The disabling behaviour symptoms of military combatants, who were involved in the Viet Nam war, provided the impetus to characterise those psychological disturbances as post-traumatic stress disorder (PTSD). This psychiatric diagnosis first appeared in the third edition of the APA's Diagnostic and Statistical Manual (DSM-III), with its seminal feature being a specific precipitating stressful event. Subsequently, two major revisions of the DSM better characterised PTSD's behavioural diagnostic features. There are three clusters of behaviour symptoms in DSM-IV 1 and four cluster groups in DSM-5. 2 These four clusters comprise re-experiencing, avoidance, negative mood and cognitions (such as depressive, hostile, aggressive and paranoid thinking), and behavioural arousal including sleep disturbances. In addition to its behavioural features, military medical personnel have described a variety of chronic disabling physical symptoms following exposure to combat. Reference Palmer3 These troublesome physical complaints lack any objective physical pathology. Where the focus is on unexplained widespread variable musculoskeletal pain and chronic fatigue, the current preferred diagnosis is fibromyalgia (formerly known as rheumatism or fibrositis). This diagnosis overlaps with equally medically unexplained syndromes such as Gulf War syndrome, chronic fatigue syndrome, combat fatigue (with previous labels including Da Costa's syndrome, disordered action of the heart, neurasthenia or neuro-circulatory asthenia, and effort syndrome). Patients with fibromyalgia have unrefreshing sleep with disturbances in EEG sleep physiology. Moreover, experimentally induced sleep physiological disturbances in normal individuals and animals result in increased pain sensitivity and pain symptoms. Clinical and epidemiological studies show that chronic sleep disturbances are integral to a vicious self-perpetuating cycle of chronic pain, fatigue, negative mood and cognition in patients with fibromyalgia. These symptoms may be precipitated by a distressing traumatic industrial or motor vehicle accident. Reference Moldofsky4 Therefore, the purpose of this study was to determine whether the disordered physiology of the sleeping/waking brain may provide the link between the behavioural and somatic symptoms of those military personnel experiencing post-combat chronic PTSD. The analyses comprised a two-part strategy: first, to establish differences between treatment-resistant PTSD and non-PTSD military personnel in multiple domains comprising EEG sleep physiology, behavioural and the medically unexplained pain and fatigue somatic symptoms; and second, to generate a decision-tree probability model for PTSD based on these differences. That is, the key behavioural elements that distinguish chronic PTSD from non-PTSD military comprise a specific stressful traumatic event (i.e. combat), altered sleep EEG physiology, persistent unexplained somatic symptoms and negative cognitive behaviour.
We employed a case-controlled study of PTSD in which both case and control groups included veterans with and without experience in combat zones, enabling us to separate PTSD symptoms from those that might be associated with combat experience alone and to assess combat experience along with other patient measures when building models. We measured behaviour symptoms, including negative cognition (depressive, hostile, aggressive and paranoid thinking), and chronic physical health concerns, including disturbances in EEG sleep physiology, sleep quality and medically unexplained somatic symptoms (e.g. musculoskeletal pain and fatigue).
Method
Participants
The participants comprise consecutive patients referred between 2008 and 2014 from an Operational Stress Injury (OSI) clinic of the Department of Veteran Affairs of Canada. The OSI clinic specialises in the assessment and treatment of PTSD and other operational stress injuries in the Canadian military and veteran forces (CF). The diagnosis of PTSD was determined by a psychiatrist employing a comprehensive psychiatric examination and the Clinician-Administered PTSD Scale (CAPS) for DSM-IV, Reference Weathers, Keane and Davidson5 where the CAPS mean rating was 77.38 and s.d. 21.36. To control for commonly disrupted and insufficient sleep problems in the military, we compared sleep and symptoms in CF veterans with persistent symptoms of PTSD with CF personnel without a history PTSD who complained of chronic insomnia. This latter group of participants were referred from Canadian Department of National Defence (DND) medical ambulatory clinics for diagnostic assessment of a possible chronic primary sleep disorder such as sleep apnoea or restless legs/sleep-related periodic limb movement disorder.
The participants consented to be assessed in the Centre for Sleep and Chronobiology in Toronto for specialised comprehensive sleep/wake clinical and laboratory investigations of their chronic treatment-resistant symptoms. The research was reviewed and approved by the DND, Institute of Military and Veterans Research Institute in association with Defence Research and Development Canada.
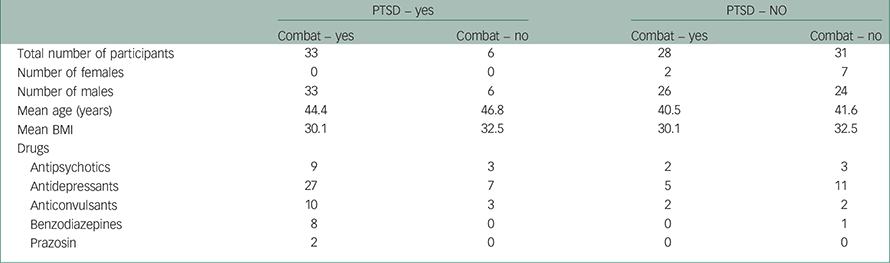
Table 1 shows the description of the participants: gender, mean age, mean BMI and the independent variables: combat experience (combat 1=yes and 0=no or unknown) and the presence of treatment-resistant PTSD (1=yes and 0=no) were independent variables. All patients with PTSD (33 with combat and 6 without) were referred from the OSI. No patients with PTSD were referred from the DND (28 with combat and 31 without). There were no significant differences in the two groups’ ages and BMI values. There were no females with PTSD in the study. SAS Software Multivariate Analysis of Variances (MANOVAs) tests showed no significant gender differences when comparing non-PTSD females (n=9) with non-PTSD males (n=50). Both genders were included in the study. Combat experience included those patients who had served in combat zones (e.g. Gulf War and Afghanistan) and those who developed treatment-resistant PTSD symptoms following deployment to the so-called ‘U.N. peace-keeping missions’ in dangerous combative environments, that is, the Balkans (Bosnia and Croatia) and Africa/Middle East (Suez Canal zone, Rwanda, Somalia and Cyprus). Six cases of PTSD occurred in sleep-disturbed, non-combat CF patients. Distressing non-combat traumatic events were quite varied. They included sexual assault, witness to a major airplane crash, engagement in dangerous service with the military police, witness to a suicide of a soldier and participation in a military training exercise.
Table 1 Sample sizes, participant descriptions and drug use
| PTSD – yes | PTSD – NO | |||
|---|---|---|---|---|
| Combat – yes | Combat – no | Combat – yes | Combat – no | |
| Total number of participants | 33 | 6 | 28 | 31 |
| Number of females | 0 | 0 | 2 | 7 |
| Number of males | 33 | 6 | 26 | 24 |
| Mean age (years) | 44.4 | 46.8 | 40.5 | 41.6 |
| Mean BMI | 30.1 | 32.5 | 30.1 | 32.5 |
| Drugs | ||||
| Antipsychotics | 9 | 3 | 2 | 3 |
| Antidepressants | 27 | 7 | 5 | 11 |
| Anticonvulsants | 10 | 3 | 2 | 2 |
| Benzodiazepines | 8 | 0 | 0 | 1 |
| Prazosin | 2 | 0 | 0 | 0 |
All patients with treatment-resistant PTSD (n=39) were functionally impaired despite psychological and lengthy pharmacological treatments (median 32.5 months (range 6–249 months)).
All patients with PTSD had received cognitive–behavioural treatments, which included trauma-focussed behaviour therapy such as prolonged exposure and various types of psychological support, for example, individual, marital, family counselling and group therapies.
At the time of the sleep study, patients with PTSD were taking at least two drug class combinations of antipsychotics (e.g. risperidone, quetiapine, aripiprazole; n=12), various antidepressants (e.g. mirtazapine, bupropion, venlafaxine, citalopram; n=34), anticonvulsant mood modulators (sodium valproate or topiramate; n=13), and benzodiazepine sedative hypnotics (n=8). In comparison with the patients with PTSD, the 59 with unremitting sleep problems and no PTSD used fewer antidepressants (n=16), anticonvulsants (n=4), antipsychotics (n=5) and sedative hypnotics (n=1) (all χ2 test, P<0.05). Table 1 provides a detailed description of the groups of participants, with and without PTSD, their use of classes of medications and whether or not they had been exposed to combat. The medical records that were made available did not specify the psychotropic medications before their treatments at the time of the polysomnographic sleep study. In particular, of the unknown number of individuals who had previously received the alpha1-adrenergic receptor blocker (prazosin) for disturbing dreams, only two participants continued to find that this drug satisfactorily reduced their recurrent nightmares. Moreover, the psychotropic treatments were not withdrawn to avoid potential adverse effects and permit full cooperation of the patients to participate in the various physiological and behavioural proceedings.
In addition, to the psychotropic drugs shown in Table 1, other medications that were consumed include the following.
-
Patients with PTSD received pain medication (opiates: 3; NSAIDs: 6). Sixteen patients received medication for cardiovascular problems. Three patients were treated for type 2 diabetes.
-
Those without PTSD were prescribed more NSAIDs (n=11) and no opiates, but similar cardiovascular drugs (n=13) and antidiabetic drugs (n=4).
Those individuals who had been previously identified by the military physicians as having problems with alcohol and/or substance abuse/dependency had been treated for their condition(s) before the time of referral for their sleep study. For this study, all participants completed a self-rated standardised questionnaire on the types, amount and frequency of use of alcohol and recreational drugs over the previous year and during 2 weeks before their sleep study. During the 2 weeks before the sleep study, neither group abused alcohol and there were no differences in their alcohol habits. During the previous year, eight patients with PTSD used cannabis, whereas none of the patients without PTSD used cannabis (χ2 test, P<0.01). Within the 2 weeks before the sleep study, only two patients with PTSD had occasionally taken cannabis drugs, whereas none in the sleep-disordered group used cannabis drugs (χ2 test, P=NS).
The individuals with and without PTSD completed psychological and physical symptom self-reports before a single overnight EEG sleep study. Because sleep disturbance has the potential to promote negative cognitions such as irritability, aggressive behaviour, paranoid and depressive thinking that may occur with PTSD, Reference Freeman, Thompson, Vorontsova, Dunn, Carter and Garety6–Reference Freeman, Stahl, McManus, Meltzer, Brugha and Wiles8 we employed the Symptom Checklist-90 (SCL-90) subscales for hostility and paranoid thinking Reference Freeman, Stahl, McManus, Meltzer, Brugha and Wiles8,Reference Derogatis and Cleary9 and the Beck Depression Inventory (BDI). Reference Beck, Ward, Mendelson, Mock and Erbaugh10 Physical symptoms were self-rated from 1 to 5 with the Wahler Physical Symptom Inventory (WPSI). Reference Blanchard, Jones-Alexander, Buckley and Forneris11,Reference Wahler12 The number of symptoms self-rated as 4 or 5 on the WPSI scale (corresponding to ‘about twice a week’ or ‘nearly every day’) was tallied to determine the prevalence of troublesome symptoms. Frequency of regional pain symptoms, Reference Saskin, Moldofsky and Lue13 severity of fatigue (range from 1 ‘full of energy’ to 7 ‘totally physically exhausted’) Reference Moldofsky and Patcai14 and Stanford Sleepiness Scale Reference Hoddes, Zarcone, Smythe, Phillips and Dement15,Reference van den Hoed, Kraemer, Guilleminault, Zarcone, Miles and Dement16 were self-rated before and after the overnight sleep.
The frequency of sleep-related behavioural disturbances during the previous 2 months was rated on the Sleep–Wake questionnaire with a graded scale of 0 – 3 (0=never, 1=sometimes, 2=often and 3=always). The behavioural disturbances during sleep included frequency of awakening during the night because of nightmares or unpleasant dreams, told that you had screamed or shouted and disturbing (bad) dreams during sleep. Abnormal cognition included self-ratings of frequency of harmful behavioural to self and others noted in BDI Q9 (10): thoughts of killing self-rated on a scale of 0–3. Abnormal temper outbursts that could not be controlled included urges to beat, injure or harm someone; urges to break or smash things; shouting or throwing things (questions from the SCL-90, which were rated on a scale of 0–4: 0=not at all, 1=a little bit, 2=moderately, 3=quite a bit, 4=extremely). Reference Blanchard, Jones-Alexander, Buckley and Forneris11
Physiological assessment of sleep comprised standardised EEG polysomnography (PSG). The PSG included electroencephalogram (EEG C3 and C4), electro-oculogram, submental and bilateral anterior tibialis electromyogram, single anterior lead electrocardiogram, measures of respiration comprising measures of airflow with oral–nasal thermistors, respiratory impedance plethysomography and pulse oximetry. An experienced registered PSG technologist completed blind ratings of sleep physiological indices. Reference Rechtshaffen and Kales17,Reference Iber, Ancoli-Israel, Chesson and Quan18 The sleep EEG stages were scored according to the original Rechtschaffen and Kales criteria, Reference Rechtshaffen and Kales17 which were needed for a computerised physiological sleep measure of EEG sleep stability known as the Cyclical Alternating Pattern (CAP). Reference Terzano and Parrino19–Reference Rosa, Alves, Brito, Lopes and Tufik21 Sleep EEG CAP is characterised by sequences of transient EEG changes in non-REM sleep that occur distinctively from the background EEG activities. They comprise three subtypes: CAPA1 is related to favourable sleep stability. CAPA2 and CAPA3 are progressive indices of poor sleep quality or sleep instability. Reference Terzano and Parrino19 These abnormal physiological features have been observed in patients who have chronic widespread musculoskeletal pain and fatigue, Reference Rizzi, Sarzi-Puttini, Atzeni, Capsoni, Andreoli and Pecis22,Reference Moldofsky, Harris, Archambault, Kwong and Lederman23 as well as sleep-related breathing disorders. Reference Bao and Guilleminault24 Analysis of nocturnal sleep EEG CAP was determined by an Embla computerised automatic detection system. Reference Rosa, Alves, Brito, Lopes and Tufik21,Reference Rizzi, Sarzi-Puttini, Atzeni, Capsoni, Andreoli and Pecis22
Data analyses
MANOVAs were fit into four groups of dependent variables. These included PSG and sleep EEG CAP indices; self-ratings of behaviour, including negative cognition; pre- and post-PSG sleepiness and fatigue; and physical symptom severity. Combat experiences (Combat 1=yes, 0=no or unknown) were the independent variables that were compared to whether or not they had treatment-resistant PTSD (PTSD 1=yes, 0=no) (see Table 1). Because there were only six patients with PTSD=1 and Combat=0, we did not test for PTSD × Combat experience interactions. EEG sleep measures included sleep architecture and computerised measure of EEG sleep stability, that is, sleep EEG CAP rate. MANOVA permitted the testing of the significance of suites of measures, while accounting for relationships among the dependent variables as well as the relationships between independent and dependent variables. Initial data exploration showed skewed distributions, and outliers, particularly for the sleep physiology measures. Normality assumptions were likely violated. For significance testing, MANOVA and ANOVA models were fitted to ranked measures; tests on ranked data provided non-parametric tests. Reference Harrar and Bathke25–Reference Hochberg and Benjamini28 All inferences were based on these ranked non-parametric tests. Because multiple testing inflates Type 1 error, or the probability of rejecting the null hypothesis when true, P-values from ANOVAs were adjusted for multiple testing within each of the four independent variable groups by the adaptive step-down Holm method. Reference Schenker and Taylor29 This method controlled for the error rate within a family of tests (family-wise error). The test does not assume independence between the various dependent variables in these ANOVAs.
We used three methods to identify distinguishing characteristics of PTSD and non-PTSD patients: canonical discriminant analysis (CDA), logistic regression and decision trees. PTSD was the dependent variable. Patient measures and combat experience were the independent variables.
We fitted a chi-square automatic interaction detection (CHAID)-like decision-tree algorithm by SAS Enterprise Miner. Reference Hochberg and Benjamini28 The CHAID approach finds splitting rules that create pure segments of data by a chi-squared test of association with a significance cut-off (alpha of 0.05), where all P-values are adjusted for multiple testing by a Bonferroni method. Missing values were addressed through multiple imputations by the predictive mean matching method, which is more appropriate for non-normal measures. Reference Schenker and Taylor29,Reference Horton and Lipsitz30 BDI was excluded from decision-tree and regression modelling because this self-rated test includes both somatic symptoms such as sleep, pain and fatigue, and abnormal cognitive behavioural data.
Results
Patients with PTSD differed from patients without PTSD in prevalence of suites of physical and behavioural health problems, while controlling for the effects of combat exposure. MANOVA tests showed significant differences between patients with PTSD and without PTSD on sleep measures (P<0.01), psychological symptoms self-reports (P<0.0001) and physical symptoms self-reports (P<0.01). The MANOVA test showed no significant difference between PTSD and non-PTSD on subtypes of sleep EEG CAP measures (P>0.05).
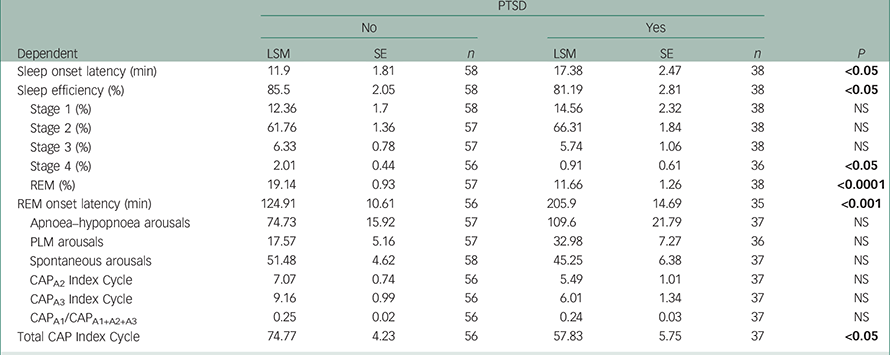
While controlling for the effects of combat exposure, individual ANOVAs showed differences between PTSD and non-PTSD groups in their distributions of onset to EEG sleep, sleep efficiency, EEG stage 4%, REM sleep onset latency, REM% and EEG CAP indices measures including CAP frequency, CAPA2, CAPA3/CAPA1+A2+A3 Reference Rizzi, Sarzi-Puttini, Atzeni, Capsoni, Andreoli and Pecis22 (see Table 2). Those with PTSD took longer to fall asleep, had less efficient sleep (i.e. less time asleep relative to time in bed), less deep (stage 4) sleep, lower percent of time in REM sleep and a prolonged delay to onset of REM sleep. The higher CAP frequency index in the non-PTSD group is consistent with their complaint of chronic poor quality of sleep in the absence of a specific combat-related traumatic experience. These sleep EEG differences may be influenced by more frequent use by the PTSD group of psychotropic drugs.
Table 2 Results of ANOVAs sleep physiology measures
| Dependent | PTSD | P | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| LSM | SE | n | LSM | SE | n | ||
| Sleep onset latency (min) | 11.9 | 1.81 | 58 | 17.38 | 2.47 | 38 | <0.05 |
| Sleep efficiency (%) | 85.5 | 2.05 | 58 | 81.19 | 2.81 | 38 | <0.05 |
| Stage 1 (%) | 12.36 | 1.7 | 58 | 14.56 | 2.32 | 38 | NS |
| Stage 2 (%) | 61.76 | 1.36 | 57 | 66.31 | 1.84 | 38 | NS |
| Stage 3 (%) | 6.33 | 0.78 | 57 | 5.74 | 1.06 | 38 | NS |
| Stage 4 (%) | 2.01 | 0.44 | 56 | 0.91 | 0.61 | 36 | <0.05 |
| REM (%) | 19.14 | 0.93 | 57 | 11.66 | 1.26 | 38 | <0.0001 |
| REM onset latency (min) | 124.91 | 10.61 | 56 | 205.9 | 14.69 | 35 | <0.001 |
| Apnoea–hypopnoea arousals | 74.73 | 15.92 | 57 | 109.6 | 21.79 | 37 | NS |
| PLM arousals | 17.57 | 5.16 | 57 | 32.98 | 7.27 | 36 | NS |
| Spontaneous arousals | 51.48 | 4.62 | 58 | 45.25 | 6.38 | 37 | NS |
| CAPA2 Index Cycle | 7.07 | 0.74 | 56 | 5.49 | 1.01 | 37 | NS |
| CAPA3 Index Cycle | 9.16 | 0.99 | 56 | 6.01 | 1.34 | 37 | NS |
| CAPA1/CAPA1+A2+A3 | 0.25 | 0.02 | 56 | 0.24 | 0.03 | 37 | NS |
| Total CAP Index Cycle | 74.77 | 4.23 | 56 | 57.83 | 5.75 | 37 | <0.05 |
All P-values from ranked (non-parametric) tests are adjusted by the adaptive Holm method to control family-wise error rate. Least-square means (LSM) for PTSD, no (0) and yes (1), are adjusted to remove combat effects. Bold denotes significance.
There were no group differences in prevalence of primary sleep disorders such as obstructive sleep apnoea or sleep-related periodic leg movements (PLM). The prevalence of patients with PTSD with an index of obstructive sleep apnoeas/hypopnoeas per hour of sleep >5 per hour (n=35) did not differ from patients without PTSD (n=25, χ2=NS). Furthermore, there were no statistical differences in their frequencies of sleep EEG arousals from sleep apnoea/hypopnoeas or with sleep-related PLM (see Table 2).
Behavioural disturbances during sleep were more common among the PTSD veterans. Of the 39 patients with PTSD, 32 were more likely to be awakened by nightmares versus 11 of 59 patients without PTSD (χ2 test, P<0.05). Twenty-one patients with PTSD versus six patients without PTSD reported screaming or shouting during sleep (χ2 test, P<0.05). Seven of those in the combat-related PTSD group also reported inadvertently striking, but not seriously injuring, their partner during sleep, whereas none of the non-PTSD group described any abnormal sleep-related behaviour.
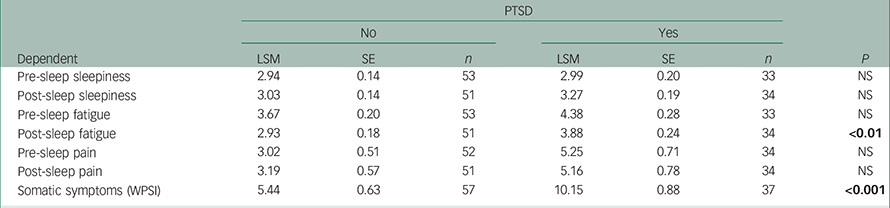
Patients with PTSD had greater mean ratings on the WPSI with more frequent physical problems (commonly musculoskeletal pain and post-sleep fatigue) (Table 3).
Table 3 Physical self-reports
| Dependent | PTSD | P | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| LSM | SE | n | LSM | SE | n | ||
| Pre-sleep sleepiness | 2.94 | 0.14 | 53 | 2.99 | 0.20 | 33 | NS |
| Post-sleep sleepiness | 3.03 | 0.14 | 51 | 3.27 | 0.19 | 34 | NS |
| Pre-sleep fatigue | 3.67 | 0.20 | 53 | 4.38 | 0.28 | 33 | NS |
| Post-sleep fatigue | 2.93 | 0.18 | 51 | 3.88 | 0.24 | 34 | <0.01 |
| Pre-sleep pain | 3.02 | 0.51 | 52 | 5.25 | 0.71 | 34 | NS |
| Post-sleep pain | 3.19 | 0.57 | 51 | 5.16 | 0.78 | 34 | NS |
| Somatic symptoms (WPSI) | 5.44 | 0.63 | 57 | 10.15 | 0.88 | 37 | <0.001 |
All P-values from ranked (non-parametric) tests are adjusted by the adaptive Holm method to control family-wise error rate. Least-square means (LSM) for PTSD, no (0) and yes (1), are adjusted to remove combat effect. Bold denotes significance.
Patients with PTSD described significantly more BDI depressive symptoms (see Table 4). Further, seven patients with PTSD reported suicidal intentions, whereas none of the patients without PTSD reported suicidal intentions.
Table 4 Results of ANOVAs on raw and ranked data for SCL-90 subscales for paranoid, hostility and BDI psychological self-reports

| Dependent | PTSD | P | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| LSM | SE | n | LSM | SE | n | ||
| Hostility score | 3.02 | 0.63 | 53 | 6.56 | 0.84 | 36 | <0.001 |
| Paranoid score | 2.05 | 0.48 | 56 | 5.68 | 0.66 | 35 | <0.0001 |
| BDI score | 9.88 | 1.05 | 56 | 22.74 | 1.47 | 36 | <0.0001 |
All P-values from ranked (non-parametric) tests are adjusted by the adaptive Holm method to control family-wise error rate. Least-square means (LSM) for PTSD, ‘no’ (0) and ‘yes’ (1), are adjusted to remove combat effects. Bold denotes significance.
More patients with PTSD reported hostility and paranoid notions on the SCL-90 (Table 4). Nineteen participants reported domestic and/or violent social behaviour with three being charged and arrested for assaultive behaviour. In contrast, none of the non-combat non-PTSD patients reported any form of social misbehaviour (χ2 test, P<0.001).
CDA and, in particular, logistic regression and decision trees identified the most salient combinations of measures that characterise PTSD. All five runs of CDA, which used multiply imputed data sets, showed significant discriminant functions (P<0.001). Excluding combat exposure, the top six variables that are most correlated (|r|>0.4) with these functions and were the most discriminating characteristics of PTSD, comprise the following features: BDI total score, paranoid score, REM onset latency, SCL-90 hostility scale score, WPSI symptom score and reduced REM% sleep. Regression models that minimise Schwarz criteria reveal that PTSD is described by REM onset latency, paranoid score and combat exposure. Individuals with combat experience had 4.7 times the odds of having PTSD compared to those without combat exposure. Because selective serotonin reuptake inhibitor (SSRI) and serotonin-noradrenaline reuptake inhibitor (SNRI) antidepressants may cause a delay in REM onset latency Reference Argyropoulos and Wilson31 and both antipsychotic as well as anticonvulsant neurotropic drugs may influence EEG sleep physiology, we also re-ran the selection routine while forcing these drugs as variables into the model. The selection routine chose the same variables. PTSD in these individuals is still described by REM onset latency, paranoid score and combat exposure, even while controlling for the use of antidepressant, antipsychotic and anticonvulsants.
The CHAID-like decision-tree analysis reveals the key traits that distinguish PTSD from non-PTSD and their relative importance. Paranoid score was the most important variable in the tree model, followed by REM onset latency, exposure to combat and WPSI severity score based on each measure's contribution to impurity reduction across the tree (relative importance: 1, 0.79, 0.77 and 0.49, respectively). For individuals who were exposed to combat, longer delays to onset of REM sleep (REM onset latency≥175.5 min) lead to higher propensity of exhibiting PTSD (0.79 or 79%). Lower REM onset latency and high paranoid scores (≥7.5) lead to high PTSD propensity (0.857 or 85.7%), whereas low paranoid scores (<2.5) lead to low PTSD propensity. With no combat experience, PTSD is still highly probable (0.83 or 83%) with higher paranoid scores (≥2.5) and higher somatic symptom ratings (WPSI≥11). On the other hand, those non-combat individuals with lower paranoid and somatic symptom ratings were likely to have a low propensity for PTSD (see Fig. 1).

Fig. 1 Chi-square automatic interaction detection (CHAID)-like decision tree with Beck Depression Inventory removed from analysis. The model shows the percentage (probability × 100) of post-traumatic stress disorder (PTSD), given patient combat experience, REM latency, and paranoid and Wahler Physical Symptom Inventory (WPSI) scores. Red segments show groups of patients with higher probabilities of PTSD than the overall average, whereas green segments show groups of patients with lower probabilities.
Note: Because both patients with and without PTSD are shown, the figure uses the DSM-IV PTSD terms to describe each of the segments.
Discussion
This study provides provisional evidence for the key clinical and physiological sleep features that characterise veterans who remain functionally disabled with PTSD despite various drug and behavioural treatments. The results are consistent with DSM-V behavioural symptomology. The association of malfunctions of the sleeping/waking brain and medically unexplained physical symptoms with DSM-IV behavioural symptoms, identified in the MANOVA and CDA analyses, broadens our understanding of chronic PTSD. These psychological and somatic symptoms are consistent with our hypothesis that EEG sleep disturbances may provide the link between negative cognition and mood, and somatic symptoms. While ANOVA and MANOVA statistics show these symptoms to be independent of combat stressor effects, the war-zone experience itself is a key characteristic of PTSD among military personnel, as shown by both the logistic regression and decision-tree models (see Fig. 1). Our decision-tree model in Fig. 1 shows that combat military service, disturbed REM sleep, paranoid thinking and medically unexplainable somatic symptoms (commonly musculoskeletal pain) characterise CF veterans with chronic PTSD. However, the regression model, while identifying combat and altered EEG sleep, did not include somatic symptoms.
Our EEG sleep results complement previous studies that show the importance of disturbances in REM and non-REM sleep. Those with chronic PTSD take longer to fall asleep, have reduced sleep efficiency, reduced stage 4 (deep) sleep, reduced and delayed onset to REM sleep. Reference Kobayashi, Huntley, Lavela and Mellman32–Reference Lettieri, Williams and Collen35 A meta-analysis of previous polysomnographic studies of military and civilian patients with PTSD versus those without PTSD showed similar findings with more stage 1 (light) sleep, less slow-wave (deep) sleep and alterations in REM. Reference Lavie34 Furthermore, our findings are consistent with previous PTSD sleep studies, which show prolongation in the onset to REM sleep. Reference Lettieri, Williams and Collen35,Reference Mellman, Nolan, Hebding, Kulick-Bell and Dominguez36 The higher EEG CAP frequency in the patients without PTSD, a sensitive marker for sleep instability, Reference Terzano and Parrino19 may be related to their primary complaint of disturbed and unrefreshing sleep. Such differences in EEG CAP frequency are unlikely to be affected by psychotropic drugs, which reduce EEG CAP rate. Reference Parrino, Spaggiari, Boselli, Giovanni and Terzano37 Unlike previous studies that associate sleep-related breathing disorders to sleep EEG CAP Reference Bao and Guilleminault24 and to PTSD in the military, Reference Lettieri, Williams and Collen35 this study found no statistical differences in apnoea/hypoponea index, sleep-related respiratory arousals or frequency of periodic limb movement arousals between those with combat-related PTSD and those with sleep-disturbed, non-PTSD military individuals.
In this study of CF veterans with chronic post-combat PTSD, a prolonged delay in the onset to REM sleep is accompanied by negative mood and negative cognitive symptoms. Whereas most tricyclic, SSRI and SNRI antidepressant drugs may reduce and delay the onset to REM sleep, Reference Argyropoulos and Wilson31 the delay in onset to REM sleep persists when the use of antidepressants, as well as anticonvulsants and psychotropic drugs, is statistically controlled. A previous sleep EEG study, however, that compared the sleep of drug-free combat-related PTSD patients with patients with major depression and normal individuals showed that the PTSD group had similar reduction in REM sleep and no delay in onset to REM sleep. Reference Mellman, Nolan, Hebding, Kulick-Bell and Dominguez36 Overall, our results are supportive of Germain's hypothesis and previous EEG sleep research that disturbed REM and non-REM sleep contribute to maladaptive stress and trauma responses in PTSD. Reference Germain38
Ideally, further studies of sleep physiology should involve comparisons between a group of non-sleep-altering medicated sleep-disturbed individuals with PTSD and age-matched normal healthy sleepers who are acclimatised to the novelty of the laboratory environment. To affirm the importance of a disorganisation of the REM/non-REM sleep as a possible key factor to the emergence of PTSD, it would be preferable to examine the EEG sleep before any drug administration, which may influence sleep physiology. The design of the research did not permit a comparative study with those who had benefitted from specific pharmacological and/or behavioural treatment. A future study employing similar physiological and behavioural methodology should comprise a large group of randomised, early-identified combat-exposed versus non-combat-exposed individuals who are free of potentially sleep EEG confounding psychotropic drugs. Such research may pave the way to determining what pharmacological and behavioural methods would be useful in those early-detected post-combat military with PTSD.
Our decision to include measures of negative cognition and mood in patients with PTSD, diagnosed by DSM-IV criteria, highlights their importance in the current DSM-5 criteria. This finding highlights the importance of paranoid and hostile behaviour in those with PTSD following a major traumatic experience. Reference Freeman, Thompson, Vorontsova, Dunn, Carter and Garety6 Their presence in these veterans with chronic PTSD, despite their various drug and behavioural treatments, may stem from an inability in civilian life to overcome their combat-ready vigilant mindset, which becomes ingrained in military training. Such cognitive and behavioural abnormalities may contribute to the increased prevalence of dysfunctional domestic and social behaviour in U.S. veterans with PTSD, Reference Jakupcak, Conybeare, Phelps, Hunt, Holmes and Felker39 and in post-combat British veterans. Reference Macmanus, Dean, Jones, Rona, Greenberg and Hull40
In conclusion, the results of this study and previous clinical research support the notion that chronic disturbed sleep physiology, unexplained musculoskeletal pain and fatigue symptoms should be considered to be integral to irremediable post-combat PTSD. Moreover, paranoid and hostile ideation among those with post-combat PTSD, as well as in civilians with PTSD, Reference Freeman, Thompson, Vorontsova, Dunn, Carter and Garety6 may be a cautionary signal to the potential for antisocial behaviour. Further studies are needed to determine whether civilians who are victims of psychologically stressful events (e.g. sexual assault, non-physically injurious MVA, and industrial and environmental disasters) may demonstrate similar distinguishing features that predispose to PTSD as noted in Fig. 1.









eLetters
No eLetters have been published for this article.