The cinnamon tree is a member of the laurel family. Cinnamon is the inner bark of the cinnamon tree and is used as a spice. Ceylon cinnamon, with the botanical name Cinnamomum zeylanicum/verum is also known as ‘true cinnamon’. However, the related Cinnamomum cassia/aromataticus, Cinnamomum burmannii and Cinnamomum loreirii are also sold as cinnamon. According to an investigation of various herbs and medicinal plants, C. cassia and C. zeylanicum extracts are among the most effective in the regulation of blood glucose in vitro (Reference Broadhurst, Polansky and Anderson1). A water-soluble polyphenol type-A polymer, isolated from C. burmannii, has been shown to enhance insulin action by stimulating the insulin receptor in vitro (Reference Anderson, Broadhurst and Polansky2, Reference Imparl-Radosevich, Deas and Polansky3). The effects on insulin sensitivity of different species of cinnamon, C. cassia, C. burmannii, C. loreirii and C. zeylanicum, have been reported not to differ(Reference Anderson, Broadhurst and Polansky2). However, in another experiment it was found that extract of C. cassia had a better effect on glucose and insulin levels in rats than an extract of C. zeylanicum (Reference Verspohl, Bauer and Neddermann4). In a previous study on healthy subjects, we found that the ingestion of 6 g C. cassia powder reduced the postprandial glucose concentration and gastric emptying rate(Reference Hlebowicz, Darwiche and Björgell5). We also found that an intake of 3 g C. cassia powder reduced postprandial insulin concentrations more noticeably than the ingestion of 1 g C. cassia powder, without affecting the postprandial glucose level or the gastric emptying rate in healthy subjects(Reference Hlebowicz, Hlebowicz and Lindstedt6). However, ingestion of capsules containing the equivalent of 5 g C. cassia powder 12 h before, or in conjunction with, an oral glucose test in healthy men has been found to reduce the glucose response, while no difference was observed in the insulin response(Reference Solomon and Blannin7). The intake of capsules corresponding to 3 g C. cassia powder the night before an oral glucose tolerance test (OGTT) has been found to reduce the glucose response in healthy men, while no difference was observed in the insulin response(Reference Solomon and Blannin8). These effects were lost within 2 d of ceasing cinnamon intake after 2 weeks of daily C. cassia ingestion(Reference Solomon and Blannin8).
Evidence of the positive effect of C. cassia supplementation on glycaemic control in people with type 2 diabetes is still inconclusive. Owing to the presence of a toxic component called coumarin, the Federal Institute for Risk Assessment (BrF) in Europe has recently warned against consuming large amounts of C. cassia powder. On the basis of experimental studies on hepatotoxicity in dogs(Reference Hagan, Hansen and Fitzhugh9), the BrF established that 0·1 mg coumarin/kg body weight per d could be ingested over a lifetime without posing a risk to health. In more recent studies on the use of coumarin as a medical product, nine out of 114 patients exhibited elevated levels of transaminases in serum(Reference Burian, Freudenstein and Tegtmeier10–Reference Vanscheidt, Rabe and Naser-Hijazi12). Analyses of cinnamon powder have shown levels of coumarin between 0·7 and 12·2 g/kg spice(Reference He, Qiao and Han13, Reference Miller, Poole and Chichila14). True Ceylon cinnamon, C. zeylanicum, has negligible amounts of coumarin(Reference Verspohl, Bauer and Neddermann4, Reference Miller, Poole and Chichila14). The BrF has therefore suggested the replacement of C. cassia by C. zeylanicum, or the use of aqueous extracts of C. cassia in order to lower coumarin exposure. However, the effects of C. zeylanicum on glucose and insulin levels have not previously been studied in human subjects.
Changes in lifestyle, such as increased energy intake and decreased physical activity, are causing overweight and obesity, leading to an epidemical increase in type 2 diabetes. Low glycaemic index (GI) and/or low glycaemic load diets are associated with a reduced risk of type 2 diabetes(Reference Barclay, Petocz and McMillan-Price15), comparable with the risk reduction observed with a high intake of dietary fibre and whole-grain products. The GI was originally introduced by Jenkins et al. (Reference Jenkins, Wolever and Taylor16) to classify carbohydrate-containing foods according to their effects on blood glucose. The GI is defined as the increase in the area under the curve (AUC) over 2 h, above the fasting blood glucose, after the ingestion of a test meal, divided by the response to a reference meal such as white bread or glucose(17). Impaired glucose tolerance (IGT) is associated with increased risk of developing type 2 diabetes(18). Studies suggest that IGT is associated with muscle insulin resistance and defective insulin secretion, resulting in less efficient disposal of the glucose load during the OGTT(Reference Abdul-Ghani, Jenkinson and Richardson19). Type 2 diabetes can be prevented by changes in the lifestyles of subjects with IGT(Reference Tuomilehto, Lindstrom and Eriksson20). The present study was therefore designed to determine whether C. zeylanicum lowered postprandial glucose, insulin levels, GI and glycaemic insulinaemic index (GII) in subjects with IGT.
Experimental methods
A total of ten subjects with IGT (six male, four female; age 61 (sd 16) years (range 29–73 years); BMI 26·3 (sd 4·2) kg/m2 (range 20·1–32·7 kg/m2)) were included in the present crossover study. All subjects were recruited from the population in southern Sweden. Patients were selected for the present study on the basis of the diagnosis of IGT (fasting glucose < 7·0 mmol/l, 2 h venous glucose ≥ 7·8 mmol/l and < 11 mol/l). Glucose tolerance status and fasting plasma glucose levels were evaluated using the criteria established by the WHO(18). A standard 75 g OGTT was performed within 12 months before enrolment. Subjects who had thyroid disorders, or who had taken insulin or oral anti-diabetic drugs within 60 d before enrolment were excluded. Four subjects were smokers. The fasting glucose concentration of each subject was checked on the day of the examination to ensure that it was normal ( < 7·0 mmol/l).
Capsules containing either 560 mg lactose (Apoteket, Produktion & Laboratorier, Gothenburg, Sweden) or 400 mg C. zeylanicum and 100 mg lactose (Svampbutiken; Mediapoint AB, Västerås, Sweden) were prepared in advance by the Malmö University Hospital Pharmacy. Although both cinnamon and placebo capsules appeared identical, it is possible that some of the participants could discern a difference between the two types of capsules. The subjects were examined between 07.30 and 10.30 hours after a 12 h fast. Smoking and snuff taking were prohibited 8 h before and during the test. The OGTT consisted of 75 g glucose to which 6·9 g lactose was added, and was ingested with fifteen capsules containing C. zeylanicum. The reference OGTT consisted of 75 g glucose, and was ingested with fifteen placebo capsules. The OGTT were served in random order at intervals of 1 week. Randomisation was performed using a table of random numbers.
Finger-prick capillary blood samples were taken to determine glucose levels, and venous blood for the determination of insulin concentrations, before and at 15, 30, 45, 60, 90, 120, 150 and 180 min after the start of the OGTT. Glucose concentrations were measured with the HemoCue Glucose system (HemoCue AB, Ängelholm, Sweden), which converts blood glucose to plasma-equivalent glucose concentrations by multiplying by a constant factor of 1·11(Reference Burnett, D'Orazio and Fogh-Andersen21). The precision of the HemoCue Glucose system was better than 0·3 sd between 0 and 22·2 mmol/l. Insulin concentrations were measured using an immunoassay with an alkaline phosphatase conjugate (Access Ultrasensitive Insulin; Beckman-Coulter AB, Bromma, Sweden). The sensitivity of the insulin immunoassay was 0·03 mU/l, and the intra-assay CV was less than 10 % in the interval 0·03–300 mU/l.
All subjects gave their written informed consent. The present study was approved by the Ethics Committee of Lund University, and was performed according to the Helsinki Declaration. The present study started on 11 May 2009 and ended on 11 September 2009. The trial registration no. is NCT01027585.
The incremental AUC were measured for plasma glucose and insulin in each subject using GraphPad Prism version 3.0 (GraphPad Software, San Diego, CA, USA). The AUC above the baseline was calculated. The GI and the GII were calculated from the 0–180 min AUC using each subject as their own reference. The GI and GII were calculated by expressing the increase in glucose level of each participant following the test meal as a percentage of the same participant's response after the reference meal. All statistical calculations were performed using SPSS for Windows (version 14.0, 2005; SPSS, Inc., Chicago, IL, USA). Differences in the plasma glucose levels, insulin levels and glycaemic index were evaluated with Wilcoxon's t test. Values of P < 0·05 were considered significant. Differences in GI in ten subjects can be detected with 80 % power at a level of P < 0·05.
Results
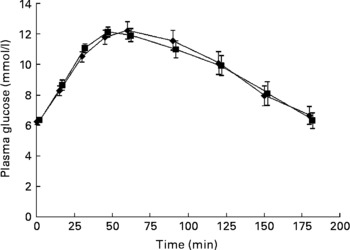
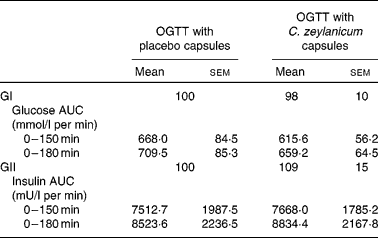
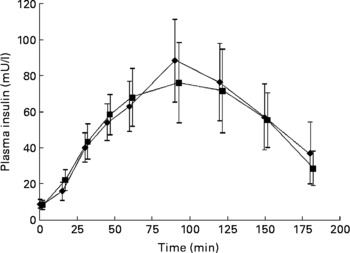
No significant differences were seen in glucose responses at different time periods, or in the incremental areas under the postprandial glucose curves, between the OGTT with and without C. zeylanicum (Fig. 1, Table 1). Neither were any significant differences seen in insulin responses at different time periods nor in the incremental areas under the postprandial insulin curves, between the OGTT with and without C. zeylanicum (Fig. 2, Table 1). No significant differences were seen in GI or GII between the meals with and without C. zeylanicum (Table 1).
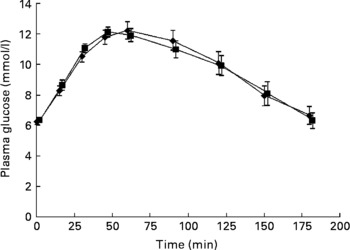
Fig. 1 Plasma glucose concentration in ten subjects with impaired glucose tolerance following an oral glucose tolerance test with placebo capsules (reference; ■) or Cinnamomun zeylanicum capsules (♦). Values are means with their standard errors represented by vertical bars. Mean values were not significantly different between the two conditions when evaluated with Wilcoxon's t test.
Table 1 Postprandial plasma glucose area under the curve (AUC), plasma insulin AUC, the glycaemic index (GI) and insulinaemic index (GII) in subjects with impaired glucose tolerance following an oral glucose tolerance test (OGTT) with placebo capsules or Cinnamomum zeylanicum capsules*
(Mean values with their standard errors, n 10)

* Mean values were not significantly different between postprandial blood glucose AUC, plasma insulin AUC, the GI and GII when evaluated with Wilcoxon's t test.
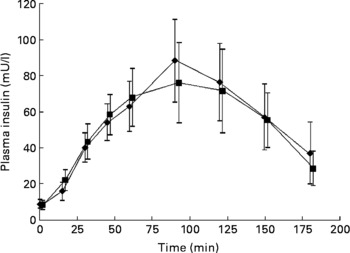
Fig. 2 Plasma insulin concentration in ten subjects with impaired glucose tolerance following an oral glucose tolerance with placebo capsules (reference; ■) or Cinnamomun zeylanicum capsules (♦). Values are means with their standard errors represented by vertical bars. Mean values were not significantly different between the two conditions when evaluated with Wilcoxon's t test.
Discussion
The aim of the present study was to elucidate the effect of C. zeylanicum on postprandial glucose and insulin levels in subjects with IGT. The present hypothesis was that an intake of C. zeylanicum would lower the postprandial glucose and insulin response. We were not able to verify this hypothesis. The present findings agree with those of Verspohl et al. (Reference Verspohl, Bauer and Neddermann4) who found that the effect of extract of C. cassia on glucose and insulin in rats was superior to that of the extract of C. zeylanicum. However, the effects of C. zeylanicum on glucose and insulin levels have not been studied previously in human subjects. Regarding cinnamon, a distinction must be made between C. zeylanicum and C. cassia. C. zeylanicum contains hardly any coumarin in contrast to C. cassia (Reference Jayatilaka, Poole and Poole22). Administration of coumarin to diabetic rats indicates alterations in the metabolism of glucose, resulting in a reduction in plasma glucose levels(Reference Pari and Rajarajeswari23, Reference Guerrero-Analco, Hersch-Martínez and Pedraza-Chaverri24). It has also been suggested that coumarin may change the glucose metabolism in the rat liver, leading to reduced plasma glucose levels(Reference Feuer, Golberg and Gibson25). The effects of coumarin on glucose and insulin levels in human subjects have not been reported. However, it can be assumed that coumarin ingestion will also affect glucose metabolism in human subjects. In a total of forty-four C. cassia samples from various Asian countries, He et al. (Reference He, Qiao and Han13) identified levels of more than 1 g coumarin per kg dry cinnamon powder in ten samples; the maximum level measured was 12·2 g/kg. Miller et al. (Reference Miller, Poole and Chichila14) reported coumarin levels from below the detection level to 0·19 g/kg in twelve C. zeylanicum samples, and between 0·7 and 12·2 g/kg in twelve C. cassia samples. Unfortunately, the coumarin levels or structure in cinnamon powder were not measured in the present study or in our previous studies on the effects of cinnamon on glucose and plasma lipids in patients with type 2 or type 1 diabetes and in healthy human subjects. Studies on animals indicate that, depending on the structure of coumarins, there may be diverse toxicity(Reference Kleiner, Xia and Sonoda26). Coumarin is poorly soluble in water, and the BrF has therefore suggested the replacement of C. cassia by aqueous extracts of C. cassia to lower coumarin exposure.
In a study in Pakistan by Khan et al. (Reference Khan, Safdar and Ali Khan27), it was found that the ingestion of 1, 3 and 6 g C. cassia powder daily for 40 d lowered the levels of fasting glucose, TAG, LDL-cholesterol and total cholesterol in women and men with type 2 diabetes receiving oral glucose-lowering treatment. Mang et al. (Reference Mang, Wolters and Schmitt28) found that treating women and men with type 2 diabetes with oral glucose-lowering medication or diet and/or physical activity and 3 g aqueous extract of C. cassia daily for 4 months resulted in a reduction in fasting plasma glucose levels, while no difference was observed in HbA1c, total cholesterol, LDL, HDL or TAG concentration. Vanschoonbeek et al. (Reference Vanschoonbeek, Thomassen and Senden29) found no improvement in HbA1c, fasting glucose or insulin concentration, TAG, LDL, HDL, total cholesterol or insulin resistance or sensitivity in overweight, postmenopausal women with type 2 diabetes, following either oral blood-glucose-lowering medication or controlled diets supplemented with 1·5 g C. cassia powder per d for 6 weeks. In a study performed in the USA, Blevins et al. (Reference Blevins, Leyva and Brown30) found no significant changes in fasting glucose, lipid, HbA1c or insulin levels in people with type 2 diabetes when 1 g C. cassia powder was consumed daily for 3 months. In the study in Pakistan by Khan, the fasting serum glucose concentrations of the subjects were between 11·4 and 16·7 mmol/l, which is approximately HbA1c values of 8·0–10·5 %(Reference Khan, Safdar and Ali Khan27). These results suggest that those with poorly controlled diabetes may benefit from cinnamon intake more than those receiving adequate treatment. Patients with type 1 diabetes treated with 1 g C. cassia powder per d for 3 months showed no difference in HbA1c, total daily insulin intake or number of hypoglycaemic episodes compared with the placebo group(Reference Altschuler, Casella and MacKenzie31). This may be explained by the fact that cinnamon decreases insulin resistance, which is not involved in the pathology of type 1 diabetes. However, a recent meta-analysis of the above-mentioned five randomised, placebo-controlled trials on patients with type 1 and type 2 diabetes did not show any significant changes in HbA1c, fasting glucose or lipid levels(Reference Baker, Gutiereez-Williams and White32). In women with polycystic ovary syndrome without diabetes, a dietary supplement of 333 mg C. burmannii extract, three times a day for 8 weeks, has been found to lower insulin resistance and fasting glucose levels(Reference Wang, Anderson and Graham33). A dietary supplement of 1·5 g C. cassia powder for 12 weeks in subjects with type 2 diabetes led to no significant changes in HbA1c, fasting glucose or lipid levels(Reference Suppapitiporn, Kanpaksi and Suppapitiporn34).
Conclusions
The insulin-like biological activity of different species of cinnamon, C. cassia, C. burmannii, C. loreirii and C. zeylanicum, has been reported not to be different in vitro in rat epididymal fat cells(Reference Imparl-Radosevich, Deas and Polansky3), and the BrF has suggested the replacement of C. cassia by C. zeylanicum, or the use of aqueous extracts of C. cassia to lower coumarin exposure for human subjects. However, the results of the present study show that the ingestion of C. zeylanicum does not affect postprandial plasma glucose or insulin levels in human subjects.
Acknowledgements
The present study was supported by Gifts and Foundations-Research Per Håkansson's Foundation, Erhold Lundström's Foundation for Medical Research and Diabetes Foundation Sydwest Skåne. J. H., J. N. and J. W. contributed to the design of the study; J. W. and K. B. were responsible for recruiting the subjects; J. W. carried out the practical aspects of the study. J. W., S. L. and J. H. conducted the statistical calculations; J. W. and J. H. created the graphs. J. W. and J. H. wrote the first draft of the manuscript and J. N., S. L. and K. B. critically reviewed the manuscript. All authors read and approved the final manuscript. The authors declare that they have no competing interest.