Implications
Handling of semen, particularly the 0.25 ml straw, is critically important. Thawed semen needs to be protected from cold and heat shocks and an inseminated within 6 to 8 min of thawing. Uterine horn insemination give a modest improvement in conceptions rates (CRs) particularly in situations where CRs are low following uterine body inseminations. Because many of the studies that evaluated heterospermic insemination were conducted in the era of fresh semen and/or lacked adequate replication, it is difficult to deduce if there are real benefits from using heterospermic semen. Best CRs are achieved when cows are inseminated from mid oestrus to a few hours after the end of oestrus.
Introduction
The low and variable CRs following AI is a major cause of production inefficiency and involuntary culling rates in cattle herds and ultimately reduces profitability. For heifers, beef and moderate yielding dairy cows, it appears that fertilisation rate generally lies between 90% and 100% (see review by Diskin et al., Reference Diskin, Waters, Parr and Kenny2016). The objective of an insemination is to ensure that there is an adequate reservoir of competent, capacitated, motile sperm in the caudal region of the oviductal isthmus, the site of the main sperm reservoir in the cow, at the time ovulation to ensure the highest possible fertilisation rate. This is a prerequisite to achieving a high embryo survival and pregnancy rate. There are many ‘cow’ and ‘semen’ factors that affect fertilisation rate. Other factors that impact on fertilisation include inseminator competency, handling of semen, site of semen deposition, heterospermic insemination and timing of AI. These are the focus of this review.
Handling of semen
Currently, most bull semen is now frozen in either 0.25 or 0.50 ml French straws and stored in liquid nitrogen at −196°C. Besides facilitating semen packaging, labelling, storage and transport, straws also facilitate a more uniform control of freezing and thawing which ultimately leads to improved sperm recovery post thawing. However, a major disadvantage with straws is their vulnerability to mishandling particularly the 0.25 ml straws which are most popular in Europe and Canada. The 0.25 ml straws have a large surface-to-volume ratio, compared with the 0.5 ml straws, which makes them vulnerable to rapid temperature fluctuations. Seidel (Reference Seidel2011) recommended that 3 s be the maximum time for moving 0.25 ml straws from one liquid nitrogen tank to another without damaging the sperm and similarly for moving from tank to thaw flask.
Thawing of frozen semen should be at maximum speed. Rapid thawing decreases the harmful effects of water recrystallisation and rehydration, thus preventing damage to sperm membrane and cytoplasm. The critical temperature zone for ice crystals to form is between ~ −50°C and ~0°C. Rapid progression though this temperature zone means that the semen switches from glassy to a liquid state and ice crystals have insufficient time to form. Numerous studies have been conducted to determine the thawing rate that gives the highest post-thaw percentage of viable sperm (Correa et al., Reference Correa, Rodriguez, Patterson and Zavos1996). In some studies thawing temperatures as high as 60°C to 80°C for 6 to 7 s have given improved post-thaw sperm motility and viability (Senger, Reference Senger1980; Lyashenko, Reference Lyashenko2015). However, the duration of exposure to such high temperature is critically important and there appears to be very little scope for error unlike the situation when straws are thawed at lower temperatures.
It is not uncommon that recommendations for handling of frozen semen vary among AI organisations, often using the same packaging, and this has, at times, led to confusion within the industry. Ideally, semen should be thawed according to the recommendations of the organisation supplying it.
From the literature and based primarily on the recommendation of Saacke (Reference Saacke1974) the following general recommendations can be deduced:
∙ Within the liquid nitrogen storage tank have the canisters containing the straws clearly labelled.
∙ Maintain an up-to-date inventory of semen straws, bull codes and their location within the liquid nitrogen tank.
∙ When locating straws keep the canister below the frost line and avoid lifting the canister for too long in the process. If a semen straw cannot be located within 10 s lower the canister into the liquid nitrogen before continuing the search.
∙ Remove the straw using a forceps, remove any excess liquid nitrogen and place in water at 35°C for 45 s (range 30 to 60 s). Thaw only sufficient straws that can be inseminated with 10 min (see later). If multiple straws are being thawed avoid direct straw-to-straw contact during the thawing process.
∙ Before loading the AI gun, pre-warm it, particularly if the environment is cold. This can be done by stroking it vigorously with clean paper towel, placing it close to your body for a few minutes in advance or using a purpose designed AI gun warmer unit.
∙ Remove the straw from the water bath, wipe dry, insert into the AI gun, cotton plug end first and cut the straw about 7 mm below the crimped end.
∙ Securely attach the AI sheath over the loaded gun.
∙ If there is a delay (>10 to 15 s) before insemination wrap the loaded gun in in a towel to provide hygienic and thermal protection or place in a gun warmer.
∙ In very warm environments it is important that loaded AI guns are protected from solar radiation and elevation and temperatures above 35°C.
∙ Clean the vulva of the cow with paper towel before insemination.
Batch thawing of semen straws
Frequently in large herds and or following oestrous or ovulation control programmes, multiple cows are presented for insemination at the same time. This has led to the practice of frequently thawing multiple straws simultaneously. A frequently asked question is whether the order the straws are subsequently used, or the interval that has elapsed between thawing and actual insemination, affect the probability of conception? Early laboratory studies by Brown et al. (Reference Brown, Senger and Becker1991) reported no difference in post-thaw sperm motility and percentage of sperm with intact acrosomes when up to 10 straws were thawed simultaneously. These authors concluded that up to 10 straws could be safely thawed simultaneously in either a thaw bath provided the straws are agitated immediately after plunging to prevent direct contact and them temporarily refreezing during the thawing process. Field results from some early studies (Lee et al., Reference Lee, Huang and Sagayaga1997; Goodell Reference Goodell2000) have suggested that thawing more than two straws at once resulted in reduced CRs of the third and fourth insemination in sequence. These studies stimulated a number of laboratories to re-examine the above question. The results from four studies with dairy cows are summarised in Table 1. These data consistently show that batch thawing of up to four straws at once does not reduce the probability of a pregnancy when the last of these straws is inseminated. The divergent results between the studies of Lee et al. (Reference Lee, Huang and Sagayaga1997) and Goodell (Reference Goodell2000) and the results reported in Table 1 may be partly explained by the relatively small numbers of cows used in the former studies (269 cows combined for the studies of Lee et al. (Reference Lee, Huang and Sagayaga1997) and Goodell (Reference Goodell2000). More importantly, the study of Lee et al. (Reference Lee, Huang and Sagayaga1997) was conducted in a hot tropical environment (Hawaii) with up to four semen straws thawed simultaneously, loaded into AI guns which were exposed to direct solar radiation during transport to the cow. Temperatures of >46°C were recorded in the inseminating gun due to direct solar radiation. Acrosome integrity was reduced (75% v. 43%) following exposure due to lack temperature control and was probably the main factor that contributed to the observed reduced CRs.
Table 1 Summary of the effects of sequential insemination number on conception rates in from four studies involving dairy cows (number pregnant/number inseminated)

AI=artificial insemination.
In an extension of these studies, Oliveira et al. (Reference Oliveira, Arruda, de Andrade, Santos, Beletti, Peres, Martins and Hossepian de Lima2012) evaluated the effects of sequence of insemination, following the simultaneous thawing of 10 semen straws (0.5 ml) on CR following timed AI in oestrous synchronised suckled multiparous Nelore beef cows. A total of 10 semen straws were thawed simultaneously in a thermostatically controlled water bath at 36°C. Thirty seconds after insertion of the 10 straws into the water bath, one straw was removed (first straw) and loaded into an AI gun and a randomly selected cow inseminated with it. During insemination of the first straw a second straw was removed from the water bath, prepared and immediately used for AI. Similarly, the other straws were removed in sequence from the water bath, loaded and inseminated as the second straw until all 10 straws were used. The mean interval from insertion of the 10 straws to removal of the last straw was 6 min and 29 s. In that study and, in contrast to other studies, the semen straws were retained at a constant temperature of 36°C until within about 40 s of insemination. Two inseminators and semen from three bulls were used in the study. For analysis of effect of sequential insemination on CR, cows were, retrospectively, separated into three groups, according to the distribution of incubation time by straw. Because most of straws 1, 2 and 3 were removed from the thawing bath between 0 and 2.5 min and cows inseminated with 1st, 2nd and 3rd semen straws were designated as Straw Group 1, cows inseminated with straws 4, 5 and 6 (incubated for 2.5 to 5.0 min) were designated Straw Group 2 and cows inseminated straws 7, 8, 9 and 10 (incubated for 5.0 to 7.0 min) were designated Straw Group 3. All cows were examined by transrectal ultrasonography 40 days after timed AI to determine the outcome of the inseminations. The results of this study are summarised in Table 2. There was clear evidence of a significant interaction between sire group and Straw Group on CR. Sire 1 had reduced CRs for the group of straws associated with the longest interval from thawing to AI (Straw Group 3; straws 7, 8, 9 and 10). The results of the laboratory analysis of the semen from the three bulls were unable to explain the findings of the insemination study, confirming the importance of field studies when evaluating bull fertility. The semen from the other two bulls used in the study was not significantly different for either Straw group. This led the authors (Oliveira et al., Reference Oliveira, Arruda, de Andrade, Santos, Beletti, Peres, Martins and Hossepian de Lima2012) to conclude that sequence of insemination after simultaneous thawing of 10 straws of semen differentially affect CRs following timed AI depending on sire. However, there was no effect of bull when six or fewer straws were thawed simultaneously and consequently, the interval from thawing to insemination kept shorter.
Table 2 Conception rate (%; n/n) 40 days after timed artificial insemination (AI) in suckled multiparous Nelore cows (Oliveira et al., Reference Oliveira, Arruda, de Andrade, Santos, Beletti, Peres, Martins and Hossepian de Lima2012)

AI=artificial insemination.
Within a column, values without a common superscript letter (x, y) differ (P<0.05). Within a row, values without a common superscript letter (a, b) differ (P<0.05).
Interestingly, in all of the studies summarised in Table 1, 0.5 ml plastic straws were used which is the standard container for commercially frozen semen in the United States, Central and South America and Asia. In Europe and Canada 0.25 ml French plastic straws are utilised for packaging of bovine frozen semen. The author could find no published studies that examined the effects of batch thawing and sequence of insemination for semen stored in 0.25 ml straws. Because of the much higher surface-to-volume ratio in 0.25 ml straws heat dissipation or accumulation occurs much faster than with 0.5 ml straws. Consequently, greater caution is warranted with 0.25 ml straws.
From the foregoing it is clear that the interval between thawing and insemination is more important than the absolute number of straws of semen thawed simultaneously. Competent, experienced inseminators should thaw not more than six straws simultaneously, ensure thawed straws are protected from either cold or warm shock and, and that all six straws are inseminated within 15 min.
Site of insemination
In the initial insemination studies in cattle in the 1940s, the semen was typically deposited in the vagina or inside the external os of the cervix. This was followed by studies where a hand was placed in the rectum to hold the cervix and the semen was deposited deep into the anterior cervix and/or uterus. The results of several studies (Knight et al., Reference Knight, Patrick, Anderson and Branton1951; Salisbury and VanDemark Reference Salisbury and VanDemark1951; Stewart and Melrose Reference Stewart and Melrose1952; Olds et al., Reference Olds, Seath, Carpenter and Lucas1953) showed little differences in fertility when semen was deposited in the uterine horns, uterine body or indeed in mid-cervix. This resulted in the uterine body been the accepted site of semen deposition when AI is employed with cattle. Subsequently, Macpherson (Reference Macpherson1968) recorded a 10 percentage point lower CR following cervical when compared with uterine body deposition indicating the importance of body insemination. The use of fresh semen and high numbers of sperm per inseminate in these early studies may have masked possible site of deposition effects which are likely to be more pronounced with frozen-thawed semen and particularly where low sperm numbers are used.
While good insemination technique would appear to be straight forward, nevertheless it appears to be done with a low degree of precision and correct insemination technique has a low repeatability. A number of studies, which utilised dye deposition technique (Wright, Reference Wright1964) or radiographs (Senger et al., Reference Senger, Becker, Davidge, Hillers and Reeves1988) to accurately determine the site of deposition all showed high error rates with uterine body insemination. Correct positioning the tip of the AI gun in the uterine body was recorded at 39%, 37% and 53% for studies by Peters et al. (Reference Peters, Senger, Rosenberger and O’Connor1984), Swain et al. (Reference Swain, Senger, Hillers and Tare1986) and Senger et al. (Reference Senger, Becker, Davidge, Hillers and Reeves1988), respectively. Interestingly, in the study of Senger et al. (Reference Senger, Becker, Davidge, Hillers and Reeves1988), which used radiographic techniques to evaluate placement technique, the success rate for correct positioning of the AI gun tip increased to 95% after retraining but regressed to 75% 6 months after the retraining. This would suggest that ongoing retraining of inseminators is critically important. In the same study, the success rate for correct positioning of the AI gun tip was 95% with no evidence of a drop off after 6 months for intra cornual insemination. This would suggest that anatomical location is more definitively identified with a higher degree of repeatability for cornual (uterine horn insemination) than for uterine body insemination. This has led a number of laboratories to re-evaluate site of deposition particularly in relation to inseminator and sperm dose effects.
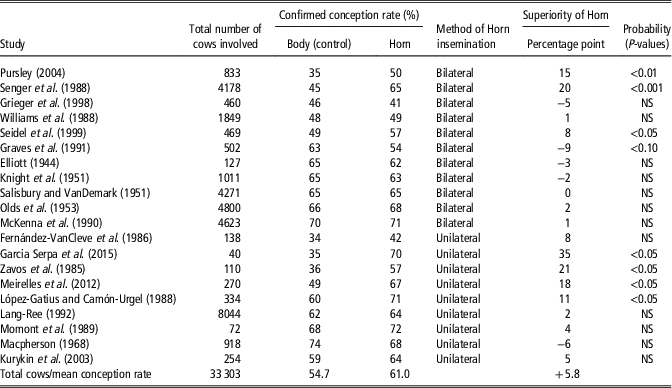
The deposition of semen near the utero tubal junction would be hypothesised to reduce sperm loss either by retrograde flow of uterine mucus or by phagocytosis and would, therefore, be expected to enhance the reservoir of sperm in the caudal region of the oviductal isthmus, the site of the main sperm reservoir in the cow (Hawk, Reference Hawk1987). However, Gallagher and Senger (Reference Gallagher and Senger1989) observed no reduction in retrograde sperm loss after semen deposition in the uterine horn or uterine body. A summary of the studies, involving 33 689 inseminations, that evaluated the effects of site of semen deposition on fertility in artificially inseminated cattle, ranked from the lowest CRs for body insemination and within method (bilateral v. unilateral) of horn insemination is presented in Table 3. It is clear from Table 3 that uterine horn insemination did not consistently result in an improvement in CR though an overall 6.3 percentage point improvement in CR was recorded following horn inseminations. While no individual study showed a statistically significant reduction in CR following uterine horn insemination 7 of the 20 studies showed a statistically significant increase in CRs following uterine horn insemination. It also is clear that with many of the early studies, CRs following body insemination were high and these studies invariably showed no improvement following uterine horn insemination. This might suggest that an upper threshold for CR to a single service exists that lies between 60% and 70% and to exceed this threshold on a consistent basis is difficult or impossible to achieve. The highest percentage point increases in CR following uterine horn insemination were invariably recorded in studies where the lowest CRs were recorded following body insemination.
Table 3 Summary of the effects of site of semen deposition on fertility in artificially inseminated cattle, ranked from the lowest conception rates for body insemination and within method (bilateral v. unilateral) of horn insemination (largely based on DeJarnette et al Reference DeJarnette, Shepard, Kaproth, Michael, Dalton, Goodell and Lee2004)

NS=non-significant.
Irish experience
This study (Diskin et al., Reference Diskin, Pursley, Kenny, Mee, Corridan and Sreenan2005) was carried out over two years with six commercial inseminators involved in each year, four of which were involved in both years. All inseminators were chosen by the AI Centre and trained before the start of the experiment in each year. The inseminators chose the co-operating herds. Each alternate cow presented for AI in co-operating herds was inseminated by placing all of the inseminate either in the body of the uterus (Body) or by placing 50% of the inseminate beyond the curvature in each uterine horn (Horn). Frozen-thawed semen in 0.25 ml straws was used throughout. Records were kept and data collected on a total of 1860 inseminations in 37 herds in Year 1 and on 1586 inseminations in 24 herds in Year 2. Conception rate was determined by ultrasonography at 28 to 60 days after AI. Data were analysed with terms for AI treatment, year, inseminator, and their interactions included in the model. There were no AI treatment×inseminator×year, treatment×year or inseminator×year interactions for CR (P>0.05). However, there was a significant effect (P<0.02) of AI treatment×inseminator on CR, with evidence of either an increase (+11.4%; P<0.05), decrease (−4 to −6%; P<0.05) or no effect (P>0.05) of Horn AI on CR for individual inseminators. Results are presented in Figure 1. A retrospective analysis of the data for each inseminator for each year showed that that there was an inverse relationship (P<0.005) between the improvement in CR recorded following Horn insemination and CR achieved following Body AI (Figure 2). This study indicates that the effect of uterine horn AI on CR is not uniform and may be inseminator dependent. For individual inseminators there was an inverse relationship between the improvement in CR recorded following Horn insemination and CR achieved following Body AI. The results further suggest that non-return rates could be improved for individual inseminators by adopting the practice of placing half of the inseminate beyond the curvature of each uterine horn as opposed to body insemination which is normal practice. Interestingly, and consistent with the results summaries in Table 3 the largest improvements in CR were recorded by inseminators with the lowest CR following Body insemination.

Figure 1 Conception rate in dairy cows following body artificial insemination (AI) for each of the eight inseminators arranged from lowest to highest conception rates (upper panel) Superiority or inferiority of Horn AI, relative to Body AI for each of the eight inseminators (lower panel) (Diskin et al., Reference Diskin, Pursley, Kenny, Mee, Corridan and Sreenan2005).
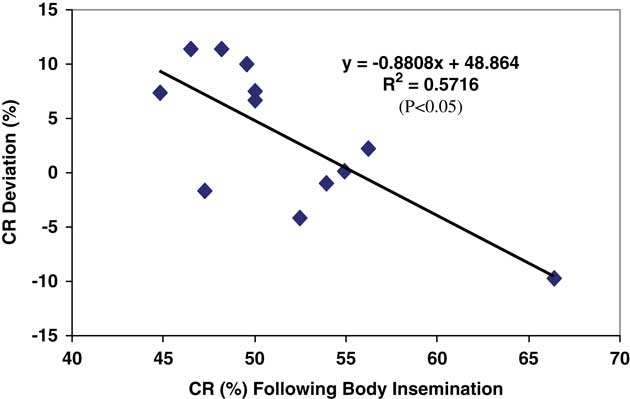
Figure 2 Conception rate deviation (CR following Horn AI – CR following Body AI) (y-axis) and CR for Body AI for each inseminator in each year (x-axis) (Diskin et al. Reference Diskin, Pursley, Kenny, Mee, Corridan and Sreenan2005).
Deposition of semen in the uterine horn ipsilateral to the side of the impending ovulation
A number of studies have evaluated the effect of placement of the inseminate deep into the uterine horn ipsilateral to the side of the impending ovulation;with the side of ovulation been determined by rectal palpation (see review by López-Gatius, Reference López-Gatius2000). A number of these studies reported statistically significant improvements in CRs of +11 percentage points (López-Gatius and Camón-Urgel, Reference López-Gatius and Camón-Urgel1988) 19% percentage points (Meirelles et al., Reference Meirelles, Kozicki, Weiss, Segui, Souza, dos Santos and dos Santos Breda2012) +21 percentage points (Zavos et al., Reference Zavos, Johns, Heersche and Miksch1985) following unilateral deep intrauterine AI ipsilateral to the side of the impending ovulation compared with uterine body insemination (see Table 3). However, others (Fernández-VanCleve et al., Reference Fernández-VanCleve, Zavos, Heersche and Miksch1986; Momont et al., Reference Momont, Seguin, Singh and Stasiuknas1989; Lang-Ree Reference Lang-Ree1992; Kurykin et al. Reference Kurykin, Aakma, Majas, Jalakas, Aidnik, Waldmann and Padrik2003) have all reported numerically smaller but non-significant improvements in CRs following unilateral uterine horn insemination on the side of the impending ovulation (see Table 3).
While the above would suggest more precise deposition of the semen in the uterine horn ipsilateral to the side of the impending ovulation would result in improved CRs, the AI technician would require a higher level of skill to accurately determine the side of the impending ovulation by rectal palpation and to avoid any risk of prematurely bursting the dominant follicle.
From the foregoing, if inseminators are achieving consistently high CR following body insemination there would appear to be little benefit in switching to uterine horn insemination. However, if CRs are low uterine horn insemination will result in a modest (almost 6% percentage points based on data summarised in Table 3) improvement in CR. However, extra care is required with deep uterine body insemination to avoid trauma to the uterine epithelium. One possible explanation for the benefits of uterine horn insemination may be the avoidance of cervical insemination which has been shown to reduce CRs by 10% percentage points (Macpherson, Reference Macpherson1968) compared with uterine body deposition. Interestingly, it would appear that deep bilateral horn deposition of semen, as opposed to common body deposition, has little effect on fertilisation rates in superovulated cows or heifers (Carvalho et al., Reference Carvalho, Souza, Dresch, Vieira, Hackbart, Luchini, Bertics, Betzold, Wiltbank and Shaver2012; Carvalho et al., Reference Carvalho, Souza, Sartori, Hackbart, Dresch, Vieira, Baruselli, Guenther, Fricke, Shaver and Wiltbank2013).
Sex-sorted semen
With sex-sorted semen the number of sperm in the inseminate is much lower, at 1 to 2×106, compared with sperm numbers of 15 to 30×106 used with conventional semen. Because of the lower numbers of sperm used with sex semen, it is reasonable to hypothesise that placement of the semen within the reproduction tract would be critically important and that deep intrauterine horn insemination would lead to higher CRs. However, Seidel et al. (Reference Seidel, Schenk, Herickhoff, Doyle, Brink, Green and Cran1999) and Seidel and Schenk (Reference Seidel and Schenk2008) found no evidence that deposition of sexed semen in the uterine horns was superior to uterine body deposition.
Irrespective of whether conventional or sexed semen is used it is important that inseminations are carefully carried out, avoid deposition of semen in the cervix, inseminators are monitored on a an ongoing basis and that retraining is regularly provided.
Heterospermic insemination
Heterospermic insemination is the insemination of a female with a mixture of semen from more than one male in contrast to the more normal homospermic insemination which uses semen from a single male. Some early studies reported improved fertility in cattle (Hess et al., Reference Hess, Ludwick, Rickard and Ely1958; Beatty et al., Reference Beatty, Bennett, Hall, Hancock and Stewart1969; Nelson et al., Reference Nelson, Pickett and Seidel1974; Stewart et al., Reference Stewart, Sponer, Bennett, Beatty and Hancock1974) and rabbits (Beatty, Reference Beatty1960; Napier, Reference Napier1961a, 1961b) when heterospermic inseminations were compared with single sire inseminations. Consequently, the results from the above papers would suggest that the use of heterospermic semen could increase reproductive rates in commercial herds.
For specific sires, the binomial nature of the outcome of an insemination (pregnant or non-pregnant) make detecting statistically significant differences in fertility among sires difficult and requires large numbers of animals be inseminated. One strategy to make more accurate comparisons of fertility among bulls is to eliminate the confounding effect of the individual cow and compare bulls within the same environment which can be achieved by heterospermic insemination. If numbers of sperm from two males of equal fertility are combined in a 1 : 1 ratio, then the ratio of the resulting offspring would theoretically be 1 : 1. Research across numerous species, has indicated that heterospermic insemination results in ratios of greater or less than 1 : 1. In cattle, Beatty et al. (Reference Beatty, Bennett, Hall, Hancock and Stewart1969) calculated that heterospermic insemination was 170-fold greater inefficiency at measuring differences in fertility among bulls compared with homospermic insemination. Heterospermic performance, defined by a competitive index (CI) (Beatty et al., Reference Beatty, Bennett, Hall, Hancock and Stewart1969), was positively correlated to non-return rate which is a very good measure of CR. Because of this, heterospermic insemination has been widely used to investigate the relationship between bull fertility and specific sperm attributes such as motility, acrosome integrity, DNA integrity, plasma membrane integrity, motility, and oxidative stress (Saacke et al., Reference Saacke, Vinson, O’Connor, Chandler, Mullins and Amann1980; Ballachey et al., Reference Ballachey, Evenson and Saacke1988; Kasimanickam et al., Reference Kasimanickam, Nebel, Peeler, Silvia, Wolfe, McAllister and Cassell2006), and those attributes were correlated to fertility.
There are a comparatively small number of published studies that have compared in field trials the fertility following homo and heterospermic inseminations and these are summarised in Table 4. A feature of many of the studies was the small numbers of cows inseminated with the heterospermic semen and the large numbers of cows in some studies inseminated with the homospermic semen. This makes interpretation of the data somewhat problematical as it over powers the study for statistical purposes. Five of the studies recorded similar CRs following homo- and heterospermic insemination while three of the studies recorded significant improvements in CRs following heterospermic insemination. In two of these latter studies the numbers of cows (n=508; Beatty et al., Reference Beatty, Bennett, Hall, Hancock and Stewart1969 and 160; Stewart et al., Reference Stewart, Sponer, Bennett, Beatty and Hancock1974) inseminated with heterospermic semen was small compared with the number of control homospermic inseminated cows. Based on the results summarised in Table 4, it is likely that heterospermic will give a small improvement in CR. However, it is clear that further large scale well designed studies with sufficient statistical power to comprehensively compare homo- and heterospermic insemination in cattle are required.
Table 4 Summary of studies that compared homo- and heterospermic semen on fertility in cattle

a Based on 508 heterosepermic insemination.
b Based on 160 heterosepermic insemination.
In pigs heterospermic insemination has been widely used in commercial practice but a few studies have compared its efficiency against homospermic insemination. In a recent study, Ferreira et al. (Reference Ferreira, Savio, Guarise, Flach, Gastal, Goncalves, Dellagostin, Alonso, Bianchi, Corcini and Lucia2014) recorded similar reproductive performance following homospermic and heterospermic inseminations, but did record differences in performance among boars undetected with homospermic AI were only evident following genotyping of piglets sired through heterospermic AI. In an earlier study, Godet et al. (Reference Godet, Mercat and Runavot1996) recorded reproductive performance on 617 litters from heterospermic insemination and on 621 litters from homospermic insemination showed that litter size at birth and farrowing rate were slightly but non-significantly higher with heterospermic semen than with homospermic semen (+0.24 alive born piglets,+0.26 total born piglets and +0.8 percentage increase in farrowing rate). Interestingly these authors point out that to be significant, these differences would require a four times as large sample.
Use of heterospermic insemination to evaluate fertility differences between males
The poor correlations between in-vitro semen tests and subsequent field fertility and the large numbers of inseminations required under field conditions to adequately test a male has proven problematical for decades. Beatty (Reference Beatty1960) in rabbits and Beatty et al. (Reference Beatty, Bennett, Hall, Hancock and Stewart1969) in cattle were the first to advocate that heterospermic insemination could be used to evaluate fertility differences between males. Usually, semen is mixed such that sperm numbers from each of the competing males are equal and it is presumed that paternity of the offspring from such inseminates should also be equal among competing males. Therefore, the outcome of such a competitive fertilisation trial is not per cent fertility or CR as in a homospermic study, rather, it is the ratio of offspring based on their paternity. This ratio is usually converted to a CI which is given to each sire being evaluated. One of the advantages of this technique is it overcomes the masking influences of variation in female fertility due to such factors as: (i) Management or health of the female, (ii) inseminator competence, (iii) sperm numbers in the inseminate dose, (iv) environmental and herd factors. This is because only females conceiving and giving birth provide data and the question is simply the paternity of the offspring. Offspring colour marking and single nucleotide polymorphisms are now the methods of choice to link individual offspring to sire.
Timing of ovulation and time of insemination
The objective of an insemination is to ensure that there is an adequate reservoir of competent, capacitated, motile sperm in the caudal region of the oviductal isthmus, at the time of ovulation to ensure the highest possible fertilisation rate. The viable lifespan of the sperm in the bovine female tract has not been accurately determined, reflecting the technical difficulty in quantifying it and also because an inseminate is a heterogeneous population of cells each with different lifespan. Furthermore, it is uncertain whether the viable lifespan of the sperm represents an intrinsic property of the sperm cell or whether it is influenced by the uterine environment. From data derived from mated animals Laing (Reference Laing1945) and Trimberger and Davis (Reference Trimberger and Davis1943) the estimated average lifespan of bull sperm is about 30 h. However, there is likely to be some variation about this mean of 30 h. An interval of 6 to 12 h has been estimated for the sperm to get from the site of semen deposition to the caudal region of the oviductal isthmus (Hawk, Reference Hawk1987).
The cattle ovum would appear to have a short lifespan after ovulation before degenerative changes are initiated. Estimates of the lifespan of the ovulated ovum vary from 8 to 12 h (Laing, Reference Laing1945; Casida Reference Casida1950) to 20 to 24 h (Thibault, Reference Thibault1967). In the latter study the longer interval is probably related to the maximum period during which penetration of the zona pellucida may be found rather than the shorter interval during which normal fertilisation can occur. Dalton et al. (Reference Dalton, Ahmadzadeh, Shafii, Price and DeJarnette2001), using HeatWatch technology to pinpoint heat onset, examined the relationship between time of insemination, fertilisation rate and embryo quality in single ovulating dairy cows. Cows were inseminated (25 million sperm) at 2.0, 12.1 and 24.2 h after heat onset. Median accessory sperm numbers were greatest in embryos recovered following the insemination at 24 h (mean±SD of 33.0±52.7) and were lowest following the insemination at 2 h (mean±SD of 9.5±23.1) . Fertilisation rates were 66% and 82% when cows were inseminated at 2 and 24 h following the onset of oestrus, respectively. Interestingly, embryo quality declined with increasing intervals after onset of oestrus. They concluded that insemination at 12 h after onset of oestrus provided a compromise in terms of maximising fertilisation and embryo survival rates and this would agree with data from Dransfield et al. (Reference Dransfield, Nebel, Pearson and Warnick1998), in which the optimal time for insemination was 4 to 16 h after oestrus onset. Dalton et al. (Reference Dalton, Ahmadzadeh, Shafii, Price and DeJarnette2001) suggested that embryo quality following late insemination may be impaired due to an ageing ovum at the time of fertilisation.
The application of both electronic methods of oestrous detection and ovarian ultrasound allowed for better estimates of time of ovulation relative to oestrous onset. For dairy cows estimates of the mean interval (±SD) from heat onset to ovulation was recorded 27.6±5.4 h (Walker et al., Reference Walker, Nebel and McGilliard1996), 28.7±8.1 (Valenza et al., (Reference Valenza, Giordano, Lopes, Vincenti, Amundson and Fricke2012) and, 26.4±5.3 h (Roelofs et al., Reference Roelofs, van Eerdenburg, Soede and Kemp2005). For beef cows the average interval from the onset of oestrus to ovulation is about 31 h (30.8 h; Yelich et al., Reference Yelich, Barnett, Fullenwider, Kempfer, Lemaster and Chase1999, 31.1 h; White et al., Reference White, Wettemann, Looper, Prado and Morgan2002) and would appear to be somewhat shorter for beef heifers (27.4 h; Lynch et al., Reference Lynch, Kenny, Childs and Diskin2010). However, all of these studies report significant variation (consistent standard deviations of 5 to 6 h) around these mean values. In the study of White et al. (Reference White, Wettemann, Looper, Prado and Morgan2002) time of ovulation after the onset of oestrus ranged from 21.5 to 42.8 h with 64% of cows ovulating between 28 and 33 h after the onset of oestrus. In the study of Lynch et al. (Reference Lynch, Kenny, Childs and Diskin2010) the range in time of ovulation, relative to oestrous onset, was between 16.4 and 46.4 h. These differences might suggest that the optimal time of insemination may be earlier for beef cows compared with either dairy cows or beef heifers. However, in practice, the exact time of oestrous onset is rarely known and combined with the inter-animal variation in timing of ovulation it is not practical to recommend an exact timing for insemination.
The early pioneering work of Trimberger and Davis (Reference Trimberger and Davis1943) and Trimberger (Reference Trimberger1948) showed that maximum CRs were obtained when cows were inseminated from midway to the end of standing oestrus. Satisfactory results were also obtained when inseminations were performed during the 6 h following the end of standing oestrus. This work lead to the well-established and recommended ‘am-pm’ rule which still stands. However, in practice and particularly in herds employing an inseminator service, cows are inseminated at a similar time each day. Nebel et al. (Reference Nebel, Walker, McGilliard, Allen and Heckman1994) found that there was no difference in non-return rates in cows inseminated after the a.m./p.m. rule or on a once-daily basis. Similarly, the data of Lynch et al. (Reference Lynch, Kenny, Childs and Diskin2010) would question the need for following the a.m./p.m. particularly if heat detection is accurate, insemination technique is good and semen fertility is high. Equally, these latter studies question the benefit of using more than one insemination on the same cows to enhance fertility particularly when heat detection is inaccurate regarding time of heat onset.
Acknowledgements
The Author was in receipt of support from Science Foundation Ireland, Proposal ID: 13/IA/2025.
Declaration of Interest
None.
Ethics statement
No required as this is a review of already published information.
Software and data repository resources
None involved.