Carotenoids are potent anticarcinogenic substances involved in antioxidant activity, stimulation of gap-junction intercellular communication and inhibition of cellular proliferation. Besides scavenging radical substances, carotenoids may stimulate the immune system and protect against breast cancer( Reference Garattini, Bolis and Garattini 1 ).
Compared with estimates of dietary intake, serum or plasma carotenoids are better indicators of the biological availability of carotenoids. Some epidemiological studies have revealed the anti-cancer effect of circulating carotenoids. However, the protective role of individual specific serum/plasma carotenoids remains controversial. A retrospective case–control study found an inverse association( Reference Ito, Ryuichiro and Sasaki 2 ), whereas cohort studies( Reference Tamimi, Colditz and Hankinson 3 – Reference Dorjgochoo, Gao and Chow 5 ) were more likely to represent modest or null associations between serum carotenoids and breast cancer risk. In a pooled analysis of eight prospective studies of circulating carotenoids( Reference Eliassen, Hendrickson and Brinton 6 ), significant negative associations with breast cancer were observed for α-carotene, β-carotene, lutein/zeaxanthin, lycopene and total carotenoids. β-cryptoxanthin was not significantly associated with risk.
However, seven of eight studies included in this pooled analysis were conducted in western countries. Compared with western women, in whom the median age at diagnosis is 60–64 years, the age at cancer diagnosis among Chinese women was much younger. Mean age at diagnosis is 48 years( Reference Bhoo-Pathy, Yip and Hartman 7 ), and 65 % of women were premenopausal( Reference Cui, Dai and Tseng 8 ). Chinese women exhibited a significantly advanced average stage on diagnosis (stage IIA v. stage I) on the basis of primary tumour size( Reference Sivasubramaniam, Zhang and Zhang 9 ). Moreover, although the incidence rate of female breast cancer in China was still significantly lower than that in western countries (age-standardised incidence rate of 22·1/100 000 women-years for Chinese women, 92·9/100 000 women-years for American women, 69·9/100 000 women-years for European women), breast cancer had a rapid increase in China( Reference Sung, Rosenberg and Chen 10 ). Therefore, there is an urgent need for efficient prevention strategies among Chinese women.
To the best of our knowledge, only one previous study has investigated the association between specific circulating carotenoids and the risk for breast cancer in the Chinese population( Reference Dorjgochoo, Gao and Chow 5 ), and evidence for the protective effect of each individual serum carotenoid is inconsistent. People living in Guangdong, China, follow the ‘traditional southern’ dietary pattern( Reference He, Ma and Zhai 11 ) characterised by high intakes of vegetables and fruits, which is different from the dietary pattern in Shanghai.
The purpose of the present study was to examine whether serum carotenoids including α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin may lower the risk for breast cancer among Chinese women. As the aetiologies of breast cancer may differ by receptor status, analyses were stratified by menopausal status, oestrogen receptor (ER) status, or progesterone receptor (PR) status to examine any protective effect of each carotenoid in these subgroups.
Methods
Study subjects
Details of this ongoing hospital-based case–control study, which began in 2011, have been reported previously( Reference Zhang, Pan and Li 12 ). Female subjects aged 25–70 years were consecutively recruited from three teaching and general hospitals in Guangzhou, China. All had been histologically diagnosed with breast cancer within 3 months of the recruitment interview. Subjects were natives of Guangdong province or had lived in Guangdong for at least 5 years. Women were excluded if they had a history of other cancers. From September 2011 to May 2014, a total of 521 (96·30 %) of 541 eligible cases were included in the study.
Control subjects were females with no history of any type of cancer who had been admitted to the same hospitals during the same period as the case subjects. They were frequency-matched by age (5-year interval) and were recruited from the departments of Plastic and Reconstructive Surgery, Vascular Surgery, and Ear, Nose and Throat. In total, 521 of 537 (97·02 %) controls participated. The controls were recruited from the above departments because we had no prior reason to believe that several conditions from these departments had apparent association with a dietary cause.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by The Ethical Committee of School of Public Health, Sun Yat-sen University. A written informed consent form was signed by all study subjects.
Data collection
The data were collected by trained interviewers through face-to-face interviews. A structured and previously validated questionnaire was used( Reference Zhang and Ho 13 ). Information on socio-demographic situation, current weight, height, menstrual and reproductive history, menopausal status, use of exogenous hormones, use of contraceptive drugs, family history of cancer, medical history, medication treatment, dietary habits, active and passive smoking, alcohol drinking and physical activities was obtained. BMI was calculated by dividing body weight (kg) by height (m) squared. Regular smoking was defined as smoking at least 1 cigarette/d for >6 consecutive months. Passive smoking was defined as exposure to others’ tobacco smoke for at least 5 min/d in the previous 5 years. Regular drinking was defined as drinking alcohol at least once per week over the past year. Postmenopausal status was defined as at least 12 months since the last menstrual cycle. Relevant medical diagnoses and pathological findings were abstracted from the medical records.
Measurement of serum carotenoids
Fasting serum samples (5 ml) were collected in pro-coagulation tubes on the 2nd day after subjects had been admitted and kept fasting for at least 12 h. Samples were put in a box filled with dry ice and sent to the laboratory. Sera were separated from blood cells by centrifugation (3000 rpm at 4°C for 15 min) within 1 h of collection. Serum samples were stored at −80°C until analysis. Concentrations of α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin were measured using HPLC. Serum carotenoids (200 μl) were deproteinated with ethanol, and α-tocopherol acetate was added as an internal standard before extraction. After extraction with hexane-butylated hydroxytoluene (2 ml) solution, carotenoids were evaporated to dryness with N at room temperature. The extract was dissolved in acetonitrile–methanol–tetrahydrofuran–ammonium acetate (mobile phase B, 200 μl, 55:35:5:5, v/v) and then a C18 HPLC column (Shiseido) and a Waters 2998 diode-array detector (Waters) were used to detect carotenoids. Mobile phase A included acetonitrile–methanol–tetrahydrofuran–ammonium acetate (85:5:5:5, v/v). A sample was injected into the column every 30 min. Retinol and carotenoids were measured at 325 nm and 325/450 nm, respectively. All procedures were performed by the same technician, and peaks were calculated automatically. The median between-batch inter-assay CV were 7·8 % for α-carotene, 8·6 % for β-carotene, 9·7 % for β-cryptoxanthin, 10·6 % for lycopene and 8·0 % for lutein/zeaxanthin. The with-run CV were 1·40 % for α-carotene, 1·50 % for β-carotene, 4·00 % for β-cryptoxanthin, 3·30 % for lycopene and 1·70 % for lutein/zeaxanthin.
Statistical analysis
Statistical analyses were performed using SPSS 19.0, and results were considered significant when P<0·05 (two-sided). For continuous variables, data are shown as means and standard deviations. For categorical variables, frequencies are presented as percentages. The quartiles of the measured carotenoids were defined according to the distribution of the control subjects. The socio-demographic characteristics and potential risk factors between the two groups were compared using Student’s t tests or Wilcoxon’s rank-sum test for continuous variables and χ 2 tests or Fisher’s exact tests for categorical variables.
Unconditional logistic regression was used to estimate the OR and 95 % CI of each quartile (Q1–Q4) of serum levels of specific carotenoids, setting the lowest quartile group as the reference. The association between the risk for breast cancer and the serum levels of specific carotenoids was further examined after adjusting for several potential confounders using multivariate logistic regression models. BMI (continuous variable), residence (urban/rural), education level (primary school or below/junior high school/senior high school/secondary technical school/ college or above), income (<2000, 2001–5000, 5001–8000, >8000 yuan/month), regular drinker (yes/no) and history of benign breast disease (yes/no) were regarded as potential confounders according to a comparison of baseline characteristics between cases and controls. Tests for trends were performed by entering the categorical variables (Q1–Q4) as continuous variables in the models.
As certain risk factors for breast cancer may exert different influences on premenopausal and postmenopausal women( Reference Colditz and Rosner 14 ), the association between specific serum carotenoids and breast cancer risk may be altered by menopausal status. Therefore, an analysis stratified by premenopausal or postmenopausal status was performed. Additionally, breast cancer defined by ER and PR status appears to be aetiologically heterogeneous( Reference Althuis, Fergenbaum and Garcia-Closas 15 ). Stratified analyses by ER status (ER+ or ER–) or PR status (PR+ or PR–) were carried out to assess whether breast cancer risk differs in accordance with ER or PR status. Our sample of 225 cases and 260 controls in two quartiles (Q1 and Q4) gave us 78 % power to detect an OR of 0·71 for the association between serum β-cryptoxanthin and breast cancer risk at P<0·05 (two-tailed). We had 100 % power to detect OR of 0·44, 0·27, 0·41 and 0·26 for the association between serum α-carotene, β-carotene, lycopene and lutein/zeaxanthin and breast cancer risk.
Results
The socio-demographic characteristics of the study subjects are presented in Table 1. Compared with control subjects, women with breast cancer were more likely to live in rural areas, have a higher BMI, be regular drinkers and have a history of benign breast disease. Case subjects were more likely to possess a lower household income and lower educational level. No significant differences were observed between the cases and controls in terms of age, number of live births, age at menarche, age at menopause, age at first live birth, menopausal status, marital status, occupation, physical activity, smoking status, history of a first-degree relative with cancer, passive smoking, oral contraceptive use or breast-feeding.
Table 1 Socio-demographic and selected risk factors for breast cancer among breast cancer cases and controls (Numbers and percentages; mean values and standard deviations)

* Among women who have had a live birth.
† Among women who had breast-fed.
‡ Among menopausal women.
The comparison of mean concentration of serum carotenoids between cases and controls is shown in Table 2. Control subjects possessed significantly higher mean concentrations of serum α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin when compared with cases.
Table 2 Concentration of serum carotenoids (μmol/l) among cases and controls in Guangzhou, ChinaFootnote * (Mean values and standard deviations; median values and 25th, 75th percentiles)
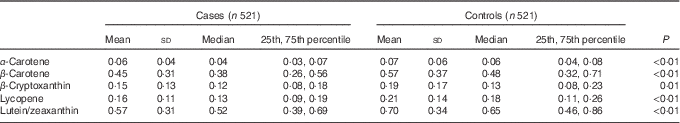
* Wilcoxon’s rank-sum test comparing the median consumption levels between cases and controls.
The OR and 95 % CI for breast cancer risk according to the serum concentration of specific carotenoids are presented in Table 3. After adjustment for various confounders, a significant inverse association was observed between serum α-carotene, β-carotene, lycopene, lutein/zeaxanthin and the risk for breast cancer. The adjusted OR for the highest quartile compared with the lowest quartile were 0·44 (95 % CI 0·30, 0·65; P trend<0·01) for serum α-carotene, 0·27 (95 % CI 0·18, 0·40; P trend<0·01) for β-carotene, 0·41 (95 % CI 0·28, 0·61; P trend<0·01) for lycopene and 0·26 (95 % CI 0·17, 0·38; P trend<0·01) for lutein/zeaxanthin. However, no significant association was found between serum β-cryptoxanthin and the risk for breast cancer, with an adjusted OR of 0·71 (95 % CI 0·48, 1·03) comparing the highest with the lowest quartile (P trend=0·07).
Table 3 Risk of breast cancer according to quartiles of serum carotenoids (Odds ratios and 95 % confidence intervals)

* OR adjusted for BMI, residence, education levels, income, regular drinker and a history of benign breast disease.
Table 4 shows the association between serum carotenoid and the risk for breast cancer stratified by menopausal status. An inverse association between serum levels of α-carotene, β-carotene, lutein/zeaxanthin and the risk for breast cancer was found in both premenopausal and postmenopausal women. Serum β-cryptoxanthin was not significantly associated with the risk for breast cancer in pre- and postmenopausal women. The inverse association between serum lycopene and breast cancer risk was only observed among premenopausal women, with an adjusted OR of 0·36 (95 % CI 0·22, 0·60) comparing the highest quartile with the lowest quartile (P trend<0·01).
Table 4 Risk of breast cancer stratified by menopausal status (Odds ratios and 95 % confidence intervals)

* OR adjusted for BMI, residence, education levels, income, regular drinker and a history of benign breast disease.
Stratified analyses by ER and PR status showed that α-carotene, β-carotene, lycopene and lutein/zeaxanthin were inversely associated with breast cancer risk among all subtypes of ER or PR status. β-cryptoxanthin was not associated with breast cancer risk either in ER or in PR subjects (Table 5).
Table 5 Risk of breast cancer stratified by oestrogen receptor (ER) or progesterone receptor (PR) status (Odds ratios and 95 % confidence intervals)

* OR adjusted for BMI, residence, education levels, income, regular drinker and a history of benign breast disease.
Discussion
This study investigated the relationship between serum carotenoid concentration and breast cancer risk. High levels of serum α-carotene, β-carotene, lycopene and lutein/zeaxanthin were found to be associated with lower risk for breast cancer. Serum α-carotene, β-carotene and lutein/zeaxanthin were found to be inversely associated with breast cancer risk in pre- and postmenopausal women. A stratified analysis by ER and PR status showed that serum α-carotene, β-carotene, lycopene and lutein/zeaxanthin were inversely associated with breast cancer risk among all subtypes of ER or PR status. Serum β-cryptoxanthin was not associated with breast cancer risk.
Previous findings regarding the protective effect of circulating carotenoids on breast cancer risk have been mixed. The protective effect of serum α-carotene, β-carotene, lycopene and lutein/zeaxanthin observed in the present study is consistent with a pooled analysis of eight prospective studies published in 2012( Reference Eliassen, Hendrickson and Brinton 6 ). This analysis, which included 3055 cases and 3956 matched controls, reported inverse associations between circulating α-carotene (highest v. lowest quintile, relative risk (RR) 0·87; 95 % CI 0·71, 1·05; P trend=0·04), β-carotene (highest v. lowest quintile, RR 0·83; 95 % CI 0·70, 0·98; P trend=0·02), lycopene (highest v. lowest quintile, RR 0·78; 95 % CI 0·62, 0·99; P trend=0·02), lutein/zeaxanthin (highest v. lowest quintile, RR 0·84; 95 % CI 0·70, 1·01; P trend=0·05) and breast cancer risk. However, circulating β-cryptoxanthin was not related to breast cancer risk. Other prospective studies also showed that serum/plasma α-carotene( Reference Tamimi, Colditz and Hankinson 3 , Reference Tamimi, Hankinson and Campos 16 , Reference Toniolo, Van Kappel and Akhmedkhanov 17 ), β-carotene( Reference Tamimi, Colditz and Hankinson 3 , Reference Tamimi, Hankinson and Campos 16 , Reference Sato, Helzlsouer and Alberg 18 ), lycopene( Reference Sato, Helzlsouer and Alberg 18 , Reference Dorgan, Sowell and Swanson 19 ) and lutein/zeaxanthin( Reference Tamimi, Colditz and Hankinson 3 , Reference Tamimi, Hankinson and Campos 16 , Reference Dorgan, Sowell and Swanson 19 ) was associated with decreased risk for breast cancer. Most previous studies( Reference Ito, Ryuichiro and Sasaki 2 – Reference Pouchieu, Galan and Ducros 4 , Reference Eliassen, Hendrickson and Brinton 6 , Reference Tamimi, Hankinson and Campos 16 – Reference Dorgan, Sowell and Swanson 19 ) have pointed to a protective role for at least one individual serum/plasma carotenoid. However, three( Reference Dorjgochoo, Gao and Chow 5 , Reference Maillard, Kuriki and Lefebvre 20 , Reference Sesso, Buring and Zhang 21 ) of those recently published studies( Reference Dorjgochoo, Gao and Chow 5 , Reference Maillard, Kuriki and Lefebvre 20 – Reference Hulten, Van Kappel and Winkvist 22 ), including a study in Shanghai, China( Reference Dorjgochoo, Gao and Chow 5 ), indicated that none of the individual circulating carotenoids (including α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin) reduce breast cancer risk. One hospital-based case–control study even reported the deleterious effect of plasma β-carotene on Korean women( Reference Kim, Ahn and Son 23 ).
Those studies that failed to find evidence for a protective role of specific serum or plasma carotenoids may have been limited by a relatively small number of cases (201 cases)( Reference Hulten, Van Kappel and Winkvist 22 ) or restricted to a certain group of subjects, such as highly educated volunteers( Reference Maillard, Kuriki and Lefebvre 20 ) or middle-aged or older female subjects( Reference Dorjgochoo, Gao and Chow 5 , Reference Sesso, Buring and Zhang 21 ), rather than the general population. Contrary to our findings, the previous study in Shanghai, China( Reference Dorjgochoo, Gao and Chow 5 ), observed no association between specific plasma carotenoids and the risk for breast cancer. The distinct differences in dietary habits between Shanghai and Guangdong females may be responsible for the inconsistent reports. Vegetables, particularly dark green leafy vegetables, are good sources of carotenoids. Compared with Shanghai women( Reference Bao, Shu and Zheng 24 ), Guangdong women( Reference Zhang, Ho and Chen 25 ) were more likely to eat vegetables (total vegetables: Shanghai women v. Guangdong women, 304 (sd 174) v. 458 (sd 252) g/d), especially dark green leafy vegetables, which are rich in α-carotene, β-carotene and lutein/zeaxanthin (dark green vegetables: Shanghai women v. Guangdong women, 92 (sd 65) v. 244 (sd 163) g/d). This may account for the finding that specific carotenoids were observed to protect against breast cancer among women in Guangdong, but not among those in Shanghai. It has been reported that the association between β-carotene and cancer risk is likely to be influenced by the source of β-carotene (food or supplement) and the doses involved( Reference Larsson, Bergkvist and Wolk 26 ). In our study, most subjects attained β-carotene from dietary sources rather than supplements. This may partially explain the discrepant results.
Our study showed that serum β-cryptoxanthin was not statistically related to the risk for breast cancer. Previous reports on the association between circulating β-cryptoxanthin and breast cancer risk have been inconsistent. Several studies( Reference Dorjgochoo, Gao and Chow 5 , Reference Eliassen, Hendrickson and Brinton 6 , Reference Tamimi, Hankinson and Campos 16 , Reference Sato, Helzlsouer and Alberg 18 , Reference Kim, Ahn and Son 23 ) reported no inverse associations for circulating β-cryptoxanthin and breast cancer risk after adjustment for other carotenoids or risk factors. In contrast, one recent nested case–control study conducted in French women showed a protective effect of plasma β-cryptoxanthin on breast cancer risk( Reference Pouchieu, Galan and Ducros 4 ). Mean serum β-cryptoxanthin was 0·15 (sd 0·13) in our study, which was markedly lower than that in the Education Nationale-European Prospective Investigation into Cancer and Nutrition study (mean 0·23 (sd 0·16))( Reference Pouchieu, Galan and Ducros 4 ). Relatively low serum β-cryptoxanthin concentration among the general population in China might help to explain the discrepancies. It is also known that carotenoids provide particular protection against breast cancer among smokers, because of the aggravated oxidative stress among this group( Reference Yokusa, Mete and NMU 27 ). In the present study, the smoking rate of subjects was extremely low (1 %). The null association between β-cryptoxanthin and breast cancer risk may be related to the limited number of Chinese women smokers.
Consistent with our study, some previous studies have shown that serum α-carotene, β-carotene( Reference Kabat, Kim and Adams-Campbell 28 ), lycopene( Reference Epplein, Shvetsov and Wilkens 29 ) and lutein( Reference Hulten, Van Kappel and Winkvist 22 ) were inversely associated with breast cancer risk in both pre- and postmenopausal women. However, the protective role of serum lycopene was only observed among premenopausal women in our study. The Shanghai Women’s Health Study( Reference Dorjgochoo, Gao and Chow 5 ) also reported the parallel effect of plasma lycopene on breast cancer risk only among premenopausal women, with an OR (95 % CI) of 0·36 (0·16, 0·80) comparing the highest with the lowest quartile (P trend=0·06). Dissimilar characteristics (such as oestrogen exposure and oxidative stress status) between pre- and postmenopausal women may account for this finding( Reference Spicer and Pike 30 ). Lycopene inhibits cancer cell proliferation under the influence of oestrogen exposure( Reference Hirsch, Atzmon and Danilenko 31 ) and was found to be protective in premenopausal subjects, who have higher levels of oestrogen. Premenopausal women were also shown to be more susceptible to the protection afforded by antioxidants (including lycopene) because of aggravated oxidative stress, in comparison with postmenopausal women( Reference Victorino, Panis and Campos 32 ).
The relatively few epidemiological studies that have examined associations between individual serum carotenoids and the risk for breast cancer stratified by ER or PR status have reported differing results. The significant protective effect of serum α-carotene, β-carotene, lycopene and lutein/zeaxanthin for all subtypes of ER and PR status in the present study was consistent with some previous findings. Tamimi et al. ( Reference Tamimi, Hankinson and Campos 16 ) found a negative association with plasma α-carotene for ER– (highest v. lowest quintile, OR 0·50; 95 % CI 0·28, 0·91; P trend=0·05) and ER+ (highest v. lowest quintile, OR 0·72; 95 % CI 0·50, 1·04; P trend=0·03) breast cancer. A prospective study( Reference Sato, Helzlsouer and Alberg 18 ) conducted in the USA suggested that β-carotene, lycopene and lutein were protective in both ER– and ER+ breast cancer patients. In contrast, a pooled analysis( Reference Eliassen, Hendrickson and Brinton 6 ) reported that α-carotene and β-carotene reduced breast cancer risk in ER– patients but not in ER+ patients. No association between plasma lycopene and breast cancer risk was observed among ER+ and PR+ women in the Shanghai Women’s Health Study( Reference Sesso, Buring and Zhang 21 ). Although the anti-cancer role of carotenoids was supported by an experimental study indicating that carotenoids inhibited proliferation of different hormone-defined breast cancer cell lines( Reference Babich, Krupka and Nissim 33 ), the protective effect of serum β-cryptoxanthin on breast cancer risk was found neither in ER– nor in PR– women. The relatively small sample size might have caused a chance result or insufficient statistical power in the analysis stratified by ER or PR status. Studies with a larger sample size are warranted to clarify this association.
A protective role for carotenoids in breast cancer aetiology is biologically plausible. Carotenoids may protect against DNA damage by neutralising oxygen species( Reference Elliott 34 ) and activating the antioxidant response element transcription system( Reference Ben-Dor, Steiner and Gheber 35 ). Besides their antioxidant potential, some carotenoids such as α-carotene, β-carotene and β-cryptoxanthin are metabolised to retinol, which is involved in cell differentiation( Reference di Masi, Leboffe and De Marinis 36 , Reference Tang and Gudas 37 ). Carotenoids also contribute to intercellular communication( Reference Chalabi, Delort and Satih 38 ), cell proliferation( Reference Chryssanthi, Grehoris and Iatrou 39 ) and cell apoptosis regulation( Reference Hua, Kittler and White 40 , Reference Cui, Lu and Bai 41 ). In addition, carotenoids influence carcinogenesis through genetic mechanisms( Reference Jenab, Slimani and Bictash 42 – Reference King-Batoon, Leszczynska and Klein 44 ).
Our study had several strengths. To the best of our knowledge, only one previous study has examined the association between circulating carotenoids and breast cancer among Chinese women( Reference Dorjgochoo, Gao and Chow 5 ). This study contributes evidence for the protective role of each carotenoid in women with different menopausal and ER/PR statuses. Furthermore, serum carotenoids were chosen as the biomarkers, to reflect carotenoid levels without being influenced by food patterns, racial differences and other environmental factors( Reference Al-Delaimy, Ferrari and Slimani 45 ). More objective results can be delivered by measuring serum carotenoids instead of estimating dietary intake.
Nevertheless, the current study had some limitations that should be taken into account when interpreting the results. First, the study design did not allow causal associations to be confirmed. Serum samples were collected after the diagnosis of breast cancer, and breast cancer itself might influence circulating carotenoids levels. However, a prospective study( Reference Al-Delaimy, Natarajan and Sun 46 ) found no variation in single serum carotenoid levels between breast cancer survivors and control subjects. Second, selection and information biases could have distorted the results. To minimise selection bias, we were careful to exclude all control subjects with any diagnoses related to breast cancer or habitual dietary changes. The similar catchment areas and length of hospitalisation of all subjects, and the relatively high response rate, also reduced selection bias. To minimise information bias, serum carotenoid measurements were performed by the same trained technician. In addition, the lower inter-assay and intra-assay CV showed that the measurement of each carotenoid was relatively accurate and precise. Third, a single-sample measurement may be defective. However, a previous study found that serum carotenoid measurements were reasonably consistent over time because carotenoids are lipid soluble and relatively stable( Reference Comstock, Burke and Hoffman 47 ). Therefore, a single sample is adequately representative of an individual’s long-term exposure( Reference McEligot, Shirley and Flatt 48 ). Finally, potential confounding variables may not have been adequately excluded. It is possible that lifestyles were different among cases and controls. However, a wide range of known predictors was considered, including active smoking, regular drinking and family history.
In conclusion, this study supports the hypothesis of a protective role of α-carotene, β-carotene, lycopene and lutein/zeaxanthin but not β-cryptoxanthin on breast cancer risk. Circulating α-carotene, β-carotene, lycopene and lutein/zeaxanthin were observed to be inversely associated with breast cancer risk among all subtypes of ER or PR status.
Acknowledgements
The authors gratefully acknowledge the cooperation of the study participants.
This study was jointly supported by the New Teachers’ Fund for Doctor Stations, Ministry of Education of China (no. 20100171120057), the National Natural Science Foundation of China (no. 81102188) and the Open-Lab Fund of Sun Yat-sen University in 2011 (no. KF201140). The funders had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows: B. Y. conducted the data collection, analysed the data and wrote this paper. M.-S. L. conducted the laboratory measurement. X.-F. M. was responsible for connecting and coordinating the field work. L. W., W.-P. L. and Y.-F. D. participated in the data collection. C.-X. Z. constructed the project design, supervised and contributed to the manuscript writing.
The authors have no conflicts of interest.








