT reagent (5 μg/ml in PBS) was added to each well, and the plate was incubated for 3 h at 37°C. At the end of the incubation period, the medium was removed and cells were solubilised with dimethyl sulfoxide (200 μl). The extent of MTT reduction to formazan within the cells was quantified by measuring the absorbance at 540 nm with a microplate reader and served as an index of cell viability(Reference Romano-Carratelli, Bentivoglio and Nuzzo31).
C-reactive protein
Plasma C-reactive protein (CRP) was measured as a marker of inflammation with a commercial kit (Dunn Labortechnik, Asbach, Germany) according to the manufacturer's instructions. This is a highly sensitive two-site enzyme-linked immunoassay detecting CRP concentrations between 0·39 and 25 ng/ml. Absorbance was measured at 450 nm on a plate reader (Labsystems iEMS Reader, Helsinki, Finland).
Statistical analysis
The calculation of statistical power was based on changes in tissue α-Toc levels according to the formula n>2F (σ/d)2, where n is the number of animals per group necessary to reach sufficient statistical power, σ is the standard deviation of the outcome measurement ( = 4 nmol/g tissue), d is differences between control and treatment which are considered to be significant on the basis of literature data ( = 10 nmol/g tissue); F equals 12·99 if a P value of 0·05 is set to indicate significant differences. Thus a sample size of >4·2 is required to measure a significant change (P < 0·05). Therefore, six animals per group were included in the trial. Statistical analysis was performed using SPSS version 13.0 (SPSS, Inc., Munich, Germany). Data were analysed for normality of distribution (Shapiro–Wilk test) and equality of variance (Levene test) before ANOVA analysis. Individual group means were compared post hoc by the Scheffé or Games–Howell tests. In the absence of normally distributed data, a Mann–Whitney U test was used. Two-way ANOVA was performed to test the independent effects of apoE genotype (apoE3 and apoE4), dietary α-Toc levels (0 or 200 mg/kg diet) and their interactions on the outcomes of interest. Data are expressed as mean values with their standard errors and significance was accepted at P < 0·05.
Results
Apolipoprotein E concentrations
Plasma apoE concentrations were 0·31 (sem 0·06), 0·36 (sem 0·09), 0·13 (sem 0·08) and 0·18 (sem 0·07) μg/mg protein for apoE3 − α-Toc, apoE3+α-Toc, apoE4 − α-Toc and apoE4+α-Toc, respectively. Although no statistical differences were observed when comparing the individual group means, apoE concentrations were significantly lower in the apoE4 mice (P = 0·043; two-way ANOVA). Dietary α-Toc did not affect plasma apoE concentrations.
α-Tocopherol concentrations in plasma and tissues
α-Toc concentration was measured in liver, lung, muscle, heart and plasma (Fig. 1). In general, large differences in α-Toc content were observed between the − α-Toc and +α-Toc groups (P < 0·01 for tissues; P < 0·05 for plasma) in both genotypes. The biggest increments were observed in the liver tissue with an approximate 20-fold difference in concentration between the − α-Toc and the +α-Toc groups. Equivalent differences between the two dietary groups from about 5- to 8·5-fold were evident in lung, muscle, heart and plasma Figs. 1(B), (C), (D) and (E)). In the four tissues analysed, lower amounts of α-Toc were observed in apoE4 in comparison with apoE3 mice, particularly in the +α-Toc groups. However, differences between genotype only reached statistical significance in the lung +α-Toc (P = 0·025) (Fig. 1 (B)). In contrast, α-Toc concentrations (normalised for cholesterol) showed a tendency to be higher in plasma in apoE4 mice (Fig. 1(D)).

Fig. 1 α-Tocopherol (α-Toc) concentrations in different tissues of apoE3 (□) and apoE4 (![]() ) mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; 200 mg/kg diet) with α-Toc. (A) Liver, (B) lung, (C) muscle, (D) heart, (E) plasma. Values are given as nmol α-Toc/g tissue or μmol α-Toc:mmol cholesterol (chol). Data are mean values (n 4–6) with their standard errors represented by vertical bars. The Mann–Whitney U test was used for statistical analysis. * Mean value was significantly different from that of the apoE3 mice fed the same diet (P < 0·05). Mean value was significantly different from that of the apoE4 mice fed the − α-Toc diet: † P < 0·05, †† P < 0·01. Mean value was significantly different from that of the apoE3 mice fed the − α-Toc diet: ‡ P < 0·05, ‡‡ P < 0·01.
) mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; 200 mg/kg diet) with α-Toc. (A) Liver, (B) lung, (C) muscle, (D) heart, (E) plasma. Values are given as nmol α-Toc/g tissue or μmol α-Toc:mmol cholesterol (chol). Data are mean values (n 4–6) with their standard errors represented by vertical bars. The Mann–Whitney U test was used for statistical analysis. * Mean value was significantly different from that of the apoE3 mice fed the same diet (P < 0·05). Mean value was significantly different from that of the apoE4 mice fed the − α-Toc diet: † P < 0·05, †† P < 0·01. Mean value was significantly different from that of the apoE3 mice fed the − α-Toc diet: ‡ P < 0·05, ‡‡ P < 0·01.
We have conducted additional experiments in order to test whether apoE gene replacement may affect vitamin E status in mice. However, liver α-Toc concentrations (20–30 nmol/g tissue) were not significantly different between wild-type (C57/BL6) and apoE transgenic mice.
Oxidative stress markers
The concentration of TBARS in the liver was greatly reduced (about 6-fold) by the addition of α-Toc to the diet (P < 0·01), but was not influenced by apoE genotype (Fig. 2(A)). The plasma concentration of F2-isoprostanes (Fig. 2(B)) was also decreased by α-Toc supplementation, although the effect was smaller (30 and 40 % reduction for apoE3 and apoE4, respectively) and did not reach statistical significance. Furthermore, a non-significant trend towards increased levels of F2-isoprostanes in apoE4 relative to the wild-type apoE3 animals was evident in the − α-Toc group.

Fig. 2 (A) Malondialdehyde (MDA) equivalents (nmol/g tissue) as assessed by thiobarbituric acid-reactive substances in the liver of apoE3 (□) and apoE4 (![]() ) mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; 200 mg/kg diet) with α-Toc. Data are mean values (n 6) with their standard errors represented by vertical bars. The Mann–Whitney U test was used for statistical analysis. †† Mean value was significantly different from that of the apoE4 mice fed the − α-Toc diet (P < 0·01). ‡‡ Mean value was significantly different from that of the apoE3 mice fed the − α-Toc diet (P < 0·01). (B) F2-isoprostanes (ng/ml) in plasma of apoE3 (□) and apoE4 (■) mice fed with a diet without ( − α-Toc; about 0 mg/kg diet) or supplemented (+α-Toc; about 250 mg/kg diet) with α-Toc. Data are mean values (n 5–6) with their standard errors represented by vertical bars. The Mann–Whitney U test was used for statistical analysis.
) mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; 200 mg/kg diet) with α-Toc. Data are mean values (n 6) with their standard errors represented by vertical bars. The Mann–Whitney U test was used for statistical analysis. †† Mean value was significantly different from that of the apoE4 mice fed the − α-Toc diet (P < 0·01). ‡‡ Mean value was significantly different from that of the apoE3 mice fed the − α-Toc diet (P < 0·01). (B) F2-isoprostanes (ng/ml) in plasma of apoE3 (□) and apoE4 (■) mice fed with a diet without ( − α-Toc; about 0 mg/kg diet) or supplemented (+α-Toc; about 250 mg/kg diet) with α-Toc. Data are mean values (n 5–6) with their standard errors represented by vertical bars. The Mann–Whitney U test was used for statistical analysis.
Antioxidant enzymes and glutathione
No differences were observed in the activities of antioxidative enzymes (SOD, CAT, GR, Se-dependent GPx) or GSH concentrations in the liver, lung and erythrocytes between individual groups (Table 2). Two-way ANOVA analysis detected no significant impact of apoE genotype, α-Toc intake, or apoE genotype × α-Toc interactions.
Table 2 Antioxidant enzyme activities and glutathione concentrations in liver, lung and erythrocytes of apoE3 and apoE4 targeted replacement mice fed with or without α-tocopherol (α-Toc) supplementation (200 mg/kg diet)*
(Mean values with their standard errors)

SOD, superoxide dismutase; CAT, catalase; GR, glutathione reductase; Se-GPx, Se-dependent glutathione peroxidase; GSH, total glutathione.
* No statistical significance between the groups, and no genotype, α-Toc, or genotype × α-Toc interaction effect was observed by two-way ANOVA. Significance was accepted at P < 0·05.
Splenocyte mitogenic response
Following stimulation with LPS or Con A for 72 h, B- and T-lymphocytes proliferated in a dose-dependent manner, reaching their maximum cell viability values at 0·75 μg LPS/ml and 2·5 μg Con A/ml for B-lymphocytes and T-lymphocytes, respectively. However, no differences were observed between the groups, indicating no genotype or α-Toc supplementation effects at any of the mitogen concentrations tested (Fig. 3).

Fig. 3 Mitogen-induced proliferation of splenocytes (B- and T-lymphocytes) in apoE3 and apoE4 mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; 200 mg/kg diet) with α-Toc: (□), apoE3 − α-Toc; (![]() ), apoE3+α-Toc; (
), apoE3+α-Toc; (![]() ), apoE4 − α-Toc; (
), apoE4 − α-Toc; (![]() ), apoE4+α-Toc. Splenocytes were incubated for 72 h with (A) lipopolysaccharide (LPS) (0–1 μg/ml) for B-lymphocyte stimulation or (B) with concanavalin A (0–2·5 μg/ml) for T-lymphocyte proliferation. Data are mean values (n 6) with their standard errors represented by vertical bars. Individual group means were compared by ANOVA with the Scheffé test.
), apoE4+α-Toc. Splenocytes were incubated for 72 h with (A) lipopolysaccharide (LPS) (0–1 μg/ml) for B-lymphocyte stimulation or (B) with concanavalin A (0–2·5 μg/ml) for T-lymphocyte proliferation. Data are mean values (n 6) with their standard errors represented by vertical bars. Individual group means were compared by ANOVA with the Scheffé test.
C-reactive protein
In the − α-Toc groups, apoE4 mice showed 30 % lower levels of CRP than apoE3 mice (P = 0·014) (Fig. 4). Although the same trend was observed in the +α-Toc groups, statistically significant differences disappeared between apoE3 and apoE4 mice, as CRP levels in the apoE3 decreased. However, the differences in apoE3 mice due to dietary α-Toc supplementation did not reach statistical significance. Two-way ANOVA revealed a significant (P = 0·006) and near-significant (P = 0·052) genotype and α-Toc effect, respectively.

Fig. 4 Plasma C-reactive protein measured in apoE3 (□) and apoE4 (![]() ) mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; about 200 mg/kg diet) with α-tocopherol. Data are mean values (n 5–6) with their standard errors represented by vertical bars. Individual group means were compared by ANOVA with the Games–Howell test. * Mean value was significantly different from that of the apoE3 mice fed the same diet (P < 0·05). There was a genotype effect determined by two-way ANOVA (P < 0·01). There was a near-significant α-Toc effect determined by two-way ANOVA (P = 0·052).
) mice fed with a diet without ( − α-Toc; 0 mg/kg diet) or supplemented (+α-Toc; about 200 mg/kg diet) with α-tocopherol. Data are mean values (n 5–6) with their standard errors represented by vertical bars. Individual group means were compared by ANOVA with the Games–Howell test. * Mean value was significantly different from that of the apoE3 mice fed the same diet (P < 0·05). There was a genotype effect determined by two-way ANOVA (P < 0·01). There was a near-significant α-Toc effect determined by two-way ANOVA (P = 0·052).
Discussion
The interaction of apoE genotype with dietary lipid composition has been widely studied(Reference Minihane, Jofre-Monseny, Olano-Martin and Rimbach12). The interactions of antioxidant supplementation and apoE genotype, however, remain to be systematically examined. It was hypothesised that due to the increased oxidative stress associated with the apoE4 allele, greater sensitivity to dietary vitamin E and responsiveness to vitamin E supplementation would be evident in apoE4 carriers(Reference Peroutka and Dreon13). However, to date, this remained speculation, since there are no published reports on the effects of dietary vitamin E on tissue oxidant or antioxidant status in vivo according to the apoE genotype.
In the present study, targeted replacement mice expressing the human apoE3 and apoE4 were fed with a diet poor (about 0 mg/kg diet; − α-Toc) or rich (about 200 mg/kg diet; +α-Toc) in α-Toc for 12 weeks. This would be equivalent to marginal dietary vitamin E supply and about 1500–2000 mg/d of vitamin E in a 70 kg human. The +α-Toc dose is not achievable with a normal diet, but it is considered within the range of high-dosage supplementation(Reference Miller, Pastor-Barriuso, Dalal, Riemersma, Appel and Guallar22, Reference McGavin, Mann, Skeaff and Chisholm32).
As has been observed in human subjects(Reference Haddy, De Bacquer and Chemaly33, Reference Schiele, De Bacquer and Vincent-Viry34), targeted replacement apoE4 mice exhibited lower plasma apoE concentrations in comparison with apoE3 mice. In the present study we first investigated the impact of apoE genotype on the tissue distribution of α-Toc by measuring its concentration in plasma, liver, lung and muscle. The present results show that plasma levels of α-Toc (normalised for cholesterol) are modestly higher in apoE4 mice. These findings are in agreement with Gomes-Coronado et al. who observed a non-significant 9 % increase in lipid-adjusted plasma vitamin E levels in male E4 carriers compared with E3 homozygotes in human subjects(Reference Gomez-Coronado, Entrala, Alvarez, Ortega, Olmos, Castro, Sastre, Herrera and Lasuncion35). Furthermore, in a vitamin E kinetics study it was observed that following administration of 150 mg of 2H-labelled RRR-α-Toc, plasma levels of labelled α-Toc were much higher in apoE4 carriers(Reference Lodge, Hall, Jeanes and Proteggente36). As suggested by the authors, in apoE4 carriers α-Toc may be retained in the LDL particles and not incorporated in the tissues, which would explain the tendency towards higher plasma levels. Indeed, in all the tissues analysed in the present study, lower concentrations of α-Toc were evident, with the biggest differences observed in the lung. This finding is consistent with a previous in vitro study in macrophages, where a trend towards lower α-Toc concentrations was observed in apoE4 compared with apoE3 transfected cells(Reference Jofre-Monseny, de Pascual-Teresa, Plonka, Huebbe, Boesch-Saadatmandi, Minihane and Rimbach7). We hypothesise that the lower cell and tissue α-Toc levels in apoE4 may be due to the lower uptake of α-Toc from LDL or HDL (which represent the predominant circulating form of α-Toc) by the scavenger receptor class B type 1 (SRB1). ApoE acts as a high-affinity ligand for SRB1(Reference Li, Kan, Lavrentiadou, Krieger and Zannis37), and SRB1 mediates α-Toc uptake from mainly HDL particles in several tissues(Reference Mardones and Rigotti38). Although the binding of the apoE to SRB1 is isoform independent(Reference Li, Kan, Lavrentiadou, Krieger and Zannis37), apoE4 shows preferential association with VLDL in contrast to apoE3 which shows a preference for large HDL particles. This could have a detrimental impact on the binding of HDL to SRB1. Probably, the relative importance of the different α-Toc uptake pathways in the tissues explains why the genotype influence is stronger in the lung. In SRB1 knock-out mice, it was observed that plasma α-Toc increased, while α-Toc levels decreased in some tissues (testes, ovary, lung and brain), but not in others (liver, kidney, spleen and fat)(Reference Mardones, Strobel, Miranda, Leighton, Quinones, Amigo, Rozowski, Krieger and Rigotti39). In addition, there are further studies that support the role of pneumocyte SRB1 expression as an important mechanism of physiological regulation of pulmonary α-Toc uptake(Reference Kolleck, Schlame, Fechner, Looman, Wissel and Rustow40). Furthermore, SRB1 is abundantly expressed in atherosclerotic lesions(Reference Hirano, Yamashita and Nakagawa41). In line with this finding, Mas et al. support the hypothesis that impairment of lipophilic antioxidant delivery in neuronal cells may facilitate oxidative stress in Alzheimer's disease(Reference Mas, Dupuy, Artero, Portet, Cristol, Ritchie and Touchon42). However, it is recognised that increased tissue α-Toc utilisation due to increased oxidative stress in apoE4 carriers could also contribute to lower tissue α-Toc concentrations. In order to examine this possibility, oxidation metabolites of α-Toc such as α-tocopherylquinone should be measured in future studies.
Apart from the effects of apoE genotype on α-Toc concentrations, it could be hypothesised that apoE4-associated increases in oxidative stress may be related to depletion of the endogenous antioxidant defence system. Tissues were analysed for concentrations of GSH, the most important water-soluble intracellular antioxidant, and for the activities of antioxidant enzymes that participate in the detoxification of free radicals and recycling of GSH, including SOD, CAT, GPx and GR. None of these parameters was modified by apoE isoform or α-Toc supplementation. To our knowledge, there is limited information in the literature regarding the effects of apoE genotype on the antioxidant ‘defence system’. Available data are derived from Alzheimer's disease patients, in which no differences were observed between genotypes in plasma and erythrocyte GSH, SOD and GPx(Reference Fernandes, Proenca, Nogueira, Grazina, Oliveira, Fernandes, Santiago, Santana and Oliveira43). In contrast, Ramassamy and co-workers observed increased CAT and GPx levels in the frontal cortex of Alzheimer's disease patients, with no genotype effect on SOD and GSH levels(Reference Ramassamy, Averill and Beffert44). However, it is difficult to compare these data with the present results, as the disease has profound effects on the oxidative status of the patients. Furthermore, in the brains of healthy and not externally stressed apoE3 and apoE4 mice, enzymic measurements of oxidative stress-related enzymes (GPx, GR, GSH transferase, CAT and SOD) revealed no significant differences between apoE3 and apoE4 animals(Reference Ophir, Amariglio, Jacob-Hirsch, Elkon, Rechavi and Michaelson45), which is in accordance with the present data. In addition, no significant effects could be observed when comparing GSH concentration in apoE3 v. apoE4 macrophages in the absence and presence of α-Toc(Reference Jofre-Monseny, de Pascual-Teresa, Plonka, Huebbe, Boesch-Saadatmandi, Minihane and Rimbach7). All together, these data indicate that the depletion of GSH and the antioxidant enzyme activities may not play a role in the observed apoE genotype–chronic disease associations.
Markers of oxidative stress were measured in response to dietary α-Toc and apoE genotype. Isoprostanes are chemically stable PG isomers that result from oxidative modification of arachidonic acid through a mechanism catalysed by free radicals, and are used as markers of oxidative stress and lipid peroxidation(Reference Patrono and FitzGerald46). In a previously reported human study that included both smokers and non-smokers, apoE4 carriers with elevated plasma cholesterol concentrations exhibited 30 % higher circulating levels of F2-isoprostanes compared with non-carriers. No impact of genotype was evident in subjects with normal cholesterol levels(Reference Dietrich, Hua, Block, Olano, Packer, Morrow, Hudes, Abdukeyum, Rimbach and Minihane11). In the present trial, although a trend towards higher F2-isoprostanes was evident in the apoE4 animals, the results did not reach statistical significance.
In addition, malondialdehyde, a decomposition product of unstable peroxides derived from PUFA, was measured in the liver using the TBARS assay. One of the limitations of this assay is the lack of specificity, since thiobarbituric acid also reacts with aldehydes. The present results show clearly that α-Toc is a very strong inhibitor of the malondialdehyde production in the liver. However, although apoE is mainly produced in the liver(Reference Sullivan, Mezdour, Aratani, Knouff, Najib, Reddick, Quarfordt and Maeda47) no differences in TBARS levels could be observed between apoE3 and apoE4 mice.
The fact that we only observed trends towards increased oxidative stress in apoE4 mice is not entirely unexpected. The limited number of studies available show that the increased CVD risk associated with the apoE4 allele is most evident in smokers(Reference Humphries, Talmud, Hawe, Bolla, Day and Miller9, Reference Talmud, Stephens, Hawe, Demissie, Cupples, Hurel, Humphries and Ordovas10, Reference Talmud48, Reference Lahoz, Schaefer, Cupples, Wilson, Levy, Osgood, Parpos, Pedro-Botet, Daly and Ordovas49), suggesting that the impact of genotype is most evident when the body is in a state of increased oxidative stress. Furthermore, the present results suggest that the gene–environment interactions observed between apoE4 and smoking could be partly mediated or exacerbated by the fact that apoE4 cannot deliver α-Toc and possibly other lipophilic antioxidants to body tissues as efficiently as apoE3.
ApoE has not only antioxidative properties, but has also been shown to play a role in the homeostasis of the immune response in vivo, with macrophage-secreted apoE known to modulate the activity of adjacent lymphocytes in the developing plaque. Adaptive immunity mediated by T- and B-lymphocytes has been shown to play a role in modulating atherosclerosis(Reference Zhou, Nicoletti, Elhage and Hansson50, Reference Major, Fazio and Linton51). Proliferation of lymphocytes following exposure to mitogenic stimuli is a well-established method for assessment of cell-mediated immunity. There are a limited number of studies which show that apoE inhibits T-cell proliferation induced by several mitogens(Reference Kelly, Clay, Mistry, Hsieh-Li and Harmony52, Reference Mistry, Clay, Kelly, Steiner and Harmony53). However, to date, there is no information on potential isoform-dependent effects of endogenous apoE on lymphocyte proliferation. We performed mitogenic response assays in splenocytes isolated from apoE3 and apoE4 mice following addition of Con A, a T-cell-, and LPS, a B-cell mitogen. ApoE is abundant in the spleen of this mouse model(Reference Sullivan, Mezdour, Aratani, Knouff, Najib, Reddick, Quarfordt and Maeda47) and, therefore, splenocytes were used to assess the effects of intrinsic apoE. The present results show that there were no differences in proliferation of splenocytes between apoE genotypes. These data support the results of Laskowitz et al. (Reference Laskowitz, Lee, Schmechel and Staats54), who showed that addition of recombinant apoE had a strong effect on suppression of mononuclear cell proliferation, but no differences could be observed between the apoE3 and apoE4 isoform. Overall, these data suggest that the effects of apoE isoforms on lymphocyte proliferation may not be a major factor in the increased cardiovascular risk observed in apoE4 carriers.
We have recently shown that macrophages engineered to produce human apoE4 have an increased inflammatory response to LPS stimulation, and secrete increased cytokine levels relative to apoE3 macrophages(Reference Jofre-Monseny, Loboda, Wagner, Huebbe, Boesch-Saadatmandi, Jozkowicz, Minihane, Dulak and Rimbach8). The most widely used marker of acute inflammation in humans is CRP, which, although the data are not fully consistent, is now considered by many to be a useful independent predictor of future cardiovascular risk(Reference de Ferranti and Rifai55, Reference Paffen and DeMaat56). The majority of circulating CRP is thought to be from hepatic sources, with CRP production induced in response to systemic cytokines such as IL6(Reference de Ferranti and Rifai55). According to our in vitro results and the increased CVD risk observed in apoE4 carriers, it might be expected that apoE4 mice would have increased plasma CRP. However, the present results show that in the − α-Toc groups, apoE4 mice exhibited decreased CRP concentrations. Although these results are unexpected, they are in full agreement with recent data from human studies, indicating increasing circulating CRP in the following order apoE4 < E3 < E2(Reference Marz, Scharnagl, Hoffmann, Boehm and Winkelmann57–Reference Judson, Brain, Dain, Windemuth, Ruano and Reed59). For example, in the study of Judson and co-workers, a ratio of 1·7 was evident between circulating CRP concentrations in apoE3/apoE3 v. apoE3/apoE4 individuals(Reference Judson, Brain, Dain, Windemuth, Ruano and Reed59), which is comparable with the ratio of 1·5 observed in our − α-Toc-fed mice. The present results have two implications. On the one hand, they support the usefulness of humanised apoE transgenic mice as a model to study the impact of apoE genotype. On the other hand, they support the concept reported by Maerz and co-workers, who suggest that CRP synthesis does not respond only to cytokines, but may also be related to the mevalonate–cholesterol synthesis pathway, which might be down regulated in apoE4 carriers. In consequence, the use of CRP as a predictor of CVD risk may lead to risk underestimation in apoE4 carriers(Reference Marz, Scharnagl, Hoffmann, Boehm and Winkelmann57).
However, in the +α-Toc groups, the genotype effect disappeared. α-Toc supplementation resulted in about a 30 % decrease of CRP in apoE3 mice, with an overall α-Toc effect reaching borderline significance (P = 0·052). This finding is in accordance with a recent cross-sectional study showing reduced CRP levels in women following vitamin E supplementation(Reference Scheurig, Thorand, Fischer, Heier and Koenig60). No impact of α-Toc on CRP concentrations was evident in apoE4 animals. Whether this was attributable to the fact that the influence of the apoE4 allele ‘overwhelmed’ the potential effects of α-Toc on CRP production, or that α-Toc reached lower tissue concentrations at the site of cytokine production, remains to be determined.
Conclusions
Our data indicate that apoE genotype does not play a major role in the tissue oxidant and antioxidant status and in the modulation of lymphocyte proliferation. Moreover, it is hypothesised that in order to observe detrimental effects of apoE4 on oxidative status in vivo, an additional source of oxidative stress (such as smoking or low fruit and vegetable consumption) may be necessary. Finally, CRP is modified by the apoE genotype in targeted replacement mice in the same manner as in humans, with apoE4 mice showing lower CRP levels. This finding suggests that the increased CVD risk in apoE4 carriers associated with modestly increased circulating LDL-cholesterol, macrophage-mediated inflammation and oxidative stress may be in part negated by lower CRP levels in this genotype subgroup.
Acknowledgements
The present study was supported by a grant of the German Ministry of Education and Science (BMBF 0313856A). G. R. and A.-M. M. designed the study. L. J.-M. and P. H. conducted the feeding trial. L. J.-M. P. H., I. S., C. B.-S., J. F. and K. J. performed the analyses. L. J.-M., A.-M. M. and G. R. wrote, edited, and reviewed the final manuscript. None of the authors have a known conflict of interest.








