Zn is an essential micronutrient for all animals, including fish. It serves important functions in various metabolic pathways, such as transcriptional regulation, protein synthesis and cellular signal recognition( Reference Watanabe, Kiron and Satoh 1 , Reference Zheng, Feeney and Kille 2 ). Dietary Zn deficiency is associated with anorexia, poor appetite, weight loss and growth retardation( Reference Watanabe, Kiron and Satoh 1 , Reference Luo, Tan and Zheng 3 , Reference Zheng, Luo and Hu 4 ). However, Zn can be toxic at high concentrations( Reference Zheng, Luo and Hu 4 – Reference Kambe, Tsuji and Hashimoto 6 ). Thus, as it is an essential but potentially toxic ion, a well-maintained Zn homoeostasis is crucial for all organisms. Compared to terrestrial animals, fish can absorb Zn from the diet and water but, at least in freshwater fish, the diet is the main pathway for Zn absorption( Reference Bury, Walker and Glover 7 ). Thus, in fish as well as in mammals, regulation of intestinal Zn absorption is crucial for health and survival( Reference Bury, Walker and Glover 7 ). Therefore, understanding the mechanisms of intestinal Zn uptake in fish is of considerable interest from both nutritional and toxicological perspectives. Studies suggested a morpho-functional specialisation of intestinal regions in fish with regard to ion transport( Reference Ojo and Wood 8 – Reference Ruhr, Takei and Grosell 10 ), and fish showed anterior–middle regionalisation of ion transport( Reference Ruiz-Jarabo, Gregorio and Gaetano 11 ). However, the mechanisms of absorption of mineral elements along the intestinal tract are far less understood( Reference Klinck and Wood 9 , Reference Ojo and Wood 12 ). Highlighting the relative importance of different sections of the intestine in ion transport processes, it is worth exploring the responsiveness of intestinal regionalisation to dietary mineral element addition.
Zn metabolism in higher eukaryotes is complicated, being regulated by a complex interplay of uptake and efflux transporter proteins, coupled with metal-dependent transcriptional control of selected transport and storage proteins( Reference Laity and Andrews 13 ). Recently, there have been advances in the understanding of genes and proteins involved in these processes and their regulation. ATPases are the membrane-bound enzymes responsible for the transport of ions through a biological membrane( Reference Marshall and Grosell 14 ). Cu-, Zn-superoxide dismutase (SOD) is a Zn-requiring enzyme that represents more than 90 % of the total SOD in cells, and plays important roles in intervening in the first transformation by dismutation of the superoxide free radicals (O2−) into H2O2 ( Reference Wang, Liu and Guo 15 ). The transport of Zn ions is controlled by two families of ion transporters, the ZnT (solute-linked carrier 30 family, SLC30A/ZnT) and Zips (solute-linked carrier 39 family, SLC39A/ZIP), which function in opposite directions of maintaining cellular Zn homoeostasis( Reference Liuzzi and Cousins 16 , Reference Huang and Tepaamorndech 17 ). The ZnT proteins, including ZnT1, ZnT5 and ZnT7, play critical roles in maintaining the cytoplasmic Zn balance by either transporting Zn out of cells or sequestrating Zn into intracellular compartments( Reference Liuzzi and Cousins 16 – Reference Palmiter 18 ). By contrast, SLC39 family proteins, including ZIP4 and ZIP5, function to increase the cytosolic Zn concentration by promoting Zn import from the extracellular space or Zn release from organelles( Reference Dufner-Beattie, Kuo and Gitschier 19 , Reference Geiser, De Lisle and Andrews 20 ). Metal response element-binding transcription factor-1 (MTF-1) functions as a cellular Zn sensor that coordinates the expression of genes involved in Zn homoeostasis. Metallothioneins (MT) are small, cysteine-rich, metal-binding proteins that play an important role in Zn homoeostasis and in detoxification of toxic metals( Reference Davis and Cousins 21 ). At present, studies on teleost Zn transporters have been mainly limited to model species because of the limitation of genomic resource in non-model fish species. Recently, Jiang et al. ( Reference Jiang, Zhang and Feng 22 ) identified a set of thirty-seven Zn transporters in the common carp genome, including seventeen from the SLC30 family (ZnT) and twenty from SLC39 family (ZIP). However, the underlying molecular mechanisms involved in the regulation of intestinal Zn transporters in response to dietary Zn in non-model species remain largely unknown.
In fish, lipids are known to be used as energy reserves, and carry out a vast array of functions. Accordingly, as in mammals, lipid absorption in fish occurs predominantly in the proximal part of the intestine( Reference Tocher 23 ). In mammals, studies indicated that Zn influences lipid metabolism( Reference Cunnane 24 , Reference Huang, Yu and Kirschke 25 ), indicating a close link between Zn and lipid metabolism. In general, lipid metabolism results from the balance between synthesis of fatty acids (lipogenesis) and fat catabolism via β-oxidation (lipolysis), and many key enzymes and transcriptional factors are involved in these metabolic processes. These enzymes include lipogenic enzymes (such as glucose-6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (6PGD), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS)) and lipolytic enzymes (such as carnitine palmitoyltransferase I (CPT I), hormone-sensitive lipase (HSL), adipose TAG lipase (ATGL))( Reference Elliott and Elliott 26 ). In addition, several transcription factors, such as PPAR and sterol-regulator element-binding protein (SREBP-1), play an intermediary role in lipid homoeostasis, by orchestrating the gene transcription of enzymes involved in these pathways( Reference Spiegelman and Flier 27 ).
Yellow catfish (Pelteobagrus fulvidraco), an omnivorous freshwater fish, is considered to be a good candidate for freshwater culture in China and other Asian countries because of its delicious meat and high market value. However, excessive lipid deposition in yellow catfish, which may affect the quality of harvest, is a problem. In our laboratory, Zheng et al. ( Reference Zheng, Luo and Hu 4 ) found that dietary deficiency and excess of Zn exerted a profound effect on lipid deposition and metabolism in the liver and muscle of yellow catfish P. fulvidraco. In the present study, we hypothesise that there would be regional differences in Zn transport and lipid metabolism, due to a differential distribution of these specific carriers. Hence, the objectives of the present study were to explore the potential mechanisms of dietary Zn regulating Zn metabolism and lipid metabolism in the fore- and mid-intestine in yellow catfish, which provided new insights into Zn nutrition in fish.
Methods
The experiment performed on animals followed the ethical guidelines of Huazhong Agricultural University for the care and use of laboratory animals, and the manuscript conformed to the Animal Research Reporting In Vivo Experiments (ARRIVE) Guidelines for Reporting Animal Research.
Diet preparation
A total of three experimental diets were formulated with ZnSO4.7H2O supplemented at levels of 0, 0·04 and 0·6 g/kg diet at the expense of cellulose (Table 1). Different Zn contents were added to the diets, based on our previous study( Reference Luo, Tan and Zheng 3 ), in order to produce three different dietary Zn groups (Zn deficiency, adequate Zn and Zn excess, respectively). The formulation of the experimental diets was according to Luo et al.( Reference Luo, Tan and Zheng 3 ). The formulated diets were dried at 80°C in an oven until the moisture was reduced to <10 %. The dry pellets were placed in plastic bags and stored at −20°C until feeding. The final Zn concentrations in the experimental diets were analysed in triplicate using inductively coupled plasma (ICP) atomic emission spectrometry, and the contents were 8·83 (Zn deficiency), 19·20 (adequate Zn) and 146·65 (Zn excess) mg Zn/kg diet, respectively.
Table 1 Feed formulation and proximate analysis of experimental dietsFootnote *
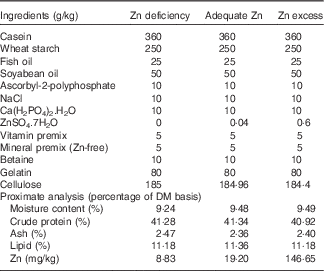
* Vitamin premix according to Luo et al. ( Reference Luo, Tan and Zheng 3 ); mineral premix according to Luo et al. ( Reference Luo, Tan and Zheng 3 ) without Zn addition. ZnSO4.7H2O (≥99·0 % in purity): Sinopharm Chemical Reagent Co. Ltd.
Experimental procedures
The experiment was conducted in Panjin Guanghe Crab Co. Ltd, Panjin, China. Yellow catfish were obtained from a local fish pond (Panjin, China). They were transferred to indoor cylindrical fibreglass tanks (90 cm height, 80 cm diameter) for 2 weeks of acclimatisation. Afterwards, 216 uniform-sized fish (initial mean weight: 0·81 (sem 0·01) g) were randomly assigned to nine fibreglass tanks with twenty-four fish per tank. The experiment was conducted in a semi-static aquarium system and continuously aerated to maintain dissolved O2 near saturation. The fish were fed to apparent satiation twice daily with two equal meals (09.00 and 16.00 hours) during the experiment. The amount of feed consumed by the fish in each tank was recorded daily and the dead fish was weighed, and feed intake (FI) and feed conversion rate (FCR)were calculated. Zn concentrations in water samples collected 10 min before and after feeding remained very low and negligible throughout the experiment. The experiment was continued for 8 weeks.
The experiment was conducted at ambient temperature and under a natural photoperiod (approximately 12 h light–12 h dark). Water quality parameters were monitored twice a week in the morning, and the ranges of the parameters were as follows: water temperature, 23·1–25·0°C; pH, 8·1–8·5; dissolved O2≥6·00 mg/l; NH4-N≤0·06 mg/l.
Sampling
At the end of the 8-week period, 24 h after the last feeding, all fish were euthanised (MS-222 at 100 mg/l), counted and weighed to determine survival, weight gain (WG) and specific growth rate (SGR). Then, fish were dissected and the contents of the intestine were gently scraped off. The fore-intestine (from the end of the stomach to the first loop of the intestine) and mid-intestine (from the first loop to the last loop) were used for the following analysis. A total of four fish per tank were randomly collected and dissected on ice to obtain the fore- and mid-intestine samples for TAG analysis. For enzyme activity and mRNA expression assays, the fore- and mid-intestine samples of twelve fish from each tank (six fish for enzymatic activities and six fish for mRNA expression) were removed immediately using sterile forceps, frozen in liquid N2 and stored at −80°C (not longer than 2 weeks) for further processing. Remaining samples were stored at −80°C for determining the Zn content.
Samples analysis
Enzymatic activity assays
For intestinal lipogenic enzyme analysis, the anterior- and mid-intestine samples were homogenised in three volumes of ice-cold buffer (0·02 m TRIS-HCl, 0·25 m sucrose, 2 mm EDTA, 0·1 m sodium fluoride, 0·5 mm phenylmethyl sulphonyl fluoride and 0·01 m-mercapto-ethanol; pH 7·4), and centrifuged at 20 000 g at 4°C for 30 min. The supernatant was collected separately, and the activities of five lipogenic enzymes were immediately assayed spectrophotometrically. The reaction was started by addition of the tissue extract. The changes in absorbance at 340 nm were monitored at intervals of 15 s for 3 min. 6PGD and G6PD activities were determined by the method of Barroso et al.( Reference Barroso, Peragón and Garcia-Salguero 28 ), malic enzyme (ME) activity following Wise & Ball( Reference Wise and Ball 29 ), isocitrate dehydrogenase (ICDH) activity according to Bernt & Bergmeyer( Reference Bernt and Bergmeyer 30 ), and FAS activity according to the method of Chang et al.( Reference Chang, Seidman and Teebor 31 ) as modified by Chakrabarty & Leveille( Reference Chakrabarty and Leveille 32 ). TAG content and activities of total ATPase and Cu-, Zn-SOD were determined using a commercial TAG assay Kit (A110-0), ATPase assay kit (A070-1) and a superoxide dismutase typed assay kit (A101-2) (Nanjing Jiancheng Bioengineering Institute), respectively. One unit of enzyme activity (U), defined as the amount of enzyme that converted 1 µmol of substrate to product per min at 30°C, was expressed as units/mg of soluble protein. Soluble protein concentration of homogenates was determined using the method of Bradford( Reference Bradford 33 ) with bovine serum albumin as the standard. These analyses were conducted in triplicates.
mRNA expression analysis (real-time fluorescence quantitative PCR)
Analyses at the gene-transcript levels were conducted using the real-time quantitative fluorescence PCR (qPCR) method. Frozen tissues were powdered in a liquid N2-chilled mortar and pestle. Total RNA was extracted from tissues using TRIzol Reagent (Invitrogen) based on the acid guanidinium thiocyanate–phenol–chloroform extraction method. A quantity of 2 µg of total RNA was used for reverse transcription with RevertAid™ Reverse Transcriptase (Fermentas) and an oligo-dT primer. qPCR assays were carried out in a quantitative thermal cycler (MyiQ™ 2 Two-Color Real-Time PCR Detection System; Bio-Rad) with a 20 μl reaction volume containing 10 μl of 2×SYBR® Premix Ex Taq™ (TaKaRa), 0·4 μl of 10 mm each of forward and reverse primers, 1 μl diluted complementary DNA (cDNA) template (10-fold), and 8·2 μl double-distilled H2O. Primers used for qPCR analysis of genes are given in Table 2. The qPCR parameters consisted of initial denaturation at 95°C for 30 s, followed by forty cycles at 95°C for 5 s, 57°C for 30 s and 72°C for 30 s. All reactions were performed in duplicates and each reaction was verified to contain a single product of the correct size using agarose gel electrophoresis. A non-template control and dissociation curve were performed to ensure that only one PCR product was amplified and that stock solutions were not contaminated. Standard curves were constructed for each gene using serial dilutions of stock cDNA. The relative expression levels were calculated using the
![]() $$2^{{{\minus}\Delta \Delta C_{T} }} $$
method(
Reference Livak and Schmittgen
34
) when normalising to the geometric mean of the best combination of two genes as suggested by geNorm(
Reference Vandesompele, De Preter and Pattyn
35
). Before the analysis, we performed an experiment to check the stability of housekeeping genes (β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein L7 (RPL7), beta-2-microglobulin (B2M), hypoxanthine-guanine phosphoribosyltransferase (HPRT), TATA-box-binding protein (TBP) and tubulin alpha chain (TUBA)), from which β-actin and B2M showed the most stable level of expression under the experimental conditions.
$$2^{{{\minus}\Delta \Delta C_{T} }} $$
method(
Reference Livak and Schmittgen
34
) when normalising to the geometric mean of the best combination of two genes as suggested by geNorm(
Reference Vandesompele, De Preter and Pattyn
35
). Before the analysis, we performed an experiment to check the stability of housekeeping genes (β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein L7 (RPL7), beta-2-microglobulin (B2M), hypoxanthine-guanine phosphoribosyltransferase (HPRT), TATA-box-binding protein (TBP) and tubulin alpha chain (TUBA)), from which β-actin and B2M showed the most stable level of expression under the experimental conditions.
Table 2 Primers used for real-time quantitative fluorescence PCR analysis

6PGD, 6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; CPT I, carnitine palmitoyltransferase I; HSL, hormone-sensitive lipase; ATGL, adipose TAG lipase; SREBP-1, sterol-regulator element-binding protein; MT, metallothionein; MTF-1, metal response element-binding transcription factor-1; RPL7, ribosomal protein L7; B2M, beta-2-microglobulin; HPRT, hypoxanthine-guanine phosphoribosyltransferase; UBCE, ubiquitin-conjugating enzyme; TUBA, tubulin alpha chain.
Zinc content
For the determination of Zn content, the fore- and mid-intestinal samples were digested in 3 ml concentrated nitric acid at 110°C for 72 h, and diluted to appropriate concentrations for Zn content using ICP-MS( Reference Chen, Luo and Chen 36 ). Quality assurance/quality control procedures included analysis of three method blanks (purified water), two certified biological reference tissues (DORM-2 and DORM-4; National Research Council of Canada) and two randomly selected duplicate samples per twenty samples. Recovery of Zn from certified biological reference tissues mentioned above ranged from 95 to 102 %.
Calculations
 $$\eqalignno{ & {\rm SR}\,\left( {{\rm survival}\,{\rm rate}} \right)\,{\equals}\,\left( {{\rm 1}00{\times}\left( {{\rm final}\,{\rm fish}\,{\rm number}} \right)/\left( {{\rm initial}\,{\rm fish}\,{\rm number}} \right)} \right) \cr & {\rm WG}\,\left( {{\rm weight}\,{\rm gain}} \right)\,{\equals}\,\left( {\left( {{\rm FBW}{\minus}{\rm IBW}} \right)/{\rm IBW}} \right){\times}{\rm 1}00\:\,\%\, \cr & {\rm SGR}\,\left( {{\rm specific}\,{\rm growth}\,{\rm rate},\,\%{\rm /d}} \right)\,{\equals}\,\left( {{\rm 1}00{\times}\left( {{\rm ln}\left( {{\rm FBW}} \right){\minus}{\rm ln }\left( {{\rm IBW}} \right)} \right)/{\rm d}} \right) \cr & {\rm FCR}\,\left( \% \right)\,{\equals}\,{\rm feed}\,{\rm intake}/\left( {\left( {{\rm FBW}-{\rm IBW}{\plus}{\rm dead}\,{\rm fish}\,{\rm weight}} \right),\,{\rm g}} \right). $$
$$\eqalignno{ & {\rm SR}\,\left( {{\rm survival}\,{\rm rate}} \right)\,{\equals}\,\left( {{\rm 1}00{\times}\left( {{\rm final}\,{\rm fish}\,{\rm number}} \right)/\left( {{\rm initial}\,{\rm fish}\,{\rm number}} \right)} \right) \cr & {\rm WG}\,\left( {{\rm weight}\,{\rm gain}} \right)\,{\equals}\,\left( {\left( {{\rm FBW}{\minus}{\rm IBW}} \right)/{\rm IBW}} \right){\times}{\rm 1}00\:\,\%\, \cr & {\rm SGR}\,\left( {{\rm specific}\,{\rm growth}\,{\rm rate},\,\%{\rm /d}} \right)\,{\equals}\,\left( {{\rm 1}00{\times}\left( {{\rm ln}\left( {{\rm FBW}} \right){\minus}{\rm ln }\left( {{\rm IBW}} \right)} \right)/{\rm d}} \right) \cr & {\rm FCR}\,\left( \% \right)\,{\equals}\,{\rm feed}\,{\rm intake}/\left( {\left( {{\rm FBW}-{\rm IBW}{\plus}{\rm dead}\,{\rm fish}\,{\rm weight}} \right),\,{\rm g}} \right). $$
Statistical analysis
The results were presented as means with their standard errors . Before statistical analysis, all data were tested for normality of distribution using the Kolmogorov–Smirnov test. Then, data from each treatment were subjected to one-way ANOVA. When overall differences were significant (P<0·05), Duncan’s multiple range test was used to compare significant differences between the treatments. Statistical analysis was performed using SPSS 19.0 for Windows.
Results
Growth performance
WG and SGR were numerically the highest in the adequate-Zn group but showed no significant differences between the other two groups (Table 3). FI, FCR and survival showed no significant differences between the three treatments.
Table 3 Effect of dietary zinc levels on growth performance of yellow catfish after 8 weeks (Mean values with their standard errors; n 3 replicate tanks, five fish were sampled for each tank)
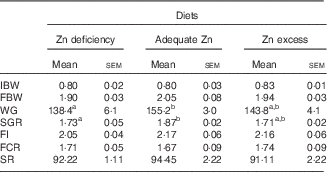
IBW, initial mean body weight; FBW, final mean body weight; WG, weight gain; SGR, specific growth rate; FI, feed intake; FCR, feed conversion rate; SR, survival rate.
a,b Mean values with unlike superscript letters were significantly different between the different dietary Zn groups (P<0·05).
Zinc and TAG contents in the intestine
In the fore-intestine and mid-intestine, Zn contents increased with increasing dietary Zn levels (Fig. 1(A)). In contrast, TAG contents in the fore-intestine and mid-intestine decreased with increasing dietary Zn levels (Fig. 1(B)).

Fig. 1 Effect of dietary zinc levels on zinc (A) and TAG (B) contents in the intestine of yellow catfish. Values are means (n 3 replicate tanks), with standard errors represented by vertical bars. For zinc content analysis, four to six fish sampled for each tank; for TAG content, four fish were sampled for each tank. ![]() , Zinc deficiency;
, Zinc deficiency; ![]() , adequate zinc;
, adequate zinc; ![]() , zinc excess. a,b,c Mean values with unlike letters were significantly different among three treatments (P<0·05).
, zinc excess. a,b,c Mean values with unlike letters were significantly different among three treatments (P<0·05).
Activities of intestinal enzymes involved in zinc transport
In the fore-intestine and mid-intestine, Cu-, Zn-SOD and total ATPase activities increased with increasing dietary Zn levels (Fig. 2).

Fig. 2 Effect of dietary zinc levels on Cu-, Zn-superoxide dismutase (SOD) (A) and ATPase (B) activities in the intestine of yellow catfish. Values are means (n 3 replicate tanks, 6 fish were sampled for each tank), with standard errors represented by vertical bars. ![]() , Zinc deficiency;
, Zinc deficiency; ![]() , adequate zinc;
, adequate zinc; ![]() , zinc excess. a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
, zinc excess. a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
mRNA expression levels of genes involved in zinc metabolism
In the fore-intestine, mRNA levels of ZnT1, ZnT5, ZnT7, MT and MTF-1 tended to increase with increasing dietary Zn levels (Fig. 3(A)). mRNA levels of ZnT1, ZnT5, ZnT7, MT and MTF-1 in the Zn-excess group were the highest and were significantly higher than those in the other two groups. By contrast, ZIP5 mRNA levels declined with increasing dietary Zn levels. ZIP4 and ZIP5 mRNA levels were the lowest for fish fed the Zn-excess diet and showed no significant difference between the other two groups.

Fig. 3 Effect of dietary zinc levels on the mRNA levels of genes involved in zinc metabolism in the fore-intestine (A) and mid-intestine (B) of yellow catfish. Values are means (n 3 replicate tanks, 6 fish were sampled for each tank), with standard errors represented by vertical bars. ![]() , Zinc deficiency;
, Zinc deficiency; ![]() , adequate zinc;
, adequate zinc; ![]() , zinc excess; MT, metallothioneins; MTF-1, metal response element-binding transcription factor-1. mRNA expression values were normalised to β-actin and beta-2-microglobulin (B2M) expressed as a ratio of the control (control=1). a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
, zinc excess; MT, metallothioneins; MTF-1, metal response element-binding transcription factor-1. mRNA expression values were normalised to β-actin and beta-2-microglobulin (B2M) expressed as a ratio of the control (control=1). a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
In the mid-intestine, dietary Zn addition up-regulated mRNA levels of ZnT1, ZnT5 and ZnT7 (Fig. 3(B)). mRNA levels of ZnT5 and ZnT7 in the Zn-excess group were the highest and were significantly higher than those in the other two groups. mRNA levels of ZIP4 and ZIP5 were the lowest for fish fed the Zn-excess diet and showed no significant differences between the other two groups. ZnT1, MT and MTF-1 mRNA levels were the highest for fish fed the Zn-excess diet and showed no significant differences between the other two groups.
Intestinal lipogenic enzyme activities
In the fore-intestine, 6PGD, ME and FAS activities tended to decrease with increasing dietary Zn levels (Fig. 4(A)). G6PD activity in the Zn-deficiency group was significantly higher than that of the adequate- and excess-Zn groups. ICDH activity was the highest for fish fed the adequate-Zn diet and showed no significant differences between the other two groups. FAS activity in the Zn-excess group was the lowest and was significantly lower than that in the other two groups.

Fig. 4 Effect of dietary zinc levels on enzyme activities involved in lipid metabolism in the fore-intestine (A) and mid-intestine (B) of yellow catfish. Values are means (n 3 replicate tanks, 6 fish were sampled for each tank), with standard errors represented by vertical bars. ![]() , Zinc deficiency;
, Zinc deficiency; ![]() , adequate zinc;
, adequate zinc; ![]() , zinc excess; 6PGD, 6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase; ME, malic enzyme; ICDH, isocitrate dehydrogenase; FAS, fatty acid synthase. a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
, zinc excess; 6PGD, 6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase; ME, malic enzyme; ICDH, isocitrate dehydrogenase; FAS, fatty acid synthase. a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
In the mid-intestine, G6PD and ME activities tended to decrease with increasing dietary Zn levels (Fig. 4(B)). G6PD and ME activities in the Zn-deficiency group was the highest and was significantly higher than that of the adequate- and excess-Zn groups. ICDH activity in the Zn-excess group was the lowest and it showed no significant difference between the adequate-Zn and Zn-deficiency groups. FAS activities were the highest in the Zn-deficiency group and they showed no significant differences between the other two groups. 6PGD showed no significant differences between the three treatments.
mRNA expression levels of genes involved in lipid metabolism
In the fore-intestine, mRNA levels of G6PD and FAS tended to decline, but mRNA levels of CPT IA and HSLa tended to increase with increasing dietary Zn levels (Fig. 5(A)). mRNA levels of G6PD, 6PGD, FAS and ACCa in the Zn-excess group were significantly lower than those of the adequate-Zn and Zn-deficiency groups. CPT IA, HSLa and ATGL mRNA levels for fish fed the Zn-deficient diet were significantly lower than those of the adequate- and excess-Zn groups. PPARα mRNA levels were the highest for fish fed the Zn-excess diet, and showed no significant differences between the other two groups. mRNA levels of PPARγ and SREBP-1 were the highest for fish fed the Zn-deficient diet and showed no significant differences between the other two groups.

Fig. 5 Effect of dietary zinc levels on the mRNA levels of genes involved in lipid metabolism in the fore-intestine (A) and mid-intestine (B) of yellow catfish. Values are means (n 3 replicate tanks, 6 fish were sampled for each tank), with standard errors represented by vertical bars. ![]() , Zinc deficiency;
, Zinc deficiency; ![]() , adequate zinc;
, adequate zinc; ![]() , zinc excess; 6PGD, 6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase; FAS, fatty acid synthase; ACCa, ACC, acetyl-CoA carboxylase; CPT I, carnitine palmitoyltransferase I; HSL, hormone-sensitive lipase; ATGL, adipose TAG lipase; SREBP-1, sterol-regulator element-binding protein. mRNA expression values were normalised to β-actin and beta-2-microglobulin (B2M) expressed as a ratio of the control (control=1). a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
, zinc excess; 6PGD, 6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase; FAS, fatty acid synthase; ACCa, ACC, acetyl-CoA carboxylase; CPT I, carnitine palmitoyltransferase I; HSL, hormone-sensitive lipase; ATGL, adipose TAG lipase; SREBP-1, sterol-regulator element-binding protein. mRNA expression values were normalised to β-actin and beta-2-microglobulin (B2M) expressed as a ratio of the control (control=1). a,b,c Mean values with unlike letters were significantly different between the three treatments (P<0·05).
In the mid-intestine, dietary Zn addition tended to down-regulate mRNA levels of 6PGD, FAS, ACCa, PPARγ and SREBP-1, but to up-regulate the mRNA expression of HSLa and ATGL (Fig. 5(B)). mRNA levels of G6PD, 6PGD and FAS in the Zn-excess group were significantly lower than those of the adequate-Zn and Zn-deficiency groups. ACCa mRNA level in the Zn-deficiency group was significantly higher than that in the other two groups. The highest PPARα expression was observed for fish fed the Zn-excess diet and showed no significant differences between other two groups. CPT IA mRNA levels were the highest for fish fed the adequate-Zn diet and lowest in the Zn-deficient group. PPARγ mRNA level for fish fed the Zn-deficient diet was significantly higher than that of the adequate-Zn and Zn-deficiency groups. SREBP-1 mRNA level was the highest for fish fed the Zn-deficient diet, but showed no significant differences between the other two groups.
Discussion
Previous studies in yellow catfish have focused on the changes in lipid deposition and metabolism in the liver, muscle and adipose tissue( Reference Hogstrand and Wood 5 , Reference Zheng, Luo and Zhuo 37 , Reference Wei, Wu and Gao 38 ), and lipid metabolism in the intestine has been neglected. To our knowledge, this is the first study aimed at deducing the basic mechanisms of intestinal lipid metabolism in combination with Zn transport in a fish species.
In the present study, dietary Zn addition induced intestinal Zn accumulation, in agreement with other reports( Reference Glover and Hogstrand 39 , Reference Feng, Tan and Liu 40 ). To explore the mechanism of Zn absorption and accumulation, we analysed the activities of enzymes and/or the mRNA expression of genes involved in Zn metabolism. Our results indicated that dietary Zn addition tended to increase total ATPase and Cu-, Zn-SOD activities. The increases in ATPase activity could possibly occur because of the maintenance of the ion flux( Reference Eroglu and Canli 41 ). Kavitha & Rao( Reference Kavitha and Rao 42 ) suggested that the induction in antioxidant enzymatic activities could be an adaptive response to toxicant stress and to neutralise the impact of ROS generated. The report demonstrated that dietary Zn excess up-regulated mRNA levels of ZnT1, ZnT5 and ZnT7, but down-regulated mRNA levels of ZIP4 and ZIP5 in the fore-intestine and mid-intestine. ZnT proteins are characterised by their ability to decrease the cytosolic Zn concentration by transporting Zn out of cells or into intracellular compartments( Reference Kirschke and Huang 43 ). In mammals and in zebrafish, dietary Zn regulates Zn-transport activities and Zn-transporter gene expression in the intestine( Reference Zheng, Feeney and Kille 2 , Reference Liuzzi, Blanchard and Cousins 44 ). Studies reported the up-regulation of mRNA levels of ZnT1( Reference Liuzzi, Blanchard and Cousins 44 , Reference McMahon and Cousins 45 ), which, in turn, reduced cytosolic Zn availability( Reference Dufner-Beattie, Kuo and Gitschier 19 ). ZnT5 and ZnT7 are localised on the membrane of the Golgi apparatus as well as the cytoplasmic vesicles, and these transporters have been shown to transport Zn into the secretory pathway for Zn sequestration and/or Zn supply to the proteins that require Zn for structural formation or activities( Reference Huang and Tepaamorndech 17 , Reference Kirschke and Huang 43 ). Thus, increased ZnT5 and ZnT7 mRNA expression will increase the transport of Zn from the cytosol into the secretory pathway for Zn sequestration and, accordingly, reduce Zn toxicity. These regulatory patterns would be consistent with functions of the corresponding proteins in Zn efflux from the cytosol, and reflect the need for greater Zn-export capacity from the cytosol, which helps delay intracellular Zn toxicity in the intestine. The present study indicated that dietary Zn addition down-regulated mRNA levels of ZIP4 and ZIP5 in the fore-intestine and mid-intestine. Increased ZIP4 mRNA expression results in increased dietary Zn absorption in response to Zn restriction, as suggested by several studies( Reference Liuzzi and Cousins 16 , Reference Dufner-Beattie, Wang and Kuo 46 ). Kambe et al.( Reference Kambe, Tsuji and Hashimoto 6 ) pointed out that ZIP5 was functional as a Zn importer and ZIP5 expression decreased in Zn-depleted environments. Taken together, these changes suggested that the fractional Zn absorbed from the diet decreased and the export of Zn from the intestinal epithelium increased as dietary Zn load increased, indicating the presence of a mechanism for regulating the metabolism of dietary Zn. Our result showed that MT mRNA levels in the fore-intestine and mid-intestine were up-regulated when fish were fed with excessive dietary Zn, in agreement with many other studies( Reference Zheng, Feeney and Kille 2 , Reference McMahon and Cousins 45 , Reference Chen, John and Lin 47 ). In the present study, dietary Zn excess induced the up-regulation of MTF-1 mRNA level in the fore-intestine and mid-intestine, together with the up-regulation of ZnT1 and MT gene expression. MTF-1 is important for the induction of ZnT1 and MT expression by Zn, via binding to multiple metal-responsive elements in the 5' regulatory regions of their respective genes’ promoter( Reference Chen, John and Lin 47 , Reference Langmade, Ravindra and Daniels 48 ). The activation of MTF-1 in response to Zn parallels increases in the relative rate of transcription of MT-1 and ZnT1( Reference McMahon and Cousins 45 , Reference Langmade, Ravindra and Daniels 48 , Reference Muylle, Robbens and De Coen 49 ). Thus, these results reported, generally, similar rates in fore- and mid-intestinal segments, which was not surprising in view of the fact that activities and gene expression related to Zn uptake and transport were similar in the two segments, although exceptions had been reported.
The present study indicated that, in the fore-intestine, dietary Zn addition reduces TAG content, activities of 6PGD, G6PD, ME and FAS as well as down-regulated mRNA levels of 6PGD, G6PD and FAS. G6PD, 6PGD, ICDH, ME and FAS are the key regulatory enzymes and genes involved in lipogenesis( Reference Elliott and Elliott 26 ). The reduction in activities of lipogenic enzymes (6PGD, G6PD, ME and FAS) and gene expression (6PGD, G6PD and FAS) in response to dietary Zn addition would contribute to the reduced TAG content. Similarly, Eder & Kirchgessner( Reference Eder and Kirchgessner 50 ) reported that dietary Zn-deficiency increased the activities of lipogenic enzymes (6PGD and G6PD) in rats. Our study also found that the changes in activities of FAS, 6PGD and G6PD were in parallel with the changes of their mRNA expression, suggesting that these enzymes were regulated by Zn at the transcriptional level. CPT I, HSLa and ATGL are three key genes involved in lipolysis( Reference Abu-Elheiga, Almarza-Ortega and Baldini 51 , Reference Kerner and Hoppel 52 ). The present study indicated that dietary Zn addition up-regulated mRNA levels of CPT IA, HSLa and ATGL in the fore-intestine. The up-regulation of these lipolytic enzymatic genes following dietary Zn addition would increase lipolysis, which, in turn, would reduce TAG content, as observed in the present study. Similarly, Zheng et al.( Reference Zheng, Luo and Hu 4 ) found that hepatic lipid contents declined with increasing dietary Zn levels, in parallel with increasing mRNA levels of CPT IA, G6PD and 6PGD, and reduced mRNA levels of ACCa and FAS, in yellow catfish. Our study indicated that, in the mid-intestine, dietary Zn addition reduced TAG contents, activities of G6PD, ME, FAS and ICDH and mRNA levels of 6PGD, G6PD, FAS and ACCa, but up-regulated mRNA levels of HSLa, ATGL and CPT IA. Thus, our study clearly suggested that dietary Zn supplementation decreased TAG content by up-regulating lipolysis and down-regulating lipogenesis in the mid-intestine of yellow catfish. We also found that changes in 6PGD activity in the mid-intestine were not attributable to the change of its mRNA expression. Similarly, enzymatic activities were not always accompanied by parallel changes in mRNA levels( Reference Ibanez, Peinado-Onsurbe and Sanchez 53 ). The mismatch between gene expression and enzyme-protein level may be involved in the time-lag effect between transcription and translation and/or RNA stability( Reference Rigault, Le Borgne and Tazir 54 ). On the other hand, our observations reveal the similar mRNA expression of genes involved in lipid metabolism between the fore- intestine and mid-intestine after diet-borne Zn exposure, indicating a similar regulatory mechanism in Zn-induced changes of lipid metabolism between the fore- and mid-intestine of yellow catfish.
It is well documented that expression patterns of lipid metabolic pathway genes are, primarily, governed by SREBP-1 and PPAR( Reference Spiegelman and Flier 27 , Reference Yahagi, Shimano and Hasty 55 ). The present study indicated that dietary Zn addition up-regulated PPARα mRNA levels, but down-regulated mRNA levels of PPARγ and SREBP-1 in the fore-intestine. PPARα plays key roles in the catabolism of fatty acids by increasing the expression of key lipolytic enzymes( Reference Ribet, Montastier and Valle 56 ). In the fore-intestine, the up-regulation of PPARα, together with the increase in the transcriptional levels of lipolytic genes CPT IA, HSLa and ATGL, might contribute to the reduction of TAG content by Zn-added diets, which resulted in reduced TAG content in the fore-intestine. Similarly, Zheng et al.( Reference Zheng, Luo and Hu 4 ) indicated that the mRNA expression of PPARα was significantly reduced during Zn deficiency and that this effect was reversible by Zn supplementation. SREBP-1 and PPARγ activate genes involved in lipogenesis( Reference Amemiya-Kudo, Shimano and Hasty 57 , Reference Barish 58 ). SREBP-1 and PPARγ mediated TAG synthesis and accumulation by the regulation of genes involved in lipogenesis at the transcriptional level( Reference Rosen, Sarraf and Troy 59 ). Thus, the reduction in TAG deposition in the fore-intestine by Zn addition might be explained by the suppression of both SREBP-1 and PPARγ, together with the concomitant reduction in the activities of lipogenic enzymes 6PGD, G6PD, ME and FAS, and mRNA levels of 6PGD, G6PD and FAS. Shen et al.( Reference Shen, MacDonald and Bruemmer 60 ) pointed out that the expression of PPARγ was significantly reduced during Zn deficiency. Amemiya-Kudo et al. ( Reference Amemiya-Kudo, Shimano and Hasty 57 ) suggested that SREBP-1 positively regulated ACC through binding to regulatory sequences in the promoter, and subsequently enhanced the transcription level. By contrast, Zheng et al.( Reference Zheng, Luo and Hu 4 ) found that mRNA levels of PPARα and SREBP-1 increased, but PPARγ mRNA levels reduced in the liver with increasing dietary Zn levels. In the mid-intestine, dietary Zn addition tended to down-regulate mRNA levels of PPAR and SREBP-1, in parallel with the reduced activities of G6PD, ME, FAS and ICDH, and mRNA levels of 6PGD, G6PD, FAS, ACCa. Thus, the present study indicated that PPARα, PPARγ and SREBP-1 mediated the regulation of lipid deposition and metabolism in the fore- and mid-intestine in yellow catfish. Similarly, several studies reported that these signalling pathways widely mediated mineral element-induced changes in hepatic lipid deposition in fish( Reference Chen, Luo and Chen 36 , Reference Wei, Wu and Gao 38 ).
In conclusion, dietary Zn supplementation increased Zn accumulation but reduced TAG content in both the fore- and mid-intestine of yellow catfish. Increased Zn accumulation was attributable to the changes in enzymatic activities and mRNA expression of genes involved in Zn absorption and transportation. The reduced TAG content in both the fore- and mid-intestine of yellow catfish were attributable to the up-regulated lipolysis and down-regulated lipogenesis. Our observations also revealed the similar mRNA expression of genes involved in Zn and lipid metabolism between the fore- and mid-intestine after dietary Zn addition, indicating presence of the same or similar regulatory mechanisms of Zn and lipid metabolism in the fore- and mid-intestine of yellow catfish.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 31422056).
Contributions of authors are as follows: Z. L. and G.-H. C. designed the experiment and analysed the data; G.-H. C. conducted the feeding trial, sample analysis and drafted the article; D.-G. Z. and S.-C. L. assisted with conducting the feeding trial and sample analysis; K. W. helped with analysis of gene expression and enzymatic activities; Z. L. and C. H. revised the manuscript; all authors read and approved the final paper.
The authors declare that there are no conflicts of interest.











