Mild traumatic brain injury (mTBI) is a frequent reason for consultation in the emergency department (ED). In Canada, the incidence of mTBI is estimated at 500–600 cases per 100,000 person-years.Reference Ryu, Feinstein, Colantonio, Streiner and Dawson1 In order to refine current clinical decision rules to avoid unnecessary CT scans or identify patients at risk of post-concussion symptoms (PCS), a number of biomarkers have been evaluated, notably S-100β protein.Reference Rodriguez-Rodriguez, Egea-Guerrero and Leon-Justel2 S-100β is a calcium-binding protein found in neural and non-neural tissues and is thought to participate in the regulation of cellular calcium homeostasis and other biological processes such as cell proliferation, differentiation and survival.Reference Michetti, Corvino and Geloso3 Serum levels of S-100β protein seem to be increased following a traumatic brain injury (TBI).Reference Hallen, Karlsson, Carlhed, Hallgren and Bergenheim4 Since this is a possible association between intracranial injury and S-100β protein, this protein was suggested as a biomarker by the American College of Emergency PhysiciansReference Jeter, Hergenroeder, Hylin, Redell, Moore and Dash5 and Scandinavian Neurotrauma Committee CT guidelinesReference Unden, Calcagnile, Unden, Reinstrup and Bazarian6 in order to avoid unnecessary CT scans in patients with an mTBI presenting low levels of S-100β serum levels (<0.10 µg/L or 100 pg/mL) within 4–6 h of injury.
Injury biomarkers such as S-100β protein have been reported to be detectable in urine,Reference Hallen, Karlsson, Carlhed, Hallgren and Bergenheim4 cerebrospinal fluid (CSF) and serum after a TBI.Reference Dash, Zhao, Hergenroeder and Moore7 Serum samples are not routinely taken in patients with mTBI. Urine samples are non-invasive and would be less burdensome in busy settings. However, we do not know if the concentration and stability of S-100β protein would interfere with its clinical use. In severe TBI, although S-100β protein levels were detectable in urine, their prognostic values (sensitivity, specificity, negative and positive predictive values) and capacity to discriminate between patients who survived or not a month following injury were systematically poorer than serum levels.Reference Rodriguez-Rodriguez, Egea-Guerrero and Leon-Justel2 Moreover, the best cut-off values for serum and urine levels were 0.461 µg/L (461 pg/mL) and 0.025 µg/L (25.1 pg/mL) respectively,Reference Rodriguez-Rodriguez, Egea-Guerrero and Leon-Justel2 suggesting that urine levels are considerably lower than serum levels. The utility of S-100β protein levels in urine remains unclear and warrants further evaluation,Reference Thelin, Nelson and Bellander8 notably in mTBI patients.
In this study, we aimed (1) to determine if S-100β protein was detectable during the acute phase in urine samples of patients who suffered an mTBI and presented at the ED, notably when it was detectable in plasma samples using commonly found Enzyme-Linked Immunosorbent Assay (ELISA) technology; (2) to assess the correlation of S-100β protein concentrations between plasma and urine samples; and (3) to compare sampling delays between patients with vs. without a detectable S-100β protein concentration for plasma and urine samples.
We conducted this prospective cohort study at the Hôpital de l’Enfant-Jésus du CHU de Québec-Université Laval, a Level 1 trauma center in eastern Quebec. Eligible patients were identified by treating physicians as having sustained an mTBI, defined as a Glasgow Coma Scale score (GCS) of 13–15 at presentation and at least one of the following: confusion or disorientation, loss of consciousness for 30 min or less, post-traumatic amnesia for less than 24 h or neurological focal symptoms following a head injury.Reference Holm, Cassidy, Carroll and Borg9 We excluded patients if they were assessed more than 24 h after their injury, were admitted to the hospital, were unable to communicate or did not speak English or French.
Once patients gave their written consent, emergency physicians collected clinical and sociodemographic data and research nurses collected blood and urine samples. Information on time of head trauma and sampling was recorded by research nurses. Information was entered into a secured database and data accuracy was ensured by a research assistant using the patient medical record. Samples were centrifuged, put into three small aliquots and stored at −20°C. Within 2 weeks, frozen samples were sent to the central lab and stored at −80°C. According to the manufacturers’ protocols, S-100β protein levels were measured using Human Protein ELISA kit from Millipore Sigma® (Germany) for plasma samples and from Signalway Antibody® (USA) for urine samples. The detection limits were respectively 2.7 and 15.6 pg/mL.
Our main outcome was the presence of S-100β protein in urine samples, defined as any detectable value as measured by ELISA. Our secondary outcomes were S-100β protein plasma and urine levels within 6 and 24 h of injury.
We presented our descriptive analyses using proportions and medians with their corresponding measures of dispersion for categorical and continuous variables, respectively. Time of sampling and S-100β protein concentrations were right-skewed (Shapiro-Wilk, p < 0.01), we thus compared distributions using Wilcoxon rank sum tests and assessed correlation with the Spearman rank correlation coefficient. We report the present study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. We performed all analyses with Statistical Analysis System® (SAS Institute Inc., Cary, North Carolina, USA, v. 9.4). The project was approved by the CHU de Québec-Université Laval Research Ethics Board.
Between May 6, 2014 and April 9, 2015, 46 patients were eligible for our study with adequate biological material for laboratory analyses. Among these, 33 (72%) were males and 37 (80%) were aged between 14 and 54 years old, and 1 person in 2 sustained a concomitant injury (Table 1).
Table 1: Clinical and sociodemographic characteristics of patients (n = 46)
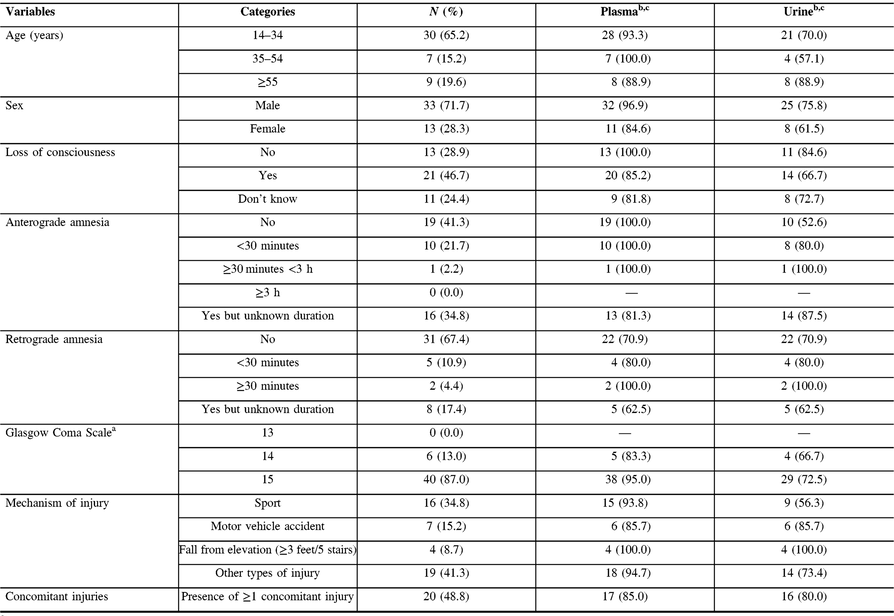
a Score on arrival at emergency department.
b Detectable values in plasma (n = 42) and urine (n = 33) samples, n (%).
c Limit of detection: plasma >2.7 pg/mL, urine >15.6 pg/mL.
S-100β protein concentrations were detectable in 42 (91%) and 33 (71%) patients in plasma and urine, respectively (Table 1). S-100β protein concentrations in plasma were not correlated with that in urine (Spearman r = 0.002, p = 0.99). In plasma, median S-100β protein concentrations in patients sampled within 6 h were similar to that in patients whose samples were taken within 24 h of trauma; however, in urine, median S-100β protein concentrations were slightly higher in patients sampled <6 h (Table 2). Median delays for plasma and urine sampling in all patients were similar (Table 3).
Table 2: Information on S-100β protein concentrations for detectable valuesa
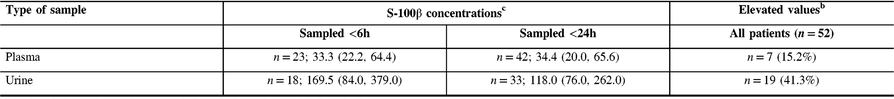
a Limit of detection: plasma >2.7 pg/mL, urine >15.6 pg/mL.
b ≥100 pg/mL.
c Median (Q1, Q3) in picograms per millilitre (pg/mL).
Table 3: S-100β protein sampling delaya

a Unknown delay in one patient for plasma samples.
b Delay between trauma and plasma or urine sampling in hours (median [Q1, Q3]).
c Detectable values of S-100β protein concentration (limit of detection: plasma 2.7 pg/mL, urine 15.6 pg/mL).
d Two-sided Wilcoxon rank sum test.
There were some limitations to our study. Our study sample included only 46 patients, but all patients had at least one symptom among amnesia, confusion, loss of consciousness or focal neurological signs, so our conclusions apply to these patients. Limits of detection of the ELISA kits used for plasma and urine differed slightly and any protein concentration below these limits could not be measured. However, S-100β protein was detectable in most plasma samples and 71% of urine samples and the corresponding S-100β protein values were not correlated with each other. So far, the potential usefulness of S-100β protein has been related to elevated values (e.g. ≥100 pg/mL) largely above our limits of detection (i.e. 2.7 or 15.6 pg/mL). Therefore, more sensitive urine assays would be unlikely to change our results.
According to some authors, S-100β protein levels could be of interest for patients with an mTBI, notably during the acute phase to determine the need to perform a head CT scan to identify intracranial lesions requiring a neurosurgical intervention.Reference Unden, Calcagnile, Unden, Reinstrup and Bazarian6, Reference Zongo, Ribereau-Gayon and Masson10 However, our results suggest that even when they could be measured in urine, S-100β protein concentrations from urine samples were not correlated with those in plasma samples of mTBI patients and are thus unlikely to yield clinically actionable information in an acute ED setting using currently available ELISA technology.
The ideal time frame for sampling remains controversial, but information on kinetic patterns and physiological pathways suggests that it has important clinical consequences. In adult patients with severe TBI, S-100β protein kinetic patterns of serum and urine levels have been shown to differ. In a study conducted by Rodriguez et al.,Reference Rodriguez-Rodriguez, Egea-Guerrero and Leon-Justel2 in both cases, a peak was reached in the first sample within 6 h of injury. Serum levels gradually decreased until 96 h while urine levels decreased until 48 h but increased up to 96 h.Reference Rodriguez-Rodriguez, Egea-Guerrero and Leon-Justel2 For practical purposes, serum and urine samples are often taken at the same time, even though S-100β protein physiological pathways suggest temporal differences. Following a TBI, the bloodbrain barrier is often disrupted and proteins are leaked into the serum. Serum half-life of S-100β protein is estimated to be 30,Reference Hallen, Karlsson, Carlhed, Hallgren and Bergenheim4 60Reference Dash, Zhao, Hergenroeder and Moore7 or even 120 min.Reference Thelin, Nelson and Bellander8 Persistent elevations of S-100β protein levels thus indicate a passive release or an active secretion from damaged tissues.Reference Rodriguez-Rodriguez, Egea-Guerrero and Leon-Justel2, Reference Thelin, Nelson and Bellander8 Urine samples taken within 2 h of injury might be uncorrelated with intracranial injury but we found insufficient evidence to draw any firm conclusions. Some authors suggest that if both samples are taken too early (e.g., <12 h in severe TBI), serum and urine samples could still be confounded by extracranial S-100β protein influx.Reference Thelin, Nelson and Bellander8 Under these conditions, the serum and urine sampling times that would allow the best potential correlation with patient’s diagnosis or prognosis might differ.
In summary, the results of our study suggest that urine samples measured with ELISA technology are unlikely to be as useful as serum samples to measure S-100β protein concentration to guide clinical management of adults with mTBI during the acute phase in the ED. This was the first study to compare S-100β protein levels in plasma and urine in this population. Future studies should assess the relevance of using urine samples with other biomarkers in the ED context.
Acknowledgements
The authors would like to thank Marilyne Dufresne for supervising patient recruitment, Daniel Robin for his support with the database, Françoise Morin and Dounia Hamoudi for laboratory analyses and all emergency physicians of the Hôpital de l’Enfant-Jésus du CHU de Québec-Université Laval for their involvement in this study.
Disclosures
Natalie Le Sage received a CIHR «Catalyst Grant – Mild Traumatic Brain Injury (#290457)» for this project. The other authors have no conflicts of interest.
Statement of authorship
NL, JF, ME, JMC, LM, PA and JP conceived the study and obtained research funding. NL undertook recruitment of patients and managed data. JF and ABP were responsible for human products and laboratory analyses. NL and PAT provided statistical advice on study design and analysed the data. PAT drafted the manuscript, NL critically revised it and all remaining authors contributed substantially to its final revision. NL takes responsibility for the paper as a whole.





