Overweight and obesity have increased at alarming rates worldwide and currently afflict 76 % of adult women in Mexico(Reference Shamah-Levy, Cuevas-Nasu and Dommarco-Rivera1), highlighting the importance of identifying the risk factors for excess weight in this population. Pregnancy and childbearing are risk factors for weight gain and obesity in women(Reference Cohen, Chaffee and Rehkopf2–Reference Mazariegos, Ortiz-Panozo and Gonzalez de Cosio5). Weight gain after childbirth may be the result of postpartum weight retention (PPWR) (i.e. retention of gestational weight), postpartum weight gain (PPWG) (i.e. weight gain that occurs entirely in the postpartum period)(Reference Kew, Ye and Hanley6–Reference Soria-Contreras, Rifas-Shiman and Aris11) or a combination of weight retention and gain (PPWR + WG) (i.e. retention of gestational weight, followed by postpartum weight gain)(Reference Kew, Ye and Hanley6,Reference Soria-Contreras, Rifas-Shiman and Aris11,Reference Abebe, Von Soest and Von Holle12) . We recently showed that PPWR, PPWG and PPWR + WG were associated with increased adiposity at 6 years postpartum(Reference Soria-Contreras, Trejo-Valdivia and Cantoral13). PPWR + WG was the only pattern directly associated with metabolic markers, such as insulin resistance(Reference Soria-Contreras, Trejo-Valdivia and Cantoral13). These adverse patterns of weight change are preventable. Therefore, it is essential to understand which characteristics make women more likely to experience them.
Different factors may predispose women to experience weight gain v. retention after delivery. Gestational weight gain (GWG) is a consistent and strong predictor of PPWR(Reference Rong, Yu and Han14,Reference Mannan, Doi and Mamun15) . Pregestational BMI has shown an inverse association with PPWR(Reference Rong, Yu and Han14,Reference Rode, Kjærgaard and Ottesen16) , and a positive association with PPWG(Reference Lipsky, Strawderman and Olson9). In some studies, exclusive breast-feeding has been associated with lower PPWR(Reference Brandhagen, Lissner and Brantsaeter17), but other studies have not found this association(Reference Chowdhury, Sinha and Sankar18,Reference Neville, McKinley and Holmes19) . To date, most of the published studies have focused on identifying the predictors of PPWR(Reference Rong, Yu and Han14–Reference Neville, McKinley and Holmes19). Few studies have identified factors associated with PPWG(Reference Lipsky, Strawderman and Olson9), and, to the best of our knowledge, no studies have looked at the predictors of PPWR + WG within the first year after delivery. Furthermore, most of the studies have included primarily white women living in the USA, western Europe or Australia. Little is known about predictors and patterns of weight change in other settings, including Mexico. The objective of the current study was to evaluate the associations of pregestational BMI, GWG and breast-feeding with four mutually exclusive patterns of weight change during the first year postpartum: PPWR, PPWG, PPWR + WG and return to pregestational weight.
Methods
Study population
This was a secondary analysis of 948 mothers participating in the prospective birth cohort Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS). A full description of the cohort is provided elsewhere(Reference Braun, Wright and Just20,Reference Tamayo, Ortiz and Téllez-Rojo21) . Briefly, PROGRESS is an ongoing prospective cohort study initiated in Mexico City in 2007. Between July 2007 and February 2011, women with a singleton pregnancy who received health insurance and prenatal care through the Mexican Social Security System were invited to participate. They had to be at least 18 years old, <22 weeks pregnant, free of renal or heart diseases, not using steroids or anti-epileptic drugs, have access to a telephone line and plan to live in Mexico City for the next 3 years. Women were excluded if they had a history of infertility, diabetes or psychosis, consumed ≥1 alcoholic drink a day or used drugs or any prescription, herbal or over-the-counter medications regularly.
For the current analysis, we used information collected by in-person interviews during the second and third trimesters of pregnancy, and at 1, 6 and 12 months postpartum. In this analysis, women who delivered a live newborn, free of congenital malformations were included (n 937). Out of the 937 women, 515 had weights available (measured or imputed) to determine their pattern of postpartum weight change, which was the outcome of interest. Out of the 515 women, those who became pregnant again during the first year postpartum were excluded (n 14). We further excluded one woman who had undergone weight loss surgery before pregnancy due to her extreme weight loss during and after pregnancy. The final sample consisted of 500 women, who were comparable in demographic and anthropometric characteristics (e.g. pregestational BMI, GWG), smoking history, lifestyle behaviours (e.g. physical activity and sedentary activities) and breast-feeding practices to the 448 women not included in the analysis. The only exception was gestational age at delivery, which was slightly higher in the analytic sample (38·4 v. 38·1 weeks).
Exposures: predictors of patterns of postpartum weight change
Pregestational BMI
Trained personnel measured women’s height at the first study visit following standardised procedures(22). All participants self-reported their pregestational weight at the initial visit. However, self-reported weight is less reliable in this setting, where few women are regularly weighed. Considering this, we used an estimated pregestational weight obtained from a linear mixed-effects model. The model used weights measured during pregnancy (second and third trimesters) that were available for most women, as well as clinical weight measurements in the 6 months prior to pregnancy through the early pregnancy period that were recovered from Mexican Social Security System clinical records. The model also included days of gestation at the time of weight collection, maternal height, age, socioeconomic status (SES), education, parity and self-reported pregestational weight.
Model performance was assessed with ten-fold cross-validation, which was based on an evaluation of how well the model predictions at the last menstrual period agreed with weights measured at the Mexican Social Security System clinics for a subset of women (n 87). These weights were measured within ±20 d of the last menstrual period. The predictive accuracy assessed by the root mean square error was 3·2 kg. In a post hoc analysis, the model’s predictions were compared with those obtained by a model recently proposed by Thomas et al.(Reference Thomas, Oken and Rifas-Shiman23), achieving similar results (data not shown).
We calculated pregestational BMI as estimated pregestational weight divided by height-squared, and classified women as underweight, normal weight, overweight or obese following the WHO criteria(24). Underweight women were combined with the normal weight category due to the small sample size (n 3).
Gestational weight gain
The study personnel measured women’s weight during the third trimester of pregnancy (mean gestational age 32 (sd 1) weeks, range 29–35 weeks)(22). GWG was calculated as the difference between third-trimester weight and estimated pregestational weight. It was corrected by the length of gestation following the procedure described by Perichart-Perera et al.(Reference Perichart-Perera, Muñoz-Manrique and Reyes-López25). We classified women as having adequate, insufficient or excessive GWG for their gestational age following the US Institute of Medicine (IOM) guidelines, which have been previously used in a Mexican population(Reference Perichart-Perera, Muñoz-Manrique and Reyes-López25,Reference Rasmussen and Yaktine26) .
Breast-feeding practices
At the first month visit postpartum, women reported whether they were breast-feeding or not and the exclusivity of breast-feeding (i.e. only breast milk or not). They also reported on the introduction of formula, medications and other types of milk, fluids and foods. With this information, we classified breast-feeding as exclusive (i.e. only breast milk), predominant (i.e. breast milk and certain liquids such as water and water-based drinks, but excluding non-human milk) and partial (i.e. breast milk and any food or liquid, including non-human milk)(27).
Outcome: patterns of postpartum weight change
During every visit, the study personnel measured weights with a digital scale following standardised procedures(22). We categorised postpartum weight change into four mutually exclusive patterns using weights at 1, 6 and 12 months postpartum, as well as estimated pregestational weight. We focused on the first year postpartum because weight changes associated with pregnancy are more likely to occur during this time(Reference Schmitt, Nicholson and Schmitt7), and also to be consistent with published research(Reference Provenzano, Rifas-Shiman and Herring28,Reference Siega-Riz, Herring and Carrier29) . Return to pregestational weight at any postpartum time point was defined as a weight no more than 500 g higher than the estimated pregestational weight. The following definitions were applied for this classification:
-
Return to pregestational weight: Women who returned to their pregestational weight at 12 months postpartum. Includes women who lost weight compared with the pregestational state.
-
Postpartum weight retention (PPWR): Women who, on average, lost weight through 12 months postpartum without ever reaching their pregestational weight.
-
Postpartum weight gain (PPWG): Women who reached their pregestational weight at any point during the first 6 months postpartum and gained weight after that.
-
Postpartum weight retention and weight gain (PPWR + WG): Women who did not return to their pregestational weight during the first 6 months and, on average, gained weight through 12 months postpartum.
We imputed weight at 12 months for 100 women with missing weight at this time point using a multiple regression model. For this procedure, we used the information from the subsample of women (n 345) with weights available at both 12 and 18 months postpartum. This subsample was statistically comparable in demographic and anthropometric characteristics to the imputed set (n 100) and the overall cohort (see online supplementary material, Supplemental Table S1). Weights at 12 and 18 months were logarithmically transformed to maximise their linear association, as suggested by the Box−Cox family of transformations(Reference Box and Cox30). A basic model included weight at 18 months as the only independent variable and explained 96 % of the variability of weight at 12 months. However, we included additional covariates to improve the model explanatory capacity and to increase precision in the model estimates, namely age, marital status and pregestational BMI. The final model achieved goodness of fit. To predict weight on the original scale, we used the exponential function.
Covariates
At the first study visit, women reported their age, parity (primiparous or multiparous), marital status (single/separated or married/cohabitating) and education (basic: elementary and secondary school; middle: high school; college: at least college). Women were classified into six SES categories (A/B [highest], C+, C, D+, D, E) using a validated questionnaire that included thirteen items on household assets and conditions (i.e. housing quality, services, material goods) and head of household’s education(Reference Villegas-Carrasco31). For the current analysis, the six categories were collapsed into three: high (A/B, C+ and C), middle (D+) and low (D, E). Gestational age at delivery was calculated from the child’s birth date and self-reported last menstrual period date. We used the Capurro method, which is based on the newborn’s physical characteristics, as a secondary method to estimate gestational age. In cases where the two methods differed by >3 weeks, we used the Capurro method-derived gestational age(Reference Sanders, Svensson and Gennings32).
Women provided information on smoking history, physical activity and sedentary activities via a general information questionnaire at the first study visit. Women were categorised as never smokers, smokers around pregnancy and former smokers (i.e. quit at least 1 year before pregnancy). Women reported leisure-time exercise as days per week and time per day invested in activities such as walking, running, swimming and aerobics, among others. Because of the high prevalence of women who did not engage in any leisure-time activity (87 %), we dichotomised this variable as physically active or not (i.e. reported >1 v. 0 min of leisure-time activity per week). Women also reported time spent reading or watching TV during weekdays and weekends. We computed average hours per day spent in these sedentary activities and categorised them as <2 or ≥2 h/d. These cut-offs are consistent with previous research(Reference Kirkegaard, Stovring and Rasmussen33). We did not have information on diet, which is a potential confounder on the associations of interest (i.e. GWG and postpartum weight change)(Reference Knudsen, Heitmann and Halldorsson34).
Statistical analysis
We described the distribution of participant’s characteristics by the pattern of postpartum weight change via mean and standard deviation for numeric variables, and percentages for categorical variables. The statistical significance of the associations was evaluated with multinomial logistic regression models.
To evaluate the associations of pregestational BMI, GWG and breast-feeding (all as categorical variables) with patterns of postpartum weight change (dependent variable), we fit multinomial logistic regression models. The reference group was women who returned to their pregestational weight at 12 months postpartum. We first included each predictor individually in separate models to assess their independent effects on patterns of postpartum weight change. All the models were adjusted for relevant confounders selected after a thorough literature review, including maternal age, marital status, education, parity and SES. The models, including GWG as the main predictor, were further adjusted for pregestational BMI, smoking history, physically active and sedentary activities during pregnancy. The models for breast-feeding were additionally adjusted by pregestational BMI, adequacy of GWG, smoking history, gestational age at delivery and pregnancy complications, including pre-eclampsia, gestational hypertension and gestational diabetes. The Hosmer−Lemeshow test was performed to assess the goodness of fit of the models(Reference Fagerland and Hosmer35). For comparison, we performed the same analysis (i.e. the association of pregestational BMI, GWG and breast-feeding with patterns of postpartum weight change) excluding women with imputed weight at 12 months. The results did not differ substantially; therefore, only the results on the full dataset are presented (see online supplementary material, Supplemental Table S2).
In a sensitivity analysis, we used a modified definition of patterns of postpartum weight change considering a 0 g instead of the 500 g margin as a threshold to return to pregestational weight. Additionally, to compare our proposed classification of patterns of weight change with an outcome commonly reported in the literature(Reference Lipsky, Strawderman and Olson9,Reference Siega-Riz, Herring and Carrier29,Reference Olson, Strawderman and Hinton36) , we evaluated the association of pregestational BMI, GWG and breast-feeding at 1 month with the odds of substantial postpartum weight retention using logistic regression models. Women were classified as having substantial postpartum weight retention if the difference between their weight at 12 months and their pregestational weight was ≥4·5 kg.
We performed all the analyses in STATA 15 (StataCorp LP). A P-value <0·05 was considered statistically significant.
Results
Women were 27 years on average, had basic education (42 %), were married or cohabitating (82 %), had low SES (53 %) and were multiparous (64 %) (Table 1). Pregestational overweight affected 41 %, and obesity 18 %, of the women. Mean gestational weight gain was 7·4 (sd 3·1) kg, and adequate gestational weight gain (47 %) was more common than insufficient (26 %) and excessive (27 %) gain. The majority (53 %) practiced partial breast-feeding at 1 month postpartum, and 28 % practiced exclusive breast-feeding.
Table 1 Participants’ characteristics according to patterns of postpartum weight change in 500 women participating in the Programming Research in Obesity, Growth, Environment and Social Stressors cohort*
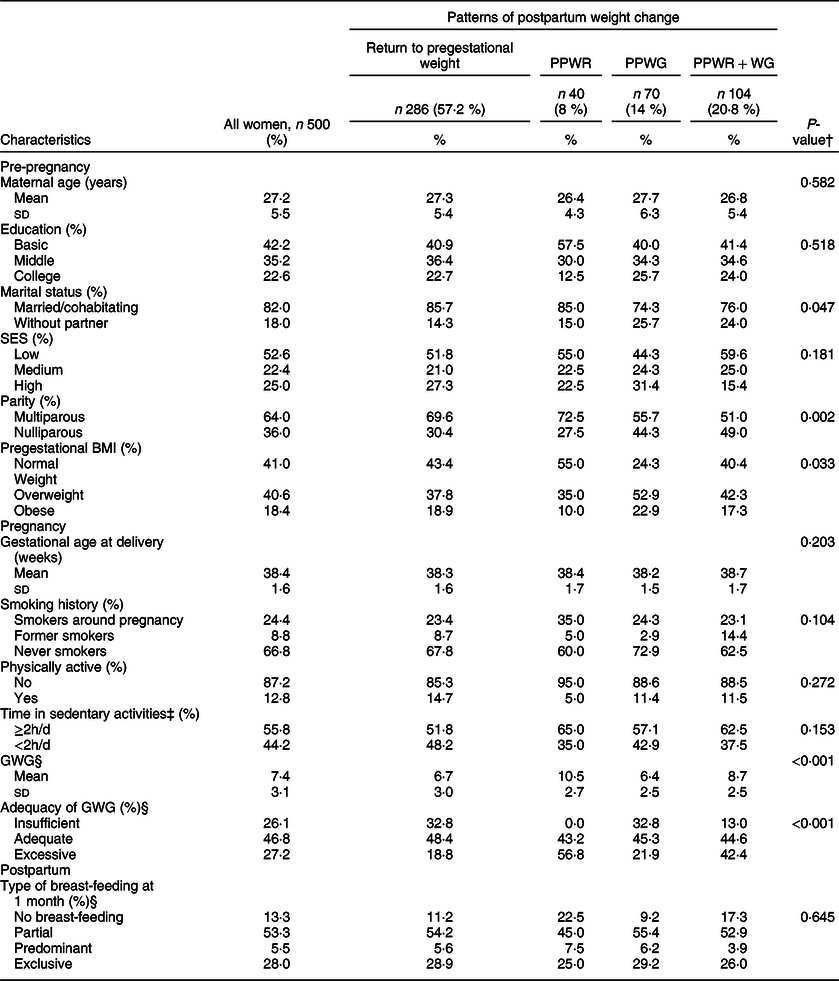
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; SES, socioeconomic status; GWG, gestational weight gain.
* Education: basic (elementary and secondary school), middle (high school) and college (at least college). SES was obtained with a validated scale and categorised as high (A/B, C+ and C), middle (D+) and low (D, E). Pregestational BMI: normal weight (<25 kg/m2), overweight (≥25 to <30 kg/m2) and obese (≥30 kg/m2). GWG: insufficient (below the expected weight for gestational age), adequate (between the expected minimum and maximum weights for gestational age) and excessive (above the expected weight for gestational age). Physically active: no (did not engage in leisure-time activity), or yes (engaged in any leisure-time activity). Type of breast-feeding at 1 month: exclusive (only breast milk), predominant (breast milk and certain liquids such as water and water-based drinks, but excluding non-human milk), partial (breast milk and any food or liquid, including non-human milk) and no breast-feeding.
† P-value for the comparison between patterns of postpartum weight change using multinomial logistic regression models. A P-value <0·05 was considered statistically significant.
‡ Time in sedentary activities includes reading and watching TV.
§ GWG and adequacy of GWG: n 449 (n 256 for return to pregestational weight, n 37 for PPWR, n 64 for PPWG and n 92 for PPWR + WG); type of breast-feeding at 1 month: n 458 (n 249 for return to pregestational weight, n 40 for PPWR, n 65 for PPWG and n 104 for PPWR + WG).
Most women returned to their pregestational weight by 12 months postpartum (57 %), while 8 % had PPWR, 14 % PPWG and 21 % PPWR + WG. Marital status, parity, pregestational BMI and GWG differed between patterns of postpartum weight change (Table 1). For example, the pattern of PPWR had a high proportion of women who were married or cohabitating (85 %), multiparous (72·5 %), normal weight prior to pregnancy (55 %) and who had excessive GWG (56·8 %). Most women who experienced PPWG had overweight or obesity before pregnancy (75·7 %), and a high proportion had adequate (45·3 %) or insufficient (32·8 %) GWG. Almost half of women with PPWR + WG were primiparous (49 %), 59·6 % had overweight or obesity before pregnancy, and 42·4 % had excessive GWG.
Mean weight change at 12 months postpartum for all women was 0·2 (sd 4·6) kg, with a range of –14·7 to 18·2 kg. Women who returned to their pregestational weight lost on average 2·8 (sd 2·7) kg by 12 months postpartum. Those with PPWR and PPWG gained 2·9 (sd 2·1) and 2·9 (sd 1·8) kg, respectively. Women classified as PPWR + WG experienced the highest weight gain from pregestational to 12 months postpartum with a mean of 5·8 (sd 3·6) kg. As shown in Fig. 1, weight trajectories from before pregnancy to 12 months postpartum also differed between the four patterns of postpartum weight change.
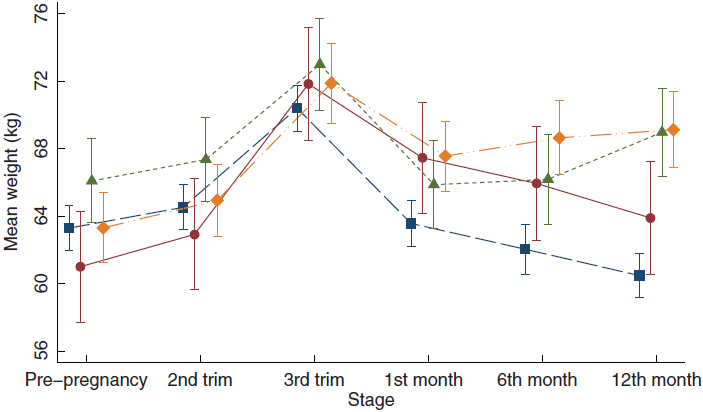
Fig. 1 Weight changes from pre-pregnancy to 12 months postpartum by patterns of weight change. The graph displays the mean weight in kg and 95 % CI at each time point within each category. ![]() , return to pregestational weight;
, return to pregestational weight; ![]() , retention;
, retention; ![]() , gain;
, gain; ![]() , retention/gain
, retention/gain
Tables 2−4 present the association between each predictor and patterns of postpartum weight change. Results are presented as relative risk ratios (RRR) and 95 % CI. In the age-adjusted model (Table 2), pregestational overweight and obesity, compared with normal weight, were each associated with a higher risk of PPWG (overweight: RRR 2·5, 95 % CI 1·3, 4·8; obesity: RRR 2·2, 95 % CI 1·0, 4·7). They were not associated with PPWR or PPWR + WG. These results persisted after adjustment for parity and sociodemographic covariates (model 2).
Table 2 Association between pregestational BMI and patterns of postpartum weight change
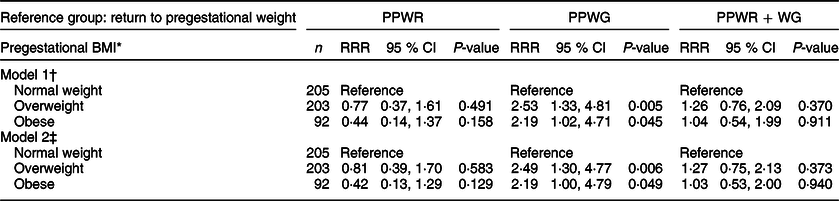
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; RRR, relative risk ratio.
Results from multinomial logistic regression models.
* Pregestational BMI: normal weight (<25 kg/m2), overweight (≥25 to <30 kg/m2) and obese (≥30 kg/m2).
† Model 1: adjusted for age.
‡ Model 2: adjusted for age, marital status (single/separated and married/cohabitating), education (basic: elementary and secondary school; middle: high school; college: at least college), parity (primiparous and multiparous) and socioeconomic status (high, middle and low).
Table 3 Association between gestational weight gain and patterns of postpartum weight change
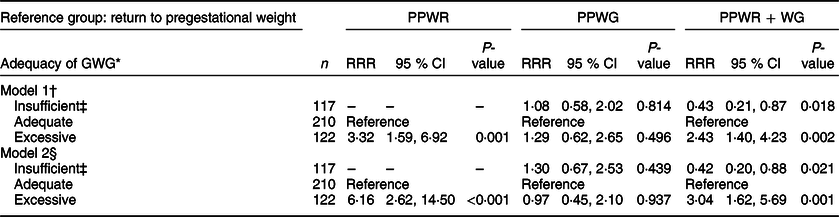
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; GWG, gestational weight gain; RRR, relative risk ratio.
Results from multinomial logistic regression models.
* GWG: insufficient (below the expected weight for gestational age), adequate (between the expected minimum and maximum weights for gestational age) and excessive (above the expected weight for gestational age).
† Model 1: adjusted for age.
‡ n 0 for insufficient GWG and postpartum weight retention.
§ Model 2: adjusted for age, marital status (single/separated and married/cohabitating), education (basic: elementary and secondary school; middle: high school; college: at least college), parity (primiparous and multiparous), socioeconomic status (high, middle and low), pregestational BMI (normal weight, overweight and obese), smoking history (never smokers, smokers around pregnancy and former smokers), physically active (yes and no) and sedentary activities in pregnancy (<2 and ≥2 h/d).
Table 4 Association between breast-feeding and patterns of postpartum weight change
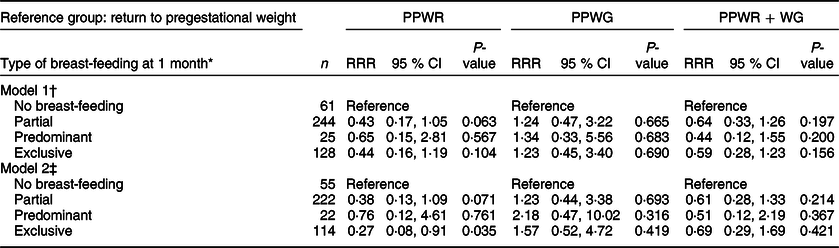
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; RRR, relative risk ratio.
Results from multinomial logistic regression models.
* Type of breast-feeding at 1 month: exclusive (only breast milk), predominant (breast milk and certain liquids such as water and water-based drinks, but excluding non-human milk), partial (breast milk and any food or liquid, including non-human milk) and no breast-feeding.
† Model 1: adjusted for age.
‡ Model 2: adjusted for age, marital status (single/separated and married/cohabitating), education (basic: elementary and secondary school; middle: high school; college: at least college), parity (primiparous and multiparous), socioeconomic status (high, middle and low), pregestational BMI (normal weight, overweight and obese), smoking history (never smokers, smokers around pregnancy and former smokers), adequacy of GWG (insufficient, adequate and excessive), gestational age at delivery and pregnancy complications (yes and no).
Excessive GWG, in comparison with adequate GWG, was associated with a higher risk of PPWR (RRR 3·3, 95 % CI 1·6, 6·9) and PPWR + WG (RRR 2·4, 95 % CI 1·4, 4·2). On the other hand, insufficient GWG was associated with a lower risk of PPWR + WG (RRR 0·4, 95 % CI 0·2, 0·9) (Table 3, model 1). These associations became somewhat stronger after adjustment for pregestational BMI, sociodemographic characteristics and lifestyle behaviours during pregnancy (model 2). GWG was not associated with PPWG in any of the models.
In the age-adjusted model, partial and exclusive breast-feeding at 1 month, compared with no breast-feeding, appeared to be protective for PPWR but not for PPWG or PPWR + WG (Table 4, model 1). In both cases, the results included the null value. After full adjustment of covariates (model 2), the association between exclusive breast-feeding and lower risk of PPWR became stronger, and the CI excluded the null (RRR 0·3, 95 % CI 0·1, 0·9).
In a sensitivity analysis using a modified definition of the outcome, we observed similar associations between pregestational BMI, GWG and patterns of postpartum weight change (see online supplementary material, Supplemental Table S3). In this supplementary analysis, exclusive breast-feeding at 1 month, compared with no breast-feeding, was not associated with a lower risk of PPWR (RRR 0·5, 95 % CI 0·2, 1·4). We also wanted to assess how our proposed classification of patterns of postpartum weight change compared with the widely used definition of substantial postpartum weight retention (i.e. retaining ≥4·5 kg at 12 months postpartum). In our sample, 17 % of women experienced substantial postpartum weight retention (see online supplementary material, Supplemental Table S4). Of these women, 13, 17 and 70 % would be classified as PPWR, PPWG and PPWR + WG, respectively, according to our classification. In age-adjusted and fully adjusted models, pregestational BMI and breast-feeding at 1 month were not associated with the odds of retaining ≥4·5 kg. Insufficient GWG, compared with adequate GWG, was associated with lower odds of substantial postpartum weight retention (OR 0·4, 95 % CI 0·2, 0·9). In contrast, excessive GWG was associated with higher odds of PPWR (OR 3·3, 95 % CI 1·8, 6·0).
Discussion
Weight gain after delivery may be the result of retaining gestational weight, gaining weight postpartum or both. These patterns of weight change are associated with potentially modifiable factors. In the current study, pregestational overweight and obesity were associated with a higher risk of PPWG during the first year postpartum, whereas excessive GWG was associated with a higher risk of PPWR and PPWR + WG. Exclusive breast-feeding at 1 month postpartum decreased the risk of PPWR.
In our study, 21 % of women experienced PPWR + WG the first year after delivery, while 14 % experienced PPWG and 8 % PPWR. To date, most of the studies have lumped all women who do not return to their pregestational weight as having PPWR. None of these studies have differentiated between patterns of postpartum weight change(Reference Siega-Riz, Herring and Carrier29,Reference Li, Teo and Morrison37–Reference Althuizen, Van Poppel and De Vries41) . Some studies have shown that PPWG occurs in the late postpartum period (≥12 or ≥18 months)(Reference Schmitt, Nicholson and Schmitt7,Reference Lipsky, Strawderman and Olson9,Reference Onyango, Nommsen-Rivers and Siyam10,Reference Kirkegaard, Stovring and Rasmussen33) . We build on these results by showing that a considerable proportion of women gain weight, either alone or combined with retention, within the first 12 months after delivery. To the best of our knowledge, only one study has characterised different patterns of weight change the first year after delivery, using repeated weight assessments(Reference Kew, Ye and Hanley6). In the current study of 305 Canadian women, 15 % weighted more at 3 months postpartum, compared with their pregestational weight, and gained weight from 3 to 12 months. Sixty-six per cent also weighted more at 3 months but lost weight from 3 to 12 months. However, on average, they still weighted more by 12 months, compared with their pregestational weight. Another 11 % had lost weight by 3 months, compared with their pregestational weight, and regained weight from 3 to 12 months(Reference Kew, Ye and Hanley6). The three groups described in the current study are comparable with our patterns of PPWR + WG, PPWR and PPWG, respectively.
Women with pregestational overweight and obesity, compared with normal weight, had a higher risk of PPWG. Similar to our cohort, in the study of Kew et al.(Reference Kew, Ye and Hanley6), women who regained weight, after having reached their pregestational weight, had the highest BMI before pregnancy. Lipsky et al.(Reference Lipsky, Strawderman and Olson9), in a study of 413 American women, found that women with overweight and obesity, compared with normal weight, had higher odds of gaining ≥2·25 kg from 1 to 2 years postpartum (overweight: OR 2·4, 95 % CI 1·3, 4·4; obesity: OR 3·0, 95 % CI 1·6, 5·6). Gunderson et al.(Reference Gunderson, Abrams and Selvin42) also showed that women with overweight and obesity were more likely to gain ≥2 kg from 6 weeks to a median of 2 years postpartum. The reason why women with pregestational overweight or obesity are more likely to gain weight postpartum is unclear. It is possible that behavioural factors associated with weight gain, such as lower physical activity, longer time in sedentary activities and unhealthy dietary patterns, are more prevalent among these groups of women(Reference Kirkegaard, Stovring and Rasmussen33,Reference Ostbye, Peterson and Krause43) .
We found that excessive GWG was associated with an increased risk of PPWR and PPWR + WG, while insufficient GWG was associated with a lower risk of PPWR + WG. Higher GWG has been consistently associated with PPWR(Reference Rong, Yu and Han14–Reference Rode, Kjærgaard and Ottesen16,Reference Siega-Riz, Herring and Carrier29,Reference Oken, Kleinman and Belfort38,Reference Nehring, Schmoll and Beyerlein44,Reference Kac, Benicio and Velasquez-Melendez45) , although none of these studies have made the distinction between retention alone v. retention and gain. In a recent meta-analysis, GWG above the IOM recommendations, compared with ‘within recommendations’, was associated with a higher weight retention (3·2 kg, 95 % CI 2·8, 3·6 kg). In contrast, GWG below the IOM recommendations was associated with a lower weight retention (−2·1 kg, 95 % CI −2·4, −1·9 kg)(Reference Rong, Yu and Han14). Few studies have evaluated the association between GWG and patterns of weight change other from PPWR(Reference Soria-Contreras, Rifas-Shiman and Aris11,Reference Abebe, Von Soest and Von Holle12) . An analysis of postpartum weight trajectories in Norwegian women found that excessive GWG was associated with two weight trajectories characterised by high initial weight retention (6 months) followed by either weight loss or sustained weight gain through 3 years postpartum. Insufficient GWG was associated with a lower risk of these trajectories(Reference Abebe, Von Soest and Von Holle12). A recent study showed that excess GWG represents mostly gains in maternal fat(Reference Berggren, Groh-Wargo and Presley46). This additional fat mass may persist beyond delivery and explain the increased risk of PPWR or PPWR + WG observed in our study. Our results of lower risk of PPWR + WG among women with insufficient GWG must be interpreted with caution. Insufficient GWG, although it may provide some benefits in terms of postpartum weight, has been associated with adverse neonatal outcomes, including a higher risk of small-for-gestational-age and preterm birth(Reference Goldstein, Abell and Ranasinha47).
In the current study, exclusive breast-feeding at 1 month was associated with a decreased risk of PPWR. Martin et al.(Reference Martin, Hure and Macdonald-Wicks40), in a study of Australian women, did not find any association between the type of breast-feeding at 3 months and weight retention at 12 months. Consistent with our findings, López-Olmedo et al.(Reference López-Olmedo, Hernández-Cordero and Neufeld48), in a group of Mexican women, found that those who breastfed exclusively until the third month postpartum had greater weight loss in comparison with those non-breast-feeding. One recent meta-analysis did not find an association between breast-feeding and postpartum weight change(Reference Chowdhury, Sinha and Sankar18). Methodological differences between studies, including different exposure times or definitions of breast-feeding, and lack of adjustment by relevant covariates might explain the inconsistency in findings.
Our study has several implications. To modify the impact of pregnancy and childbearing on women’s weight and health status, we need to improve our understanding of the course of weight change after delivery and the characteristics associated with the different patterns of weight change. In the current study, we proposed a different approach to characterise postpartum weight trajectories in women. Although some authors have suggested using the term PPWR within a limited period following delivery, for example, 12−18 months(Reference Schmitt, Nicholson and Schmitt7,Reference Lipsky, Strawderman and Olson9) , we showed that women also gain weight during this time. We found that PPWR + WG and PPWG were more common than PPWR among women who did not reach their pregestational weight during the first year after delivery. In a previous study, we found that women with PPWR, PPWG and PPWR + WG, compared with those who returned to their pregestational weight by 1 year postpartum, had increased adiposity 6 years after delivery. We also found that PPWR + WG was associated with metabolic alterations such as insulin resistance(Reference Soria-Contreras, Trejo-Valdivia and Cantoral13). In the current study, we extend these results by identifying the contribution of specific modifiable factors to the risk of each of the patterns of weight change.
Our findings should be evaluated within the strengths and limitations of the study. Generalisability may be limited because our population consisted primarily of women of low SES living in Mexico City. Only 53 % of the original cohort had information available for this analysis, which may have increased the possibility of selection bias. This type of bias is unlikely because analysed and non-analysed women were identical in all characteristics, including the exposures of interest (except for gestational age at delivery). The potential for confounding was minimised by adjusting each of the associations studied for relevant covariates. However, we did not have information on diet, which likely plays a role in the variability of postpartum weight change(Reference Knudsen, Heitmann and Halldorsson34,Reference Oken, Taveras and Popoola49,Reference Boghossian, Yeung and Lipsky50) . In specific, diet during pregnancy has been associated with both GWG and PPWR(Reference Knudsen, Heitmann and Halldorsson34); therefore, it is a potential confounder of this association. We cannot completely rule out the possibility of residual confounding for the lack of adjustment by diet. However, other studies have shown that the association between GWG and postpartum weight change is strong and persists after adjustment by dietary patterns and energy intake(Reference Kirkegaard, Stovring and Rasmussen8,Reference Siega-Riz, Herring and Carrier29) . Finally, the results on the association between breast-feeding and patterns of postpartum weight change must be interpreted with caution because some categories had very small sample sizes (i.e. predominant breast-feeding, n 22). We recognise that we may have been underpowered to detect any association between these categories and the outcome.
Some strengths must be acknowledged. We categorised breast-feeding practices according to the WHO(27) and adjusted by pregestational BMI, GWG and parity, which are important confounders of the association between breast-feeding and postpartum weight change(Reference Neville, McKinley and Holmes19). Studying breast-feeding at 1 month postpartum may be seen as a limitation but, by doing this, we were confident that the exposure (i.e. breast-feeding) preceded the outcome of interest (i.e. patterns of weight change). The WHO recommends practicing exclusive breast-feeding for at least 6 months postpartum(51). Therefore, our results on the association between breast-feeding and PPWR must be interpreted with caution and should be replicated in future studies. A strength of the current study is that most weights used for the analysis were objectively measured by trained personnel. One exception was weight at 12 months that was imputed for a subset of women. The results excluding this subset of women were comparable with those of the primary analysis that included women with imputed and non-imputed weights. Self-reported pregestational weight is highly subject to error in this population, and misreporting can be substantial(Reference Thomas, Oken and Rifas-Shiman23). In the absence of measured pregestational weight, using an estimated pregestational weight rigorously validated, instead of self-reported, might be a strength of this analysis.
Conclusions
We found that while most women return to their pregestational weight 1 year after delivery, an important subset experiences retention of gestational weight, weight gain or both, which may increase their long-term risk of obesity and metabolic diseases. Although our results need to be replicated in a different population, they suggest that these adverse patterns of weight change may be prevented by targeting women with pregestational overweight or obesity and excessive GWG, and by promoting exclusive breast-feeding. Future studies should test whether targeting these high-risk women and promoting breast-feeding has an impact on postpartum weight change and long-term women’s health.
Acknowledgements
Acknowledgements: We thank the Centro Médico ABC and the National Institute of Perinatology, México, for their support with the current research. Financial support: The current work was supported by the National Institute of Environmental Health Sciences, grants nos. R01 ES013744, P30 ES023515, R01 ES021357, R24 ES028522, and partially funded by the National Institute of Public Health/Ministry of Health of Mexico. The funding sources had no role in the design of the study, in the analysis, interpretation of the data or the writing of the manuscript. Conflict of interest: The authors declare that they have no competing interests. Authorship: D.C.S.C. conceptualised and designed the study, carried out the statistical analysis, interpretation of the data and drafted the manuscript M.M.T.R., B.T.V. and R.L.R. participated in the conceptualisation and design of the study, interpretation of the data and critically reviewed the manuscript. E.O. helped in the interpretation of the data and critically reviewed the manuscript. R.O.W., A.C., M.L.P.Z., A.A.B., A.C.J., M.A.O. and I.R.S. critically reviewed the manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Committees on Ethics, Biosafety and Research at the Mexican National Institute of Public Health, as well as the Institutional Review Boards of the participating institutions. Written informed consent was obtained from all subjects/patients.
Supplementary material
For supplementary material accompanying this article visit https://doi.org/10.1017/S1368980020002803.







