Introduction
In the active limestone quarry of Winterswijk (Eastern Netherlands, Fig. 1), micritic limestone of Anisian (Middle Triassic, c. 247.2–242 Ma) age is being commercially exploited. In order to reach the Anisian strata, an overburden of respectively Rhaetian (Late Triassic) claystone, Rupelian (early Oligocene) clay and Late Pleistocene boulder clay has to be removed; it is mostly discarded. In 1989, Rhaetian infillings of a sinkhole (subrosion pipe) within the Anisian sediments were discovered and their contents studied. The sediment plug had a diameter of about 30 m and had a total height of about 2 m (Oosterink et al., Reference Oosterink, Simon and Winkelhorst2005, Reference Oosterink, Simon, Hagdorn and Winkelhorst2006; Klompmaker & Van den Berkmortel, Reference Klompmaker and Van den Berkmortel2007). Several papers described the fossil content of the subrosion infill. Herngreen et al. (Reference Herngreen, Van Konijnenburg-van Cittert and Oosterink2005) and Klompmaker et al. (Reference Klompmaker, Herngreen and Oosterink2010) described palynomorphs. Klompmaker et al. (Reference Klompmaker, Herngreen and Oosterink2010) also described bivalves. Additionally, some Hettangian (Earliest Jurassic) psiloceratid ammonites were discovered (Klompmaker & Van den Berkmortel, Reference Klompmaker and Van den Berkmortel2007). More recently, Diependaal & Reumer (Reference Diependaal and Reumer2021) described the Rhaetian chondrichthyan and actinopterygian remains found in this material. The subrosion pipe and its infill cannot be sampled anymore since its removal due to the advancing quarry face.

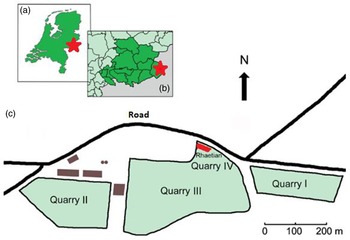
Figure 1. The location of the the Winterswijk quarry and its Rhaetian deposit. a. In the eastern Netherlands, b. In the Achterhoek area, c. Schematic plan of the quarry with the Rhaetian deposit in the northernmost part of quarry IV. Adapted after Klompmaker et al. (Reference Klompmaker, Herngreen and Oosterink2010).
In 2004, a second and considerably larger Rhaetian deposit consisting of black claystones was found in situ in the northern face of the quarry (Fig. 2). Its age was established based on discovered palynomorphs (Herngreen et al., Reference Herngreen, Van Konijnenburg-van Cittert and Oosterink2005). Thus, the age of the material is here tentatively considered to be Rhaetian (c. 208.05–201.36 Ma; Galbrun et al., Reference Galbrun, Boulila, Krystyn, Richoz, Gardin, Bartolini and Maslo2020), although no absolute dating has so far been performed and a possible latest Late Norian age cannot be explicitly excluded. An ongoing research project into the sedimentology and palynology of the deposits may provide a better age constraint. Hence, we use the term Rhaetian with some caution.

Figure 2. The exposure of the Rhaetian in the northern part of quarry IV. The unconformity between the black Rhaetian and the grey micritic Anisian limestone is well visible on the bottom the trench.
So far, the only description of macrofossils from the newly discovered Rhaetian deposits in Winterswijk concerns ophiuroid echinoderms (Thuy et al., Reference Thuy, Klompmaker and Jagt2012). Here, we describe chondrichthyan and actinopterygian remains sampled in 2018. They are compared with the material from the subrosion pipe (Diependaal & Reumer, Reference Diependaal and Reumer2021) and with the abundantly preserved Rhaetian fish material from the British Penarth Group (Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). The material here described concerns the first in situ Rhaetian from the Netherlands, and it considerably increases our knowledge of the Dutch Triassic.
Material and methods
The studied material had been collected during an excavation in 2018 organised by Naturalis Biodiversity Center and Utrecht University in collaboration with the Muschelkalk Working Group. In 2019, the collected samples that had temporarily been stored away were soaked in Mullrose cleaning vinegar (9% acetic acid), washed and sun-dried, and the residue was then hand-picked for fossils under a binocular microscope. For some of the fossils, some extra treatment was needed in order to clean them better. This was again done by using acetic acid (Jeppsson et al., Reference Jeppsson, Anehus and Fredholm1999). The fossils did not need any further treatment for their preservation. The material is stored in the collection of the Department of Earth Sciences (Utrecht University). The samples have the uniform collection code WWR18 (for Winterswijk Rhaetian 2018), and individual teeth and scales are numbered (e.g., WWR18-052).
Terminology for the Lissodus minimus teeth is after Duffin (Reference Duffin1985, Reference Duffin, Swift and Martill1998a). The terminology for the dermal denticles is after Duffin (Reference Duffin, Swift and Martill1998a).
The teeth are described/categorised based on their morphology; their size is less relevant as fishes change teeth throughout their lifetime (Botella et al., Reference Botella, Valenzuela-Ríos and Martínez-Pérez2009) and as it depends on the position of the teeth (e.g., distal or mesial) in the heterodont dentitions (Duffin, Reference Duffin1985). The remains were only counted when at least two-thirds of the fossil including the major cusp were present and recognisable. The teeth of Lissodus minimus were only counted when the central part combined with at least one of the mesial or distal ‘wings’ are present. The photographs were made by using a Keyence VHX-500 digital microscope.
Systematic paleontology
Class Chondrichthyes (Huxley, 1880)
Order Hybodontiformes (Patterson, 1966)
Genus Lissodus (Brough, 1935)
Duffin (Reference Duffin1985) published the following diagnosis: ‘Teeth up to 7 mm long, showing moderate heterodonty. The principal central cusp is highest in mesial and anterolateral teeth. The labial peg is well developed in mesial teeth, but becomes progressively weaker laterally through the dentition. The occlusal crest is moderate. Lateral cusplets may be developed (up to five pairs). The crown is robust and low in lateral teeth. The crown may be ornamented by moderate vertical ridges. The crown–root junction is deeply incised in mesial teeth, but becomes progressively less so laterally. Specialised foramina are present.’
Rees & Underwood (Reference Rees and Underwood2002) emended the diagnosis for the genus Lissodus as follows: ‘Jaws deep, lower jaw tapering anteriorly; anterior teeth with moderately to well-developed central cusp, occlusal crest and labial protuberance; occlusal face of labial protuberance sloping gently towards crown base; crown shape almost triangular in occlusal view; lateral teeth lower, larger, more mesio-distally expanded; cusps, occlusal crest, and labial protuberance poorly developed; root lingually inclined, lower than crown, not as voluminous; single, strictly horizontal row of small circular foramina near crown–root junction; basal plate of cephalic spines ‘T-shaped’ with terminally expanded lobes.’
Lissodus minimus (Agassiz, Reference Agassiz1836)
Fig. 3A–I
At least 800 teeth in our sample can be attributed to this species. They are low-crowned, wide, crescent-shaped and show moderate heterodonty. The central cusp is the highest one; depending on the position in the jaw there may be none, one, two or three lateral cusplets. A labial peg is generally present, but its development varies, as does the presence of striations.
Here, we differentiate four morphotypes. They differ morphologically in relation to the position of the teeth in the upper and lower jaws: more distal (close to the symphyseal symmetry axis) or more mesial.
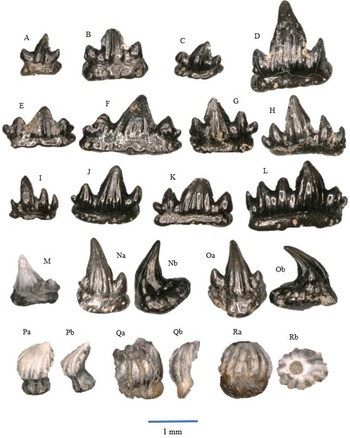
Morphotype I (Fig. 3A-C). The crown is narrow in labial–lingual direction, and in mesial–distal direction the teeth are wide. There is one centrally placed main cusp and one lateral cusplet on each side. These lateral cusplets are smaller (lower) than the main cusp. An occlusal crest runs over the apex of the main cusp and the lateral cusplets. The narrow labial peg is strongly developed. The crowns tend to be unornamented, although some specimens contain some vertical striations on the main cusp. The teeth are mesio-distally straight rather than curved; they are known as anterior teeth (Duffin, Reference Duffin, Swift and Martill1998a, text fig. 20C; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). The size ranges between about 1.5 and 3 mm in the mesial–distal direction.

Figure 3. All a = occlusal view, b = labial view. Lissodus minimus, morphotype I: A – WWR18-0081; B – WWR18-0085; C – WWR18-0087. Lissodus minimus, morphotype II: D – WWR18-0093; E – WWR18-0095. Lissodus minimus, morphotype III: F – WWR18-00720 ; G – WWR18-0075. Lissodus minimus, morphotype IV: H – WWR18-0107; I – WWR18-0112. ‘Hybodus’ cf. cuspidatus: J – WWR18-0094; K – WWR18-0074; L – WWR18-0116.
Morphotype II (Fig. 3D,E). The teeth have a large centrally placed main cusp and two or three more or less conspicuous lateral cusplets on each side of it. Ornamentation as in morphotype I and limited to the main cusp only. The teeth are straight or slightly curved, and the labial peg is mostly strongly developed. The presence of the lateral cusplets indicate that these teeth are anterolateral teeth (Duffin, Reference Duffin, Swift and Martill1998a, text fig. 20B; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). The size ranges between about 2 and 3 mm.
Morphotype III (Fig. 3F,G). These teeth have a high main cusp, but lateral cusplets are not present. The occlusal crest is shallow, but still visible. The teeth are slightly asymmetrical, and ornamentation is only present on the main cusp. A labial peg is present but smaller than the labial pegs of morphotypes I and II. The teeth, having a straight outline, are lateral teeth (Duffin, Reference Duffin, Swift and Martill1998a, text fig. 20A; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). The size ranges between about 1.5 and 3.5 mm.
Morphotype IV (Fig. 3H,I). The main cusp is not as large as in morphotypes I, II and III. Lateral cusplets are absent, and the occlusal crest is very shallow. The crowns are usually flat. The teeth are more ornamented compared to the morphotypes mentioned above. The ornamentation consists of vertical striations running from the apex of the main cusp to the lower part of the crown and at the place where lateral cusplets occur in other morphotypes. However, the ornamentation is not always visible. The labial peg is mostly weakly developed, and the teeth are either straight or slightly curved. These teeth are posterolateral teeth (Duffin, Reference Duffin, Swift and Martill1998a, text fig. 20D; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). The size ranges between about 2 and 3 mm.
Remarks
The genus Lissodus ranges from the Early Triassic (Scythian) to the Middle Cretaceous (Albian), although the literature (e.g., Duffin, Reference Duffin1985; Duncan, Reference Duncan2004) mentions the genus from the Early (Tournaisian) and Late (Westphalian) Carboniferous. Although Duffin (Reference Duffin1985, p. 118) and Duncan (Reference Duncan2004) suppose that the Carboniferous sharks they describe belong to Lissodus, the time gap of at least 150 Ma between the Westphalian and the Early Triassic seems to exclude that the Paleozoic and Mesozoic sharks are congeneric; here, we suppose it to be an example of convergent evolution. Rees & Underwood (Reference Rees and Underwood2002) provided an updated revision of the genus, excluding the Paleozoic taxa from the genus, and the British Carboniferous material was subsequently transferred to the genus Reesodus Koot et al. (Reference Koot, Cuny, Tintori and Twitchett2013) by Smith et al. (Reference Smith, Martill and Duffin2017).
Originally, this species was described as Acrodus minimus (Agassiz, Reference Agassiz1836), but it was later incorporated into the genus Lissodus by Duffin (Reference Duffin1985). The different morphotypes are sometimes difficult to distinguish; there seems to be ‘gliding scale’ regarding the shape. A single tooth may therefore feature characteristics that put it between morphotypes. The majority of the available teeth only feature a crown, the root being broken off. Only three specimens (e.g., Fig. 3A) still have their roots. The crowns themselves are often broken too. Many loose ‘wings’ (or arms) have been found in the samples. The structure and shape of the teeth indicate that this shark was durophagous and most likely fed on shelled, benthic organisms (Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022).
This species has also been mentioned from the UK (Duffin, Reference Duffin1985, Reference Duffin, Swift and Martill1998a; Cuny & Risnes, Reference Cuny and Risnes2005; Foffa et al., Reference Foffa, Whiteside, Viegas and Benton2014; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Whiteside et al., Reference Whiteside, Duffin, Gill, Marshall and Benton2016; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Whiteside & Duffin, Reference Whiteside and Duffin2017; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), Eastern Europe (Chrząstek, Reference Chrząstek2008; Michalík et al., Reference Michalík, Lintnerová, Wójcik-Tabol, Gaździcki, Grabowski, Golej, Šimo and Zahradníková2013; Ősi et al., Reference Ősi, Botfalvai, Prondvai, Hajdu, Czirják, Szentesi, Pozsgai, Götz, Makádi, Csengődi and Sebe2013; Posmoşanu, Reference Posmoşanu2015; Botfalvai et al., Reference Botfalvai, Győri, Pozsgai, Farkas, Sági, Szabó and Ősi2019; Szabó et al., Reference Szabó, Botfalvai and Ősi2019) and Western Europe (Duffin, Reference Duffin1993; Duffin & Delsate, Reference Duffin and Delsate1993; Cuny, Reference Cuny1995; Godefroit et al., Reference Godefroit, Cuny, Delsate and Roche1998; Henz & Hertel, Reference Henz and Hertel2011; Cuny et al., Reference Cuny, Hunt, Mazin and Rauscher2013; Sander et al., Reference Sander, Wintrich, Schwermann and Kindlimann2016; Diependaal & Reumer, Reference Diependaal and Reumer2021).
Genus ‘Hybodus’ (Agassiz, 1837)
‘Hybodus’ cf. cuspidatus (Agassiz, Reference Agassiz1836)
Fig. 3J,K,L
A few of our Lissodus-like specimens show secondary cuspules at the labial base of the three principal striations that run from the tip of the main cusp to the lower edge of the tooth. Agassiz (Reference Agassiz1836, tome III, p. 194) described a species as Hybodus cuspidatus and added as a diagnostic: ‘La base de la couronne est très-étroite, et les plis y déterminent souvent de petits bourrelets, comme dans le H. reticulatus (…)’ (translated: The base of the crown is very narrow, and the striations often bear small cuspules, like in H. reticulatus). In addition, the central cusp is relatively high.
Remarks
The taxon cuspidatus was not included into Lissodus by Rees & Underwood (Reference Rees and Underwood2002) in their revision of the genus; Seilacher (Reference Seilacher1943) included it into Polyacrodus. We use the species name cuspidatus here rather than cloacinus, as Dorka (Reference Dorka2003) has shown that the latter is invalid and should be considered a junior synonym of cuspidatus. As Rees (Reference Rees2008) considered the generic name Polyacrodus to be a nomen dubium and as the genus Hybodus is limited to two species including the type species H. reticulatus (Maisey, Reference Maisey1986), we here place the generic name ‘Hybodus’ between quotation marks. We think that our few specimens that show small secondary cuspules at the base of the striations cannot be considered to fall within the intraspecific variation of Lissodus minimus, but belong to a different species, to which the epiteth cuspidatus seems most appropriate. Here, we hesitatingly range them as ‘Hybodus’ cf. cuspidatus, but this conclusion may be subject to further study when more material becomes available. It should be noted that only the large lateral teeth of H. cuspidatus (senior synonym of H. ‘cloacinus’) have been described in the literature (e.g., Duffin, Reference Duffin, Swift and Martill1998a, Plate 26, fig. 5 or Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016, fig. 6); none of the anterior or posterior teeth have yet been described. We attempt this here, for the first time, using ‘Hybodus’ cf. cuspidatus. As our specimens have only one main cusp or lack secondary cusps, we think that they must be posterior teeth.
Order Synechodontiformes (Duffin & Ward, 1993)
Genus Rhomphaiodon (Duffin, Reference Duffin1993)
Rhomphaiodon minor (Agassiz, 1833–43)
Fig. 4A–M

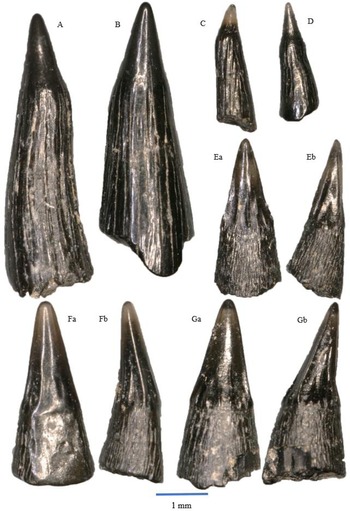
Figure 4. Rhomphaiodon minor, morphotype I: A – WWR18-0001; B – WWR18-0002; C – WWR18-0005; D – WWR18-0006. Rhomphaiodon minor, morphotype II: E – WWR18-0012; F – WWR18-0013; G – WWR18-0014; H – WWR18-0015. Rhomphaiodon minor, morphotype III: I – WWR18-0021; J – WWR18-0022; K – WWR18-0023. Rhomphaiodon minor, morphotype IV: L – WWR18-0032. Rhomphaiodon minor, morphotype V: M – WWR18-0318. Parascylloides turnerae: N – WWR18-0132, a = labial side, b = mesial–distal side, turned right; O – WWR18-0133, a = labial side, b = mesial–distal side, turned left. Chondrichthyan dermal denticles: P – placoid scale WWR18-0164, a = oblique view, b = lateral side, turned right; Q – ctenacanthoid scale WWR18-0168, a = anterior side, b = lateral side, turned right; R – hybodont scale WWR18-0169, a = lateral side, b = occlusal view.
A total of 377 teeth can be linked to this species (excluding a similar amount of 377 detached central cusps, bringing the total number of teeth to over 750). The crown and root are separated by a strong angle or indentation in labial or buccal view, of often c. 90 degrees (see e.g., Fig. 4C, F and K). The main cusp is the highest, and it is ornamented by multiple vertical striations that start at the apex and run to the lower edge at the enamel–root junction. These striations might be absent as a result of weathering. The main cusp is located at the centre of the tooth, and there are lateral cusplets that are smaller than the central cusp and emerge directly from the root. The lateral cusplets also contain vertical striations. The teeth are either roughly symmetrical or somewhat asymmetrical in shape depending on the number and development of the lateral cusplets.
Here, we distinguish five different morphotypes of these teeth, two of them (I and II) regarding the symmetrical teeth and three other types (III, IV and V) regarding the asymmetrical teeth.
Morphotype I (Fig. 4A–D) concerns teeth with one lateral cusplet on each side, and morphotype II (Fig. 4E–H) shows two lateral cusplets on each side. The outer pair is lower than the inner pair. Morphotype III (Fig. 4I–K) concerns teeth that have one lateral cusplet on one side, while the other side has two; here again, the outer cusplets are lower than the inner cusplets. In morphotype IV (Fig. 4L), the teeth have two lateral cusplets on one side, while the other side has three, and finally, morphotype V (Fig. 4M) shows teeth that one lateral cusplet on one side, while the other side does not possess one. Morphotypes IV and V are rare in our sample with three and four specimens, respectively. In all five morphotypes, the root is very wide. These roots possess foraminae that run on the horizontal surface, although they are not always present. For the symmetrical ones, the (mesio-distal) width of the teeth ranges between about 1 and 2.5 mm, and the height ranges between about 0.75 and 1.5 mm. For the asymmetrical ones, the width of these teeth ranges between about 0.75 and 2 mm. The height ranges between about 1 and 2 mm. The broken and isolated main cusps may originate from somewhat larger teeth; they range in height between 1 and 2.5 mm.
Remarks
Originally, this species was described as Hybodus minor Agassiz, 1833–43 (Duffin & Gaździcki, Reference Duffin and Gaździcki1977; Duffin & Delsate, Reference Duffin and Delsate1993; Cuny, Reference Cuny1995; Duffin, Reference Duffin, Swift and Martill1998a; Godefroit et al., Reference Godefroit, Cuny, Delsate and Roche1998; Cuny et al., Reference Cuny, Hunt, Mazin and Rauscher2013; Michalík et al., Reference Michalík, Lintnerová, Wójcik-Tabol, Gaździcki, Grabowski, Golej, Šimo and Zahradníková2013). Similarities were found with the species Rhomphaiodon nicolensis (Duffin, Reference Duffin1993). Duffin (Reference Duffin, Swift and Martill1998a) explained that ‘Hybodus’ minor is to be considered a nomen dubium, as it is based on a fin spine and the taxon thus lacks diagnostic characters. It was later reassigned to Rhomphaiodon minor, because Hybodus minor teeth possessed haphazard crystalline enameloid, which was only known to be present in Rhomphaiodon nicolensis (Cuny & Risnes, Reference Cuny and Risnes2005). Furthermore, the two species are said to be closely related as they are consistently found in association with the spine Nemacanthus monilifer (Cuny & Risnes, Reference Cuny and Risnes2005).
The shape and structure of the teeth indicate that this species was a small predator (Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021). The species has also been mentioned from the UK (Duffin, Reference Duffin, Swift and Martill1998a; Cuny & Risnes, Reference Cuny and Risnes2005; Foffa et al., Reference Foffa, Whiteside, Viegas and Benton2014; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), Slovakia (Michalík et al., Reference Michalík, Lintnerová, Wójcik-Tabol, Gaździcki, Grabowski, Golej, Šimo and Zahradníková2013) and Western Europe (Duffin and Delsate, Reference Duffin and Delsate1993; Cuny, Reference Cuny1995; Godefroit et al., Reference Godefroit, Cuny, Delsate and Roche1998; Cuny et al., Reference Cuny, Hunt, Mazin and Rauscher2013; Sander et al., Reference Sander, Wintrich, Schwermann and Kindlimann2016, Diependaal & Reumer, Reference Diependaal and Reumer2021).
Genus Parascylloides (Thies et al., Reference Thies, Vespermann and Solcher2014)
Parascylloides turnerae (Thies et al., Reference Thies, Vespermann and Solcher2014)
Fig. 4N,O
There are in total 35 teeth that can be assigned to this species. The main cusp of small teeth of Parascylloides turnerae is relatively large, and it curves significantly in a lingual direction. It tends to curve somewhat in sideways direction. The teeth may have no, one, or two symmetrically placed lateral cusplets that are small in size and emerge directly from the root of the tooth. The crown is heavily ornamented by strong widely spaced vertical ridges that start at the apex of the main cusp and continue until the root. The root has an oval shape in lingual–buccal direction, with the crown positioned on the labial side. It possesses several foraminae across its horizontal surface, although these openings are not always clearly visible. The root represents about 25% of the total tooth height, which ranges between about 1 and 1.5 mm.
Remarks
The teeth superficially resemble those of Rhomphaiodon minor. Sykes et al., (Reference Sykes, Cargill and Fryer1970, plate 17, fig. 1) described a tooth of P. turnerae from Barnstone (UK) as an ‘Indeterminate Hybodont dermal denticle of type A’, stating: ‘this resembles the minute teeth of Hybodus minor, with the typical root, nearly cylindrical crown, and inconspicuous lateral denticles’. The difference is that P. turnerae always possesses no more than one lateral cusplet on each side, while R. minor can have lateral cusplets in varying numbers. In addition, the root of P. turnerae is stretched in labial–lingual direction, while the root of R. minor is larger in the mesial–distal direction. Also, the main cusp of P. turnerae bends significantly more in the lingual direction, while the main cusp of R. minor is comparatively straight.
The teeth of Parascylloides turnerae were originally described as the symphyseals or parasymphyseals of Rhomphaiodon nicolensis (Cappetta, Reference Cappetta2012), actually R. minor (see above), but they were reassigned to a new species, due to their frequency and morphological differences (Thies et al., Reference Thies, Vespermann and Solcher2014), with the following differential diagnosis: ‘A tooth crown built by a large massive central cusp and one pair of minute lateral cusplets together with a tongue-shaped, labio-lingually expanded root showing a modified anaulacorhize vascularisation distinguishes the teeth of Parascylloides gen. nov. from the teeth of all other synechodontiform and neoselachian taxa in general’.
The frequency of these teeth that we find in our sample is quite different from that reported from Seinstedt (‘47.5% of all of the neoselachian teeth and tooth fragments identifiable’ or 144/(144 + 74) = 66% of the total R. minor and P. turnerae; Thies et al., Reference Thies, Vespermann and Solcher2014) and at Barnstone (25,2% of the total of 493 R. minor, 12 R. nicolensis and 170 P. turnerae teeth; Thies et al., Reference Thies, Vespermann and Solcher2014). In our sample, they represent only 8,45% of the total of R. minor and P. turnerae (35/(377 + 35)) and could therefore be interpreted here as symphyseal or parasymphyseal teeth of Rhomphaiodon minor. Percentages equivalent to ours were also found at Saltford (35/(547 + 35) = 6%, Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021) and at Aust (30/(387 + 30) = 7%, Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018).
P. turnerae has also been mentioned from the UK (Thies et al., Reference Thies, Vespermann and Solcher2014; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022) and Germany (Thies et al., Reference Thies, Vespermann and Solcher2014; Sander et al., Reference Sander, Wintrich, Schwermann and Kindlimann2016). In Diependaal & Reumer (Reference Diependaal and Reumer2021, fig. 1K), a tooth similar to these is described. There, it was incorrectly assigned to Pseudodalatias barnstonensis, and the tooth is here reassigned to Parascylloides turnerae (Chenal, in litt.; Duffin, in litt.).
Chondrichthyan dermal denticles
Fig. 4P–R
Three different denticle morphotypes are found in our sample.
Indetermined placoid scales (Fig. 4P): Seven of our specimens belong to this type of denticle. The denticle looks like a drop-shaped or leaf-shaped crown on a pedestal-like root. The crown is bent towards the posterior and ends in a blunt tip. It contains strong ridges and is flattened on the upper side and has a somewhat concave backside. The root does not feature any ornamentation. The height of these scales is about 1 mm. This type of scale is mentioned from many localities of the British Penarth Group (e.g., Duffin, Reference Duffin, Swift and Martill1998a; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022) and also from Poland (Duffin & Gaździcki, Reference Duffin and Gaździcki1977) and Luxemburg (Delsate & Duffin, Reference Delsate and Duffin1999).
Ctenacanthoid scales (Fig. 4Q): This type of denticle shows a wide base bearing multiple more or less similar cusps. Those cusps form the crown and are bending backwards, which results in a convex shape. There are two variations of this type of denticle. The cusps of the first type (27 specimens) are roughly the same length, while in the second type (5 specimens), the outer cusps are higher than the central ones if present. The width of these ctenacanthoid scales ranges between about 0.75 and 1.5 mm. Similar scales are found in other localities (e.g., Duffin, Reference Duffin, Swift and Martill1998a; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022).
Hybodont scales (Fig. 4R): This type of denticle appears as a thick circular knob on a flat circular or slightly elliptical root or pedestal. The knob is ornamented by thick radially oriented vertical ridges, sometimes giving it a star-like shape. The top is smooth and may contain a central depression, probably due to wear. The width ranges between about 0.5 and 1.5 mm at their widest point. This type of denticle is known as hybodont morphotype (e.g., Duffin & Delsate, Reference Duffin and Delsate1993; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022).
Remarks
Dermal denticles are known from most of the Rhaetian localities described from the UK (Sykes, Reference Sykes1974; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), and also from Poland (Duffin & Gaździcki, Reference Duffin and Gaździcki1977), France (Cuny, Reference Cuny1995; Cuny et al., Reference Cuny, Hunt, Mazin and Rauscher2013), Luxembourg (Delsate & Duffin, Reference Delsate and Duffin1999), and the Winterswijk subrosion pipe from the Netherlands (Diependaal & Reumer, Reference Diependaal and Reumer2021). Their exact taxonomic attribution is problematic.
Class Osteichthyes (Huxley, 1880)
Subclass Actinopterygii (Cope, 1887)
Order Saurichthyiformes (Aldinger, 1937)
Family Saurichthyidae (Owen, 1860) (sensu Stensiö, 1925)
Genus Saurichthys (Agassiz, Reference Agassiz1835)
Saurichthys longidens (Agassiz, Reference Agassiz1835)
Synonym: Severnichthys acuminatus (Agassiz, Reference Agassiz1835) partim
Fig. 5A–D
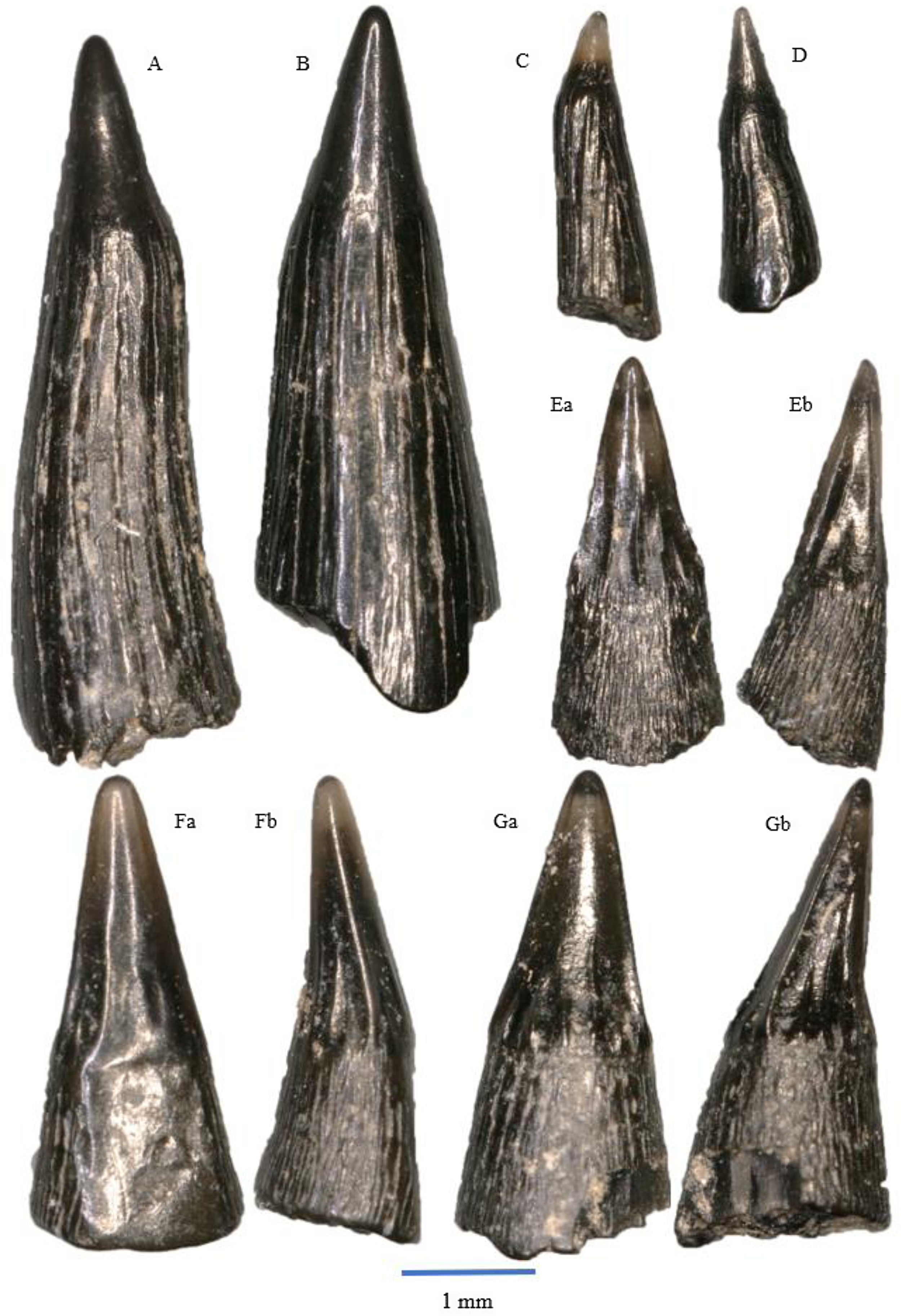
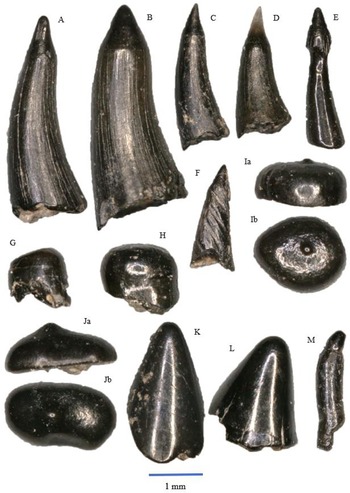
Figure 5. Saurichthys longidens: A – WWR18-0189; B – WWR18-0190; C – WWR18-0183; D – WWR18-0184. Birgeria acuminate: E – WWR18-0197, a = labial side, b = mesial–distal side, turned left; F – WWR18-0199, a = labial side, b = mesial–distal side, turned left; G – WWR18-0200, a = labial side, b = mesial–distal side, turned right.
The teeth of Saurichthys longidens are elongated and usually straight in shape, although some specimens have a slightly sigmoidal shape (Fig 5A). The teeth consist of an acrodin cap and a shaft. The cap comprises between 10 and 30% of the total preserved tooth length. It has a conical shape and lacks ornamentations. In our material it usually has a black colour, although some specimens have a translucent cap. The junction between the cap and the shaft is not well pronounced, although sometimes marked by a faint ridge. The shaft has an ornamentation of longitudinal ridges; sometimes these ridges may be worn. The ridges do not continue in the acrodin cap, thus marking the boundary cap and shaft. The base of the tooth is often flared, and the tooth usually has a somewhat oval circumference. The length of the teeth ranges between 1 and 6 mm. In our sample, a total of 589 teeth can be assigned to this species.
Remarks
Originally, this species was described as Saurichthys longidens by Agassiz (Reference Agassiz1835). In 1994, Saurichthys longidens and Birgeria acuminata were merged into the new genus Severnichthys (Storrs, Reference Storrs1994). However, the validity of this genus is disputed (see Diependaal & Reumer, Reference Diependaal and Reumer2021). Saurichthys was most likely a piscivorous predator (Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021), as indicated by their sharp teeth.
The species has also been mentioned from the various localities of the Penarth Group in the UK (Duffin, Reference Duffin, Swift and Martill1998a; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), Eastern Europe (Duffin & Gaździcki, Reference Duffin and Gaździcki1977; Michalík et al., Reference Michalík, Lintnerová, Wójcik-Tabol, Gaździcki, Grabowski, Golej, Šimo and Zahradníková2013; Botfalvai et al., Reference Botfalvai, Győri, Pozsgai, Farkas, Sági, Szabó and Ősi2019) and Western Europe (Bürgin & Furrer, Reference Bürgin and Furrer1993; Duffin & Delsate, Reference Duffin and Delsate1993; Delsate & Duffin, Reference Delsate and Duffin1999; Diependaal & Reumer, Reference Diependaal and Reumer2021).
Order Birgeriiformes (Heyler, 1969)
Family Birgeriidae (Aldinger, 1937)
Genus Birgeria (Stensiö, 1919)
Birgeria acuminata (Agassiz, Reference Agassiz1835)
synonym: Severnichthys acuminatus (Agassiz, Reference Agassiz1835) partim
Fig. 5E–G
The teeth of Birgeria acuminata are elongated and have a conical blade-like shape. They consist of an acrodin cap and a shaft. The labial side of the cap tends to be smooth and unornamented, although some specimens do contain some thick vertical ridges on this side. These two ridges can be referred to as lateral carinae, giving it a lentil-shaped cross section with the lingual side being more convex than the labial side. The lingual side of the teeth is ornamented by vertical ridges. The acrodin cap comprises about 30–50 per cent of the total tooth length. There often is a prominent ridge that separates the cap from the shaft.
On the shaft, the teeth are ornamented by very fine vertical striations. In some specimens, the fine striations on the labial side can be hardly visible or they have disappeared due to erosion. The flattening on the labial side of the acrodin cap continues further downwards but is gone at the base of the tooth, which results in a mostly circular cross section at the base. The majority of the teeth are found with both the cap and shaft together, but sometimes, only the cap is found. The total length of the teeth ranges between 1 and 3.5 mm. A total of 229 teeth can be linked to this species.
Remarks
The teeth somewhat resemble the teeth of Saurichthys longidens, but there are some significant differences. The acrodin caps of Saurichthys longidens are completely unornamented, and they have a conical shape and a circular cross section. On the other hand, the caps of Birgeria acuminata do feature ornamentation, especially on the lingual side, and the shape is more blade-like as a result of the presence of lateral edges. The shaft of Saurichthys longidens is long and features strong vertical ridges on its surface, while the shaft of Birgeria acuminata is shorter and is ornamented by very fine vertical striations.
This species was originally described as Saurichthys acuminatus by Agassiz (Reference Agassiz1835). Then, Savage & Large (Reference Savage and Large1966) reassigned the species to the genus Birgeria as Birgeria acuminata, since the large and stout lower jaw of Birgeria acuminata, as described and depicted by them, does not match with the slender and elongated morphology of the Saurichthys skull (Rieppel, Reference Rieppel1985). Bürgin & Furrer (Reference Bürgin and Furrer1993) provided a more elaborate discussion on the differences between the genera Birgeria and Saurichthys regarding their teeth. Shortly thereafter, Storrs (Reference Storrs1994) merged both Birgeria acuminata and Saurichthys longidens into the new genus Severnichthys. The morphological impossibility of this merger was discussed by Diependaal & Reumer (Reference Diependaal and Reumer2021), who separated the two taxa again into the original different genera; Tintori & Lombardo (Reference Tintori, Lombardo and Tanner2018) expressed a similar opinion. Tackett et al. (Reference Tackett, Ziere and Clement2022) also mentioned the taxonomic problems pertaining Severnichthys, Saurichthys and Birgeria, without however reaching a conclusion. B. acuminata was most likely a predator (Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021).
The species has also been mentioned from the many Rhaetian localities in the UK (Duffin, Reference Duffin, Swift and Martill1998a; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), from Eastern Europe (Duffin & Gaździcki, Reference Duffin and Gaździcki1977; Michalík et al., Reference Michalík, Lintnerová, Wójcik-Tabol, Gaździcki, Grabowski, Golej, Šimo and Zahradníková2013; Botfalvai et al., Reference Botfalvai, Győri, Pozsgai, Farkas, Sági, Szabó and Ősi2019; Szabó et al., Reference Szabó, Botfalvai and Ősi2019) and from Western Europe (Bürgin & Furrer, Reference Bürgin and Furrer1992; Duffin & Delsate, Reference Duffin and Delsate1993; Godefroit et al., Reference Godefroit, Cuny, Delsate and Roche1998; Delsate & Duffin, Reference Delsate and Duffin1999; Sander et al., Reference Sander, Wintrich, Schwermann and Kindlimann2016; Diependaal & Reumer, Reference Diependaal and Reumer2021).
Order Palaeonisciformes (Hay, 1902)
Family Palaeoniscidae (Vogt, 1852)
Genus Gyrolepis (Agassiz, Reference Agassiz1835)
Gyrolepis albertii (Agassiz, Reference Agassiz1835)
Fig. 6A–F

Figure 6. Gyrolepis albertii: A – WWR18-0177; B – WWR18-0174; C – WWR18-0173; D – WWR18-0175; E – WWR18-0311; F – WWR18-0313. Sargodon tomicus: G – WWR18-0211; H – WWR18-0202. Pycnodontiformes sp. indet.: I – molariform WWR18-0214, a = labial side, b = occlusal view; J – molariform WWR18-0212, a = labial side, b = occlusal view. ‘Lepidotes’ sp.: K – molariform WWR18-0224; L – molariform WWR18-0225; M – insiciform WWR18-0315.
The teeth of Gyrolepis albertii are elongated and slender. Like the two actinopterygian species described above, they consist of an acrodin cap and a shaft. The overall tooth has a distinct curved shape. The straight acrodin cap is unornamented and small compared with the rest of the tooth, about 10% of the total tooth length. The cap has a conical shape and is often black in colour, although some tips are translucent. The apex of the cap is often sharp, but there are specimens where the cap is more smoothened, most probably due to wear. The cap and the shaft are separated from each other by a ridge.
The curved shaft is largely smooth, but it does contain fine vertical non-branching striations. However, these striations are not always preserved. The base of the tooth might be slightly flared. The teeth have a circular cross section, and the shaft increases in diameter the further down from the cap. The length of the total tooth ranges from about 1 to 4 mm. There is a total of 1240 teeth that can be attributed to this species, making it the most abundant taxon in the sample. The sharpness of the teeth indicates that this species was carnivorous/piscivorous (Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021).
There are two specimens that look different compared with the standard Gyrolepis albertii tooth. Fig. 6E shows a tooth that shape-wise as well as based on the tip belongs to this species, but it seems that the outer layer of the shaft is missing and that this specimen shows the inner side of the shaft; it supposedly has a taphonomical origin. The specimen of Fig. 6F features no vertical striations on the shaft, but four deep diagonal furrows, that seem to be the result of some peculiar type of wear.
Remarks
Morphologically, the teeth superficially resemble those of Saurichthys longidens and Birgeria acuminata. Similarities between Gyrolepis albertii and Saurichthys longidens are that both teeth have a small conical, unornamented acrodin cap and a long shaft. The difference between these species is that the cap of Saurichthys longidens is relatively longer and less sharp than the cap of Gyrolepis albertii. Additionally, the shaft of Saurichthys longidens is straight and is ornamented by thick vertical ridges, while the shaft of Gyrolepis albertii is curved and features only thin vertical striations that may sometimes be absent.
Similarities between Gyrolepis albertii and Birgeria acuminata are that both tooth types possess a cap and a shaft. However, the acrodin cap of Birgeria acuminata is relatively large, features ornamentation and is lentil-shaped in cross section, while Gyrolepis albertii possesses a small pointy cap without ornamentations and has a circular cross section. Likewise, the shaft of Birgeria acuminata is short and features very fine vertical striations, while the shaft of Gyrolepis albertii is long and features slightly thicker striations.
Gyrolepis albertii is a widespread taxon; it has also been mentioned from many localities in the Rhaetian of the UK (Duffin, Reference Duffin, Swift and Martill1998a; Whiteside & Marshall, Reference Whiteside and Marshall2008; Van den Berg et al., Reference Van den Berg, Whiteside, Viegas, Schouten and Benton2012; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Whiteside et al., Reference Whiteside, Duffin, Gill, Marshall and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), from Eastern Europe (Duffin & Gaździcki, Reference Duffin and Gaździcki1977; Botfalvai et al., Reference Botfalvai, Győri, Pozsgai, Farkas, Sági, Szabó and Ősi2019; Szabó et al., Reference Szabó, Botfalvai and Ősi2019) and from continental Western Europe (Bürgin, Reference Bürgin1992; Duffin & Delsate, Reference Duffin and Delsate1993; Godefroit et al., Reference Godefroit, Cuny, Delsate and Roche1998; Delsate & Duffin, Reference Delsate and Duffin1999; Sander et al., Reference Sander, Wintrich, Schwermann and Kindlimann2016; Diependaal & Reumer, Reference Diependaal and Reumer2021).
Other Actinopterygian teeth
Fig. 6G–M
A small number of actinopterygian teeth cannot be ascribed to the three taxa mentioned above. They are mostly knob-like molariforms originating from durophagous fish, teeth with a circular or ovoid circumference. Some bear a small protuberance (e.g., Fig. 6J), and other ones are simply bulbous or have a flattened surface (e.g., Fig. 6G). An indentation may be centrally present. The exact taxonomic attribution is often difficult. In the literature, they are often provided with names with question marks (e.g., ?Paralepidotus sp. in Duffin & Gażdzicki, Reference Duffin and Gaździcki1977) or that are written between quotes (e.g., ‘“Lepidotes” tooth’ or ‘“Colobodus” tooth’ in Nordén et al., Reference Nordén, Duffin and Benton2015).
Here, we distinguish three types of molariform teeth: teeth with a flat surface are attributed to Sargodon tomicus, and other molariforms, not having a flat surface but a bulbous one, either with a protuberance (however vague it may appear) or without one or being provided with a small indentation, are provisionally listed as Pycnodontiformes indet (see Kriwet, Reference Kriwet2005); the somewhat cone-shaped teeth are identified as ‘Lepidotes’ sp.
Order Amiiformes (Hay, 1929)
Family Dapediidae (Lehman, 1966)
Genus Sargodon (Plieninger, Reference Plieninger1847)
Sargodon tomicus (Plieninger, Reference Plieninger1847)
Fig. 6G,H
A few teeth can be linked to this species. The molariform teeth are small circular to elliptical domes with a flat occlusal surface. There is no ornamentation present.
S. tomicus has also been mentioned from the UK (Duffin, Reference Duffin, Swift and Martill1998a, Reference Duffin1998b; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022) and Poland (Duffin & Gaździcki, Reference Duffin and Gaździcki1977).
A few dozen teeth in our sample are here tentatively attributed to this taxon. All teeth are detached black bulbous molariforms with or without any ornamentation, and some are provided with a more or less conspicuous protuberance. The occlusal surface is not flat, as it is in S. tomicus. The teeth have a circular cross section. The height of these teeth ranges between about 1 and 3.5 mm.
The cone-shaped teeth are identified as an indeterminate species of Lepidotes. One elongate tooth (Fig. 6M) is here also tentatively attributed to ‘Lepidotes’ sp. This contradicts the identification of a similar incisiform from the Rhaetian of Chipping Sodbury, UK, as S. tomicus by Lakin et al., (Reference Lakin, Duffin, Hildebrandt and Benton2016, p. 48. fig. 11E and F). The caps of both the tooth in Lakin et al. (Reference Lakin, Duffin, Hildebrandt and Benton2016) and our tooth are less wide than the body of the teeth. A cap similar in size and with a morphology somewhat resembling a matryoshka doll, but lacking the body, was published both by Korneisel et al., (Reference Korneisel, Gallois, Duffin and Benton2015, fig. 7F) and by Taylor et al., (Reference Taylor, Duffin, Hildebrandt, Parker and Benton2023, fig. 6E) as ‘Lepidotes’ sp., which attribution we here follow.
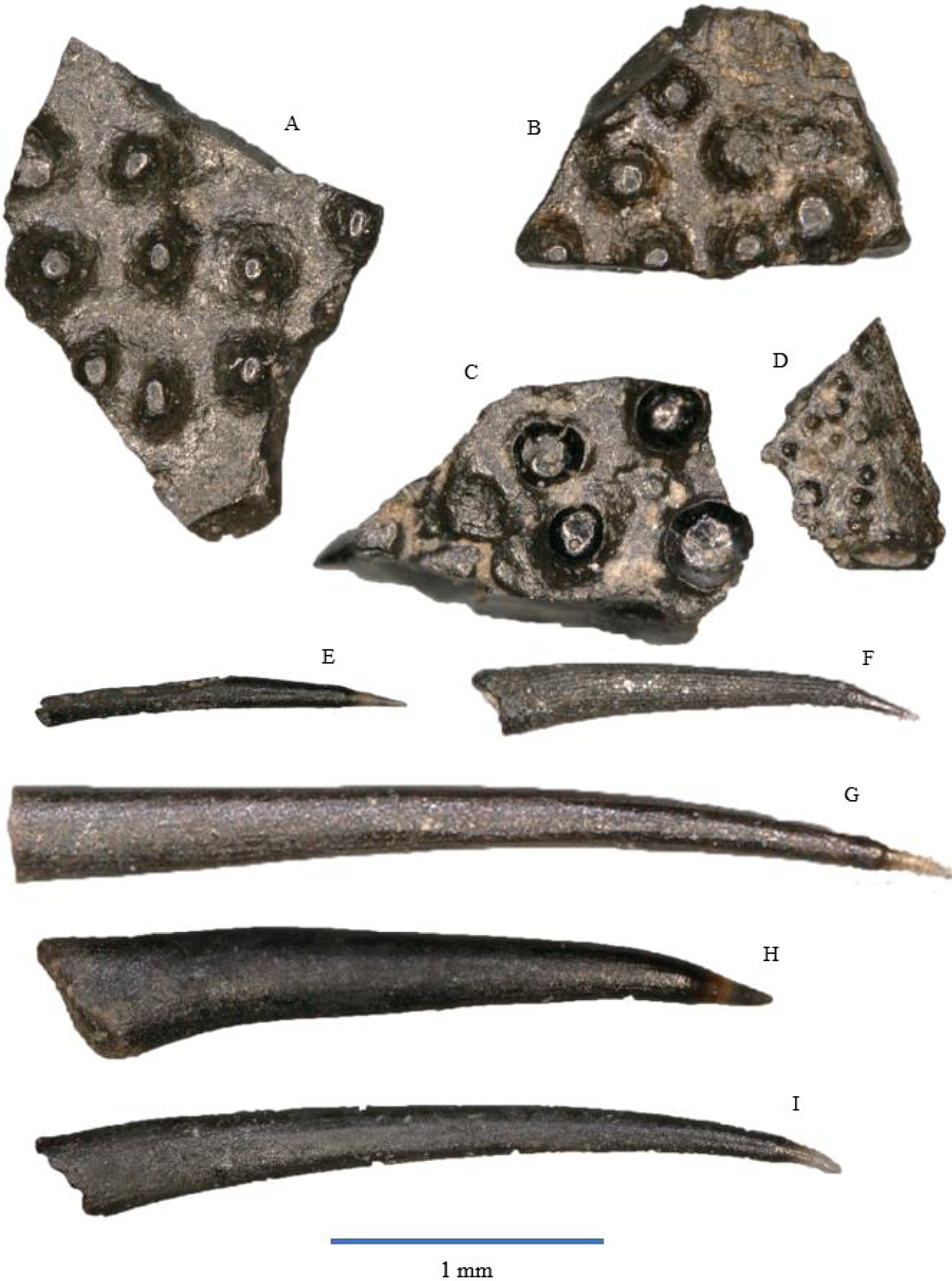
Figure 7. Actinopterygian tooth plates: A – WWR18-0237; B – WWR18-0238; C – WWR18-0239; D – WWR18-0240. Actinopterygian gill rakers: E – WWR18-0155; F – WWR18-0154; G – WWR18-0158; H – WWR18-0159; I – WWR18-0160.
Our sample contains 21 specimens of flat bony fragments bearing multiple small and low knobs on their surface. These knobs have an oval or circular circumference, and they are either rounded or flat at the top and do not show sharp tips. The knobs themselves are between 0.1 and 0.3 mm in diameter. One specimen (Fig. 7D) has much smaller knobs, which are about 0.05 mm in diameter; this could be from a more juvenile individual. According to Korneisel et al. (Reference Korneisel, Gallois, Duffin and Benton2015), Nordén et al. (Reference Nordén, Duffin and Benton2015), Mears et al. (Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016) and Slater et al. (Reference Slater, Duffin, Hildebrandt, Davies and Benton2016), who described similar fragments from the UK Rhaetian, these plates are actinopterygian jaw fragments, tooth plates, or palatal fragments, but they refrain from linking it to a specific species or genus.
Remarks
The fragments resemble the tooth plates of the genus Colobodus (Oosterink & Poppe, Reference Oosterink and Poppe1979). Colobodus sp. has been found earlier in the Anisian of Winterswijk (Oosterink & Poppe, Reference Oosterink and Poppe1979; Diedrich, Reference Diedrich2001; Oosterink & Winkelhorst, Reference Oosterink and Winkelhorst2013). However, the knobs in our specimen are too much separated from each other to belong to this genus. In addition, the genus Colobodus was most likely already extinct before the Rhaetian, since the last occurrence is recorded at the Middle Triassic Anisian–Ladinian boundary, c. 242 Ma (Mutter, Reference Mutter2004; Nordén et al., Reference Nordén, Duffin and Benton2015).
Our material contains over 500 so-called gill rakers that are extremely elongated, narrow teeth consisting of a very small and often lost acrodin cap and a very long shaft. The cap forms about less than 10% of the total tooth length. It is sharp and usually translucent, although it might sometimes be completely black. The shaft is slightly curved in lingual direction. In most of the specimens, the cap is slightly curved, but it is straight in some of them. The cap itself does not feature any ornamentation and is very thin. The shaft is usually unornamented, but some specimens show faint longitudinal striations. The cross section of the shaft is elliptical in labial–lingual direction. Gill rakers are often found without the acrodin cap, but due to the typical elongated, narrow shaft with an elliptical cross section and without major ornamentation, they are still recognisable as gill rakers. The total length of the rakers ranges between about 1 and 3.5 mm.
Remarks
Superficially, the morphology of the teeth of Gyrolepis albertii somewhat resembles that of the gill rakers. The differences are that the shaft of the gill rakers is much longer and much thinner than the shaft of G. albertii teeth. The gill raker shaft also has an elliptical cross section, while the teeth of G. albertii have a circular cross section. Additionally, the acrodin cap of the gill rakers is much smaller than the cap of G. albertii.
This type of gill rakers is often assigned to the chondrichthyan Pseudocetorhinus pickfordi (e.g., Duffin, Reference Duffin, Swift and Martill1998a; Andreev & Cuny, Reference Andreev and Cuny2012; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Whiteside et al., Reference Whiteside, Duffin, Gill, Marshall and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018). However, Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017) and Cross et al. (Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018) argue that these gill rakers might belong to a still unidentified osteichthyan. Based on this lack of consensus, Diependaal & Reumer (Reference Diependaal and Reumer2021) refrained from assigning the rakers to a taxon; yet, these gill rakers cannot originate from a chondrichthyan but must belong to an as-yet-unknown actinopterygian, as chondrichthyans do not possess such acrodin caps. Landon et al., (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017, fig. 6H, I) mention two rakers as ‘unidentified tooth’.
Gill rakers are usually associated with filter feeding (e.g., Duffin, Reference Duffin, Swift and Martill1998a; Cuny & Benton, Reference Cuny and Benton1999), and similar rakers are mentioned from several Rhaetian localities in the UK (Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018), from Luxembourg (Godefroit et al., Reference Godefroit, Cuny, Delsate and Roche1998) and from the Winterswijk subrosion pipe in the Netherlands (Diependaal & Reumer, Reference Diependaal and Reumer2021).

Figure 8. Actinopterygian osteological remains: A – unidentified bone WWR18-0323; B – vertebra WWR18-0324; C – vertebra WWR18-0325. Actinopterygian scales: Gyrolepis scales with ganoid layer: D – WWR18-0281; E – WWR18-0282; F – WWR18-0283; G – WWR18-0288; H – WWR18-0292; I – WWR18-0299. Gyrolepis scales without ganoid layer: J – WWR18-0264; K – WWR18-0269. Pholidophorid or Ginglymodian scales: L – WWR18-0302; M – WWR18-0317; N – WWR18-0309.
Six skeletal fragments have been found in the samples, one unidentified bone and five actinopterygian vertebrae. These remains cannot be assigned to any genus or species due to a lack of characteristic features.
Actinopterygian scales
Fig. 8D–N
There are more than 2200 actinopterygian scales present in the collection. Most of these are attributed to Gyrolepis albertii (Fig. 8D-K). The scales all have a variable rhomboidal to squared shape, and most of them are lozenge-shaped, but some other ones are more drop-like or ellipsoid in outline. Some specimens have more rounded edges. The exposed external surface of the scales is ornamented by thin ridges in the ganoine layer. In most specimens, the striations show some bifurcation, but others may only have straight striations. These striations, which may vary in thickness, are positioned across the widest parts of the scales, that is, they run in the longest direction of the lozenge. On average, the size of the scales ranges between 1.5 and 4 mm, although one specimen is about 9 mm at its longest. The ornamentations resemble the characteristic patterns of the scales of Mears et al. (Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016) and Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017).
Some 85 specimens of the same species (see e.g., Fig. 8J,K) have an identical rhomboidal to squared shape and size as the ones mentioned above. They are characterised by ridges that concentrically follow the outline of the scale. These are growth lines that have become visible because the ornamented ganoine layer is absent. It must have fallen off due to some taphonomic process. Landon et al., (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017, fig. 6L) depicted a similar scale; it is their morphotype M3.
A second type of scale also has a rhomboidal to squared shape, but it is characterised by the complete lack of ornamentation on the ganoine layer (Fig. 8L-N). Growth lines as in the scales of G. albertii that lost their ganoine covering are not visible. The scales are slightly concave. The size ranges between about 1.75 and 3 mm. According to Mears et al., (Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016, fig. 10g) who depicted a similar scale, their morphotype S4, it was identified as ?Pholidophorus, based on Whiteside & Marshall (Reference Whiteside and Marshall2008, fig. 5ii, jj) who described another similar scale under that name without the question mark. Here, we refrain from a taxonomical identification; they could either be from a pholidophorid teleost or from a ginglymodian fish.
Scales similar to the ones described here are also mentioned from the UK (Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Cueille et al., Reference Cueille, Green, Duffin, Hildebrandt and Benton2020; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), from Eastern Europe (Duffin & Gaździcki, Reference Duffin and Gaździcki1977; Chrząstek, Reference Chrząstek2008; Botfalvai et al., Reference Botfalvai, Győri, Pozsgai, Farkas, Sági, Szabó and Ősi2019; Szabó et al., Reference Szabó, Botfalvai and Ősi2019) and from Western Europe (Bürgin, Reference Bürgin1992; Duffin & Delsate, Reference Duffin and Delsate1993; Cuny, Reference Cuny1995; Delsate & Duffin, Reference Delsate and Duffin1999; Diependaal & Reumer, Reference Diependaal and Reumer2021).
General discussion
With the exception of a few actinopterygian scales, all fossils described in this paper are small to extremely small. Some as-yet-unknown taphonomical process apparently separated the small remains from larger ones such as hybodont fin spines or larger molariform or incisiform teeth.
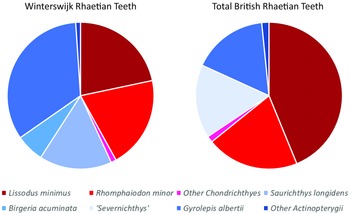
It is interesting to compare the faunal contents of our sample from the Winterswijk Rhaetian with associations from other European localities of similar age. In general, the faunal compositions of the samples from the British Penarth Group and from Winterswijk compare well. We have a total of 6577 countable specimens present in our sample, of which 3688 are individual chondrichthyan and actinopterygian teeth.
Scales are the most abundant specimens, and they count for 34.3% of the total. Close to that percentage are the actinopterygian teeth of Saurichthys longidens, Birgeria acuminata, Gyrolepis albertii, Sargodon tomicus, and indeterminate pycnodontiforms. These teeth make up 31.9% of the total. At the same time, chondrichthyan teeth of Lissodus minimus, ‘Hybodus’ cuspidatus, Rhomphaiodon minor and Parascylloides turnerae count for 24.3% of the total. These three categories form the largest part of the total amount of specimens. Furthermore, there also are actinopterygian tooth plates, gill rakers and a few skeletal remains, and some chondrichthyan dermal denticles (9.5% in total).
Comparison with the British Rhaetian deposits
Marine Rhaetian (Late Triassic, c. 208.05–201.36 Ma) sediments with an abundance of marine fossils have been described from many localities in Northwestern Europe (Klompmaker et al., Reference Klompmaker, Herngreen and Oosterink2010). The fish fauna from the British Rhaetian deposits of the Penarth Group close to the Bristol Channel has been described in many papers (Duffin, Reference Duffin, Swift and Martill1998a, Reference Duffin1998b; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallois, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018; Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020; Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021; Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). A recurring issue in those British publications is the use of the genus ‘Severnichthys’. As mentioned by Diependaal & Reumer (Reference Diependaal and Reumer2021), Storrs (Reference Storrs1994) merged Birgeria acuminata and Saurichthys longidens into the taxon Severnichthys acuminata on the basis of a single jaw. Diependaal & Reumer (Reference Diependaal and Reumer2021) argued this to be incorrect and concluded Severnichthys to be a nomen dubium. A similar conclusion was also reached by Tintori & Lombardo (Reference Tintori, Lombardo and Tanner2018), while Tackett et al. (Reference Tackett, Ziere and Clement2022) mentioned the need to reconsider the taxonomy of Birgeria, Saurichthys and Severnichthys. Williams et al. (Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022) still decided to retain what they call ‘the Severnichthys concept’ but recognised that it might be incorrect. Here, we do not use the taxon Severnichthys, which we consider a nomen dubium (and therefore put it between quotation marks). Interestingly, Birgeria teeth are almost absent in localities from French Lorraine, whereas teeth of Saurichthys are common, as for example in Saint-Nicolas-de-Port (Cuny & Ramboer, Reference Cuny and Ramboer1991).
The total count of the British Rhaetian shows that a large portion of the British Rhaetian teeth are comprised of Lissodus minimus, which add up to 43.8% of the teeth. The percentage is double the percentage of Lissodus minimus in Winterswijk, with 21.7% of the teeth. When looking at the different British sites separately, Lissodus minimus forms a large portion of the teeth, with the exceptions of Stowey Quarry (16.5%, Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018) and Hapsford Bridge (1.2%, Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020). At Chipping Sodbury and Saltford, the teeth of Lissodus minimus comprises more than half of the total tooth count, with 69.7% (Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016) and 54.7% (Moreau et al., Reference Moreau, Duffin, Hildebrandt, Hutchinson, Parker, Carpenter and Benton2021), respectively. Also in the Westbury Garden Cliff section, Lissodus minimus comprises nearly half of the total teeth count (48.9%, Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022).
A similar trend can be observed for Rhomphaiodon minor. In the total British Rhaetian, it has a presence of 20.3%, while in Winterswijk, they have a presence of 10.2%. The highest percentages of Rhomphaiodon minor can be observed in the Westbury Fm. Section, the Aust Cliff section (Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018) and the Westbury Garden Cliff section (Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022), where the teeth of Rhomphaiodon minor make up about 30% of the total. On the other hand, very low percentages are observed in Chipping Sodbury (3.0%, Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016) and Hapsford Bridge (absent, Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020). The rest of the British sections show a presence of about 10 or 15% for Rhomphaiodon minor.
Another large portion in the British Rhaetian fish fauna is made up by the actinopterygian Gyrolepis albertii, which comprise 16.6% of the total tooth count. In Winterswijk, however, their presence is double that percentage: 33.6%. Remarkably, only in Hapsford Bridge, the largest portion of teeth are comprised of Gyrolepis albertii, where 80% of the teeth belong to this species (Ronan et al., Reference Ronan, Duffin, Hildebrandt, Parker, Hutchinson, Copp and Benton2020). Gyrolepis albertii usually makes up between the 10 and 20% of the total teeth count in the separate localities, which is in line with the total British Rhaetian chart. Only in the Stowey Quarry sections, Gyrolepis albertii teeth show a low presence (3.7%, Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018).
The other actinopterygian that is common in the British samples is the obsolete genus ‘Severnichthys’, with a total of 16.2%. This percentage is not far from the combined percentage of Saurichthys longidens and Birgeria acuminata in Winterswijk, which is about 22.2%. The Stowey Quarry features the largest percentage of ‘Severnichthys’ (66.2%, Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018), while the Westbury Garden Cliff features the lowest percentage of ‘Severnichthys’ (2.2%, Williams et al., Reference Williams, Duffin, Hildebrandt, Parker, Hutchinson and Benton2022). Similarly, at Barnhill Quarry, ‘Severnichthys’ makes up nearly one-third of the total amount of teeth (Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016). The other sections, which include the Westbury Formation sections and the Aust Cliff section, show that the ‘Severnichthys’ teeth make up for about 10% of the total.
See Fig. 9 for the relative frequencies of chondrichthyans and actinopterygians. Despite local differences, the overall trend in the British Rhaetian deposits is that more teeth belong to chondrichthyans (roughly about 66%) than to actinopterygians (about 34%), which contradicts the situation in Winterswijk where the chondrichthyans comprise about 43% and the actinopterygians make up about 57%. This observation can be explained by several factors. The thinly interbedded mudstones, limestones and sandstones of the Penarth Group deposits (Gallois, Reference Gallois2007) indicate a shallow marine/coastal environment. In Winterswijk, the environment was stressed, as indicated by the absence of bivalves and crinoids, and the facies indicate a deep water environment. At the bottom waters, the oxygen concentrations were low (Klompmaker et al., Reference Klompmaker, Herngreen and Oosterink2010; Estes-Smargiassi & Klompmaker, Reference Estes-Smargiassi and Klompmaker2015). These low oxygen concentrations in the Winterswijk bottom waters could have caused a lower number of Lissodus minimus teeth. It is known that Lissodus minimus was a bottom dweller in search of hard-shelled invertebrates (Fischer, Reference Fischer2008; Fischer et al., Reference Fischer, Voigt, Franz, Schneider, Joachimski, Tichomirowa, Götze and Furrer2012). Therefore, it most likely did not favour oxygen-poor waters, which resulted in a less prominent occurrence in Winterswijk compared with the average British Rhaetian.

Figure 9. Pie charts showing the relative frequencies of taxa in Winterswijk and in the combined British Rhaetian localities. Blue colours indicate Actinopterygii; red hues are for chondrichtyans. The light blue ‘Severnichthys’ slice in the British pie combines Saurichthys and Birgeria.
Also different or low salinity values might have caused lower percentages of the chondrichthyan fauna in Winterswijk compared with the UK. It is known that both Lissodus minimus and Rhomphaiodon minor are euryhaline sharks (Duffin, Reference Duffin1985; Fischer et al., Reference Fischer, Voigt, Franz, Schneider, Joachimski, Tichomirowa, Götze and Furrer2012). Their remains are found in many different aquatic facies, which means that they migrated to different locations. There might have been differences in salinity level of the water between the two localities (Klompmaker et al., Reference Klompmaker, Herngreen and Oosterink2010), which probably resulted in a more suitable environment for these two chondrichthyans in Britain compared to Winterswijk, for which reason their teeth are more abundant in the UK.
Similarly, the percentages of Saurichthys longidens and Birgeria acuminata in Winterswijk resemble those of Severnichthys in the British deposits, while the percentage of Gyrolepis albertii is doubled compared with the British deposits. Schmitz et al. (Reference Schmitz, Åberg, Werdelin, Forey and Bendix-Almgreen1991) studied 87Sr/86Sr ratios in the phosphates of Saurichthys longidens from British deposits, and they suggested that the Westbury bone beds were either reworked or had freshwater input. Since the 87Sr/86Sr ratios of Saurichthys longidens could contain a signal of freshwater input for the Westbury bone beds, it might suggest that Saurichthys longidens, and possibly also Birgeria acuminata, could tolerate changes in salinity of the water, while Gyrolepis albertii could have had less tolerance for freshwater input and their teeth were therefore less prominent in the British samples. On the other hand, Cueille et al. (Reference Cueille, Green, Duffin, Hildebrandt and Benton2020) mentioned that many of the Gyrolepis albertii scales ended up in coprolites, and therefore, Gyrolepis albertii must have had a high presence in the British waters. However, an individual fish contains many scales that must have been indigestible, causing these scales to eventually end up in coprolites.
However, more analysis regarding parameters such as freshwater input, salinity and oxygen concentrations needs to be done in order to obtain an understanding of the differences between the two regions in Britain and the Netherlands, and similarly aged localities elsewhere.
Conclusions
The Winterswijk Rhaetian sediments yield many fossils of chondrichthyan and actinopterygian fishes. The most abundant taxon is the actinopterygian Gyrolepis albertii, followed by the chondrichthyan Lissodus minimus. Other actinopterygian fishes, including Saurichthys longidens, Birgeria acuminata, Sargodon tomicus and an unidentified pycnodontiform, have slightly more presence percentwise than the chondrichthyan teeth, which include Lissodus minimus, Rhomphaiodon minor, Parascylloides turnerae and some ‘Hybodus’ cf. cuspidatus. In addition to the teeth, also chondrichthyan dermal denticles, actinopterygian scales and gill rakers, tooth plates, and some fish bones were found. The faunal composition mirrors that of the contents of the subrosion pipe from Winterswijk described earlier (Diependaal & Reumer, Reference Diependaal and Reumer2021).
The most important taxa from the UK are the chondrichthyans Lissodus minimus and Rhomphaiodon minor, and the actinopterygians Gyrolepis albertii, Saurichthys longidens and Birgeria acuminata (the latter two taxa combined into the now obsolete genus Severnichthys). In the British Rhaetian deposits, the teeth of the chondrichthyans are more abundant than the actinopterygian teeth. That is contrary to our observations from Winterswijk, where more actinopterygian teeth are present. That difference could be caused by lower oxygen levels in bottom waters in Winterswijk and freshwater input and/or changes in salinity in the British Rhaetian, but more research is needed to falsify this.
Acknowledgements
We wish to thank Sibelco for entry to the quarry and for allowing the yearly excavation campaign. Its plant manager, Mr Gerard ten Dolle, is a great supporter of our research. Members of the Werkgroep Muschelkalk Winterswijk were helpful during the work in the quarry. We thank the crew of the 2019 excavation for sampling and initial washing of the samples, led by Prof. Anne Schulp and Mr Timo van Eldijk. Reviewers Dr C. J. Duffin and Dr W. Pawlak provided helpful suggestions that improved the manuscript.
Competing interests
This paper is based on the extensive MSc thesis of the first author (BdL) which is available for consultation on request. There are no competing interests.