Introduction
The family Berycidae consists of ten species divided into two genera, Beryx and Centroberyx (Nelson, Reference Nelson2006). The adult fishes of Beryx spp. are generally found in the Atlantic, Indian and Pacific oceans (Busakhin, Reference Busakhin1982; Vinnichenko, Reference Vinnichenko1997a; Kakora, Reference Kakora2005; Moore, Reference Moore, Carpenter and De Angelis2016). These fishes are known to have a life span of 8–69 years (Yoshino et al., Reference Yoshino, Kon and Miura1999; Friess and Sedberry, Reference Friess and Sedberry2011a). The adult length also varied among the three species, B. decadactylus reach up to 270–630 mm (Friess and Sedbery, Reference Friess and Sedberry2011a; Santos et al., Reference Santos, Novoa-Pabon, Silva and Pinho2019), while B. splendens to 200–520 mm (Adachi et al., Reference Adachi, Takagi, Tanaka, Yamada and Kitakado2000; Rico et al., Reference Rico, Lorenzo, González, Krug, Mendonça, Gouveia and Dias2001) and B. mollis to 97–330 mm (Yoshino et al., Reference Yoshino, Kon and Miura1999; Borsa et al., Reference Borsa, Akimoto, Pasco, Tehei and Watabe2011; Bineesh et al., Reference Bineesh, Nashad, Aneesh Kumar, Akhilesh and Hashim2018).
Beryx spp. can be commonly found in waters above continental shelves and slopes, rocky grounds, seamounts and oceanic ridges (Busakhin, Reference Busakhin1982; Clark and O'Driscoll, Reference Clark and O'Driscoll2003), and are known as batch spawners with spawning aggregations (Isidro, Reference Isidro1996). They occupy different water masses/layers during their life cycle. The adults live in the benthopelagic zone while juveniles are generally restricted to the mesopelagic zone. Previous studies from different regions show that spawning of Beryx spp. generally occurs at 600–900 m depths while eggs and larvae develop in the productive surface waters (Mundy, Reference Mundy1990; Isidro, Reference Isidro1996; Lehodey et al., Reference Lehodey, Grandperrin and Marchal1997; Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002; Santos et al., Reference Santos, Novoa-Pabon, Silva and Pinho2019). Volcanically originated habitats of ocean seamounts are known as popular spawning grounds for Beryx spp. (Rogers, Reference Rogers1994; Kuboshima, Reference Kuboshima1995; Lehodey et al., Reference Lehodey, Grandperrin and Marchal1997).
Pelagic early life stages are known to aid widespread dispersal via oceanic currents (Isidro, Reference Isidro1996). Beryx spp. show an aggregating behaviour during the day and scatter during the night, most notably with vertical migrations into shallower waters for feeding (Lehodey et al., Reference Lehodey, Grandperrin and Marchal1997; Vinnichenko, Reference Vinnichenko1997b). Common prey of these fishes are small crustaceans, mesopelagic fish, cephalopods, polychaetes and salps (Dürr and González, Reference Dürr and González2002; Di Blasi et al., Reference Di Blasi, Carlig, Ferrando, Ghigliotti, Psomadakis and Vacchi2018).
The genus Beryx (Beryciformes (Sawbellies); Berycidae (Alfonsinos)) contains three species: Red Bream Beryx decadactylus (Cuvier, 1829), Splendid Alfonsino Beryx splendens (Lowe, 1834) and Balloon Alfonsin Beryx mollis (Abe, Reference Abe1959). Among the three species, B. splendens and B. decadactylus are commonly recorded (Masuzawa et al., Reference Masuzawa, Kurata, Ohnishi, Masuzawa, Kurata and Ohnishi1975; Busakhin, Reference Busakhin1982) in the Pacific Ocean (Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002, Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006), Mediterranean Sea (Psomadakis et al., Reference Psomadakis, Stefanni, Merella, Ferrando, Amato and Vacchi2012; Di Blasi et al., Reference Di Blasi, Carlig, Ferrando, Ghigliotti, Psomadakis and Vacchi2018), North Atlantic Ocean (Friess and Sedberry, Reference Friess and Sedberry2011b) and in the Indian Ocean (Shotton, Reference Shotton2016).
Compared to the other two species, B. mollis has fewer observations, likely due to their low abundance (Abe, Reference Abe1959; Yoshino et al., Reference Yoshino, Kon and Miura1999). Most records of B. mollis are in the Indo-West Pacific region, mainly in Japanese waters (Yanagimoto and Chow, Reference Yanagimoto and Chow2020, Figure 1A). Recent records in the south eastern Pacific and south west coast of India have further extended have extended its known geographical distribution (Bineesh et al., Reference Bineesh, Nashad, Aneesh Kumar, Akhilesh and Hashim2018; Kimura, Reference Kimura2023).

Figure 1. Worldwide distribution of Beryx spp., modified after Source GBIF.org (25th July 2023) GBIF occurrence download; https://www.gbif.org/occurrence/search?taxon_key=2356611 and Kimura (Reference Kimura2023). (A) The green, blue and red coloured circles indicate the distribution of B. decadactylus, B. splendens and B. mollis, respectively. The orange squares in Figure 1A, B (zoomed) indicate the location of the sampling area off Sri Lanka. (B) The red filled circles show the collected locations of B. mollis in the present study – a single egg and an early larva were collected from station 568 and one post larva from station 552 during the present survey.
Extensive commercial fisheries for Beryx spp. can be seen in the Pacific Ocean, with catches up to 15,000 tons in the year 2003 (Shotton, Reference Shotton2016). Moderate fishing occurs in the Atlantic Ocean (2500 tons), often as a by-catch in mixed species fishery (Langley and Walker, Reference Langley and Walker2002; Large et al., Reference Large, Hammer, Bergstad, Gordon and Lorance2003). Fishing methods used are deep-sea trawls and longlines (Clarke and Moore, Reference Clarke and Moore2002). The Japanese coastal area is a successful spawning and breeding ground for Beryx spp. These are a common food fish in Japanese markets (Shotton, Reference Shotton2016). Akimoto et al. (Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006) emphasizes the fact that even though a wide distribution of B. mollis in Indo-West Pacific is presumed, very little data on their abundance are available. Despite B. mollis being less prevalent in most regions, it has been found to be most abundant (73%) in catches from some areas of the New Caledonia (Akimoto et al., Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006).
Although there are numerous records and literature available on B. splendens, and to a moderate extent, on B. decadactylus, very little information is available on B. mollis. Until early 1990s, B. mollis had only been recorded in Japanese waters (Kotlyar, Reference Kotlyar1993; Yoshino et al., Reference Yoshino, Kon and Miura1999; Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002). This is likely due to similarities in appearance between B. mollis and B. splendens. In fact, previously B. mollis was considered as a juvenile of B. splendens. The historical misidentification of B. mollis as B. splendens could be a plausible explanation for the late documentation of B. mollis in these waters.
Although meristic traits such as number of dorsal-fin rays are generally considered to be a distinctive character in segregating Beryx spp., this does not apply for B. mollis and B. splendens, as the numbers to a large extent overlap in these two species (Yoshino et al., Reference Yoshino, Kon and Miura1999; Yoshino and Kotlyar, Reference Yoshino and Kotlyar2001; Akimoto et al., Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006; Nishida et al., Reference Nishida, Chiba, Sakuma, Higashi, Suzuki, Miyamoto, Yonezaki, Hoshino and Sawada2022). Furthermore, length/body depth ratio and body colour traits are also very similar (Nishida et al., Reference Nishida, Chiba, Sakuma, Higashi, Suzuki, Miyamoto, Yonezaki, Hoshino and Sawada2022), making the segregation of these two species even more difficult.
Conversely, B. decadactylus is easily distinguishable due to its unique morphological features. It has a greater body depth and number of soft rays in the dorsal fin (Yoshino et al., Reference Yoshino, Kon and Miura1999; Yoshino and Kotlyar, Reference Yoshino and Kotlyar2001). Yoshino et al. (Reference Yoshino, Kon and Miura1999) confirmed B. mollis as a valid species by examination of morphological and morphometric characteristics. Later, the species is verified by observations of adult fishes from both the Indian and Pacific oceans (Yoshino and Kotlyar, Reference Yoshino and Kotlyar2001; Akimoto et al., Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006; Bineesh et al., Reference Bineesh, Nashad, Aneesh Kumar, Akhilesh and Hashim2018).
With the development of molecular techniques, identification of B. mollis and B. splendens became both easier and reliable. Recent studies using restriction enzymes or species-specific PCR primers have successfully distinguished the three species (Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002, Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006; Nishida et al., Reference Nishida, Chiba, Sakuma, Higashi, Suzuki, Miyamoto, Yonezaki, Hoshino and Sawada2022). Using molecular approaches on adults, Akimoto et al. (Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002) showed that B. splendens is more closely related to B. mollis than to B. decadactylus.
Species identification of early life stages of Beryx spp. is even more difficult than in adults. Even though there are records of early life stages of B. splendens, this is not the case for egg, larval and juvenile stages of B. mollis (Akimoto et al., Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006).
The survey area around Sri Lanka, an island in the Indian Ocean, has a unique coastal geographic structure, with a narrow border with shallower depths, and steeper incline continental shelf (De Vos et al., Reference De Vos, Pattiaratchi and Wijeratne2014). The ocean around the southern tip of Sri Lanka experiences the exchange of contrasting water masses between the low-saline Bay of Bengal and the high-saline Arabian Sea. Seasonal circulation patterns are influenced by the monsoons. During the highly productive summer (the southwest monsoon, May–September), the monsoon current flows eastward, from the Arabian Sea towards western waters off Sri Lanka (De Vos et al., Reference De Vos, Pattiaratchi and Wijeratne2014; Hood et al., Reference Hood, Beckley and Wiggert2017) and in the winter (the northeast monsoon, November–February), the East Indian Coastal Current flows westward (Shankar et al., Reference Shankar, Vinayachandran and Unnikrishnan2002; Lee et al., Reference Lee, Jinadasa, Anutaliya, Centurioni, Fernando, Hormann, Lankhorst, Rainville, Send and Wijesekera2016; Hood et al., Reference Hood, Beckley and Wiggert2017). The south western coast of Sri Lanka is the focused area in this study (Figure 1B). Spatial patterns of zooplankton and Chlorophyll-a concentrations are closely associated with the monsoons, and a coastal upwelling off the south coast of Sri Lanka is distinctly evident during the peak southwest monsoon period (Yapa, Reference Yapa2000; Dalpadado et al., Reference Dalpadado, Arrigo, van Dijken, Gunasekara, Ostrowski, Bianchi and Sperfeld2021, Reference Dalpadado, Roxy, Arrigo, van Dijken, Chierici, Ostrowski, Skern-Mauritzen, Bakke, Richardson and Sperfeld2023; Wimalasiri et al., Reference Wimalasiri, Weerakoon, Jayasinghe and Dalpadado2021) providing good feeding opportunities for planktonic organisms.
DNA barcoding techniques have greatly aided the identification of early life stages of fish, expanding existing knowledge on organisms in the Ocean (Leyva-Cruz et al., Reference Leyva-Cruz, Vásquez-Yeomans, Carrillo and Valdez-Moreno2016; Rathnasuriya et al., Reference Rathnasuriya, Mateos-Rivera, Bandara, Skern-Mauritzen, Jayasinghe, Krakstad and Dalpadado2019, Reference Rathnasuriya, Mateos-Rivera, Skern-Mauritzen, Wimalasiri, Jayasinghe, Krakstad and Dalpadado2021; Kerr et al., Reference Kerr, Browning, Bønnelycke, Zhang, Hu, Armenteros, Murawski, Peebles and Breitbart2020; Lima et al., Reference Lima, Lima, Savada, Suzuki, Orsi and Almeida2020). Precise identifications are essential for the conservation and management of these ecologically and economically important fish stocks such as B. mollis. Molecular methods combined with morphological measures have proven to be the best method for an accurate and reliable identification of earlier life stages of fish. Therefore, in this study, the morphological characteristics of early life stages of B. mollis (egg and larval stages) have been evaluated and species identification confirmed by using molecular approaches targeting the mitochondrial COI gene.
Materials and methods
Sampling
An ecosystem survey was conducted from 24 June to 16 July 2018 in Sri Lankan waters during the southwest monsoon period (Figure 1A, B) using the research vessel Dr. Fridtjof Nansen. The survey was conducted under the framework of the EAF-Nansen Programme (2017–2021) with the objects of encompassing marine resources, pollution and climate (Krakstad et al., Reference Krakstad, Jayasinghe, Totland, Dalpadado, Søiland and Cervantes2018). Ichthyoplankton samples were collected using a Manta net (0.1159 m2 opening and 335 μm net mesh size). The Manta net was towed horizontally at a speed of ~1.5 ms−1 for 15 min. Although Manta nets were primarily designed for collecting microplastic samples from the surface waters (Pasquier et al., Reference Pasquier, Doyen, Kazour, Dehaut, Diop, Duflos and Amara2022), these gears have later been recognized also as an efficient method for ichthyoplankton sampling (Jonathan, Reference Jonathan1988; Kang et al., Reference Kang, Kwon, Lee, Song and Shim2015).
Fish egg and larvae preserved in 96% ethanol were examined under a stereo microscope for morphological features and meristic measurements. Thereafter, a single egg and larvae were assigned specific sample numbers and were placed individually into 1.5 ml mocro-centrifuge tubes with 96% ethanol and stored at 4 °C prior to molecular analyses.
DNA isolation, PCR amplification and sequencing
DNA was extracted using a solution containing 75 μl of 5% Chelex 100 Resin (BioRad, CA, USA) and 15 μl of Proteinase K (Qiagen, Germany). Samples were then incubated at 56° C for 1 h and 10 min at 96° C. PCR amplification targeting the mitochondrial COI gene was performed following the protocol described by Mateos-Rivera et al. (Reference Mateos-Rivera, Skern-Mauritzen, Dahle, Sundby, Mozfar, Thorsen, Wehde and Krafft2020). The resulting sequences were manually curated and uploaded to the GenBank with the accession numbers OR234865, OR234866 and OR234867.
Phylogenetic tree
To make the phylogenetic tree, downloaded COI sequences from the GenBank were retrieved and aligned together with the sequences from this study using MUSCLE in MEGA V11 (Tamura et al., Reference Tamura, Stecher and Kumar2021). We applied Neighbour-joining analysis (Saitou and Nei, Reference Saitou and Nei1987) and Kimura's two-parameter method (Reference Kimura1980) with the percentage replicates in which the associated taxa clustered together in the bootstrap test with 1000 replicates.
An adult Gephyroberyx darwinii collected in the same Nansen survey as the current study (accession number OR378763), together with two other sequences of the same species downloaded from the GenBank were used to root the phylogenetic tree. Gephyroberyx darwinii, (Johnson, 1866) belonging to the same family Berycidae as B. mollis was caught in two bottom trawl stations (6°01′N 81°30′E, 8°45′N 79°31′E) at ~270 m depth during the Nansen survey in 2018. This reef-associated benthopelagic species is widespread in the Indo-West Pacific, Atlantic and Mediterranean subtropical waters (Andrade et al., Reference Andrade, Soares, Barreiros, Gasparini and Hostim-Silva2004; Moore, Reference Moore, Carpenter and De Angelis2016). They occupy depths up to 1200 m (Bertoncini et al., Reference Bertoncini, Souza Soares, Barreiros, Gasparini and Hostim-Silva2004).
Results and discussion
Manta trawl, a reliable method for sampling fish eggs
The manta trawl, resembling the shape of a manta ray, is effectively used to collect ichthyoplankton in surface waters (Brown and Cheng, Reference Brown and Cheng1981). This trawl is recognized as a more effective gear in the collection of ichthyoplankton (<15 mm) compared with other Neuston nets (Jonathan, Reference Jonathan1988; Kang et al., Reference Kang, Kwon, Lee, Song and Shim2015). Even though the primary objective of the manta trawl in the Nansen ecosystem survey in 2018 was to collect microplastics in the surface water layer, recent studies confirm the effectiveness of this gear in catching pelagic fish eggs in the Indian Ocean.
Molecular and morphology analyses
Based on morphological approaches and DNA sequencing, a single egg and two larval developmental stages of B. mollis were identified in the southwestern region (stations 568 and 552, Figure 1B). The egg recorded in this study was 0.98 mm in diameter and contained a single oil droplet of 0.17 mm diameter (Figure 2A, Table 1). The pre-larva and post-larva had standard lengths of 1.6 and 2.8 mm, respectively (Figure 2B, C). To our knowledge, this is the first morphological description and genetic confirmation of B. mollis early developmental stages documented so far.
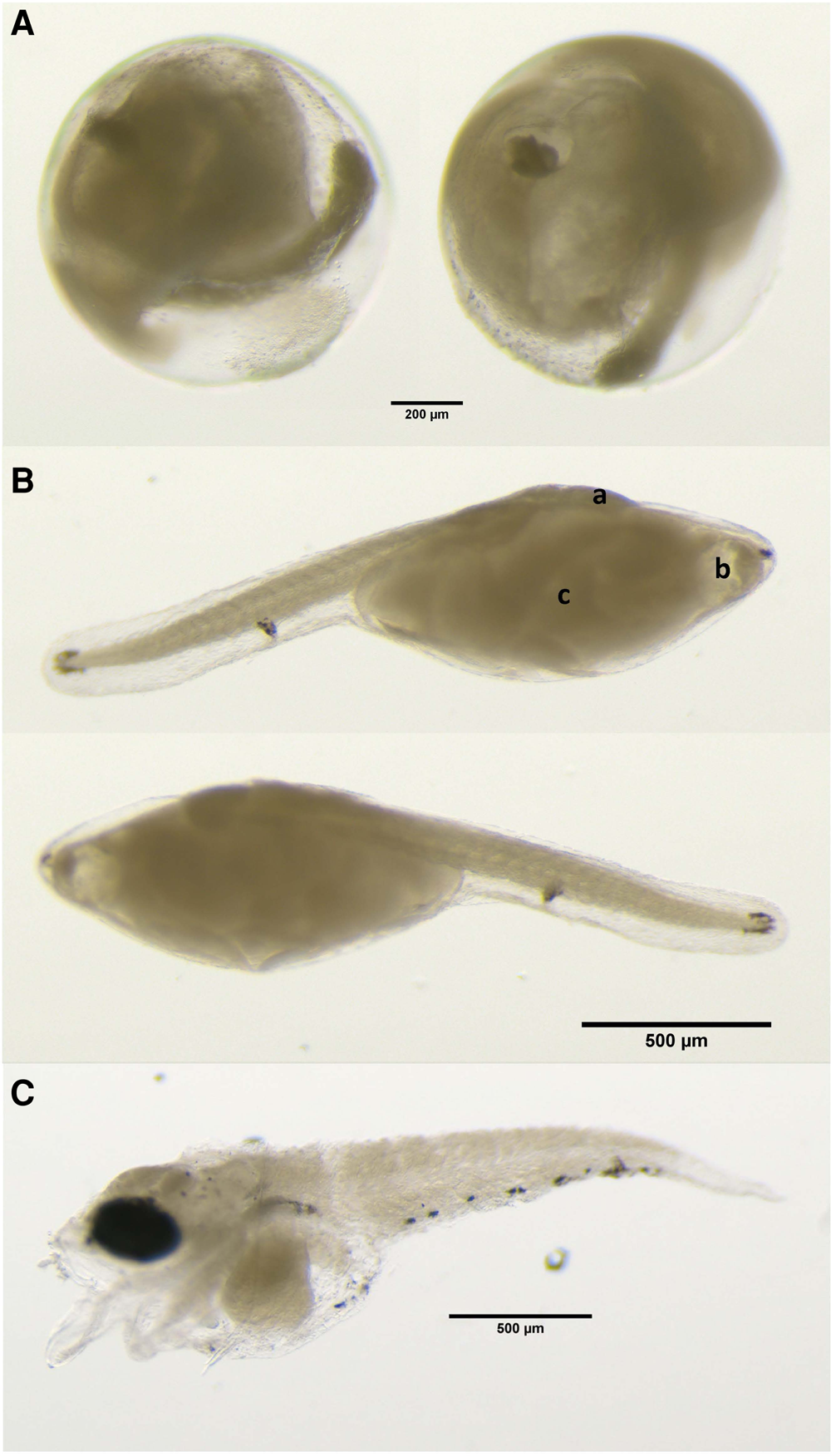
Figure 2. Microscopic images of the early development stages of Beryx mollis (A) egg; (B) pre-yolk sac absorption larva (collected in station 552); (C) post-larva (collected in the station 568). Placement of head (a), yolk sac (b) and oil globule (c) in larvae. See Figure 1B for locations. All images were taken from 96% ethanol preserved samples.
Table 1. Morphological and morphometric characteristic comparisons between Beryx mollis egg and larvae from this study and Beryx spp. from previous literature.

Egg (Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002) and larvae (Mundy, Reference Mundy1990).
Phylogenetic analysis
The phylogenetic tree constructed with the sequences from the samples collected in this study together with sequences from the GenBank database showed how the three different Beryx species are clustered. The B. mollis from this study are clustered together with individuals reported in other regions, e.g. off Japan and India. The B. mollis cluster is more closely related to the B. splendens than the B. decadactylus cluster (Figure 3). Comparison of morphological characteristics exhibited close similarities among B. mollis and B. splendens than with B. decadactylus (Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002), as can also be related to their close phylogenetic and evolutionary relationship (Figure 3).

Figure 3. Neighbour-joining phylogenetic tree. COI gene sequences of B. mollis from the present study are shown in bold and marked with a red star. The Gephyroberyx darwinii collected from the Nansen survey is shown in bold and marked with a blue star. Accession numbers are provided within parenthesis. Evolutionary distance showed in each branch with the scale of 0.02.
Occurrences of early life stages of Beryx mollis
Fish eggs and larvae of the three Beryx spp. share very similar morphological appearances (Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002). The morphological descriptions provided by Mundy (Reference Mundy1990) and Akimoto et al. (Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002), for Beryx spp. were used for comparison with our findings (Table 1). These studies have described Beryx spp. fish eggs of size 1.08–1.22 mm and having an orange oil globule ranging 0.16–0.31 mm in diameter (Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002, Table 1), which agrees with the egg characteristics recorded in our study. Most studies on measurements of fish eggs are on preserved samples. In previous studies on early life stages of Beryx spp., samples were collected first in formalin (5–10%) and thereafter transferred to ethanol (ca. 96%) for sorting of species (Mundy, Reference Mundy1990; Akimoto et al., Reference Akimoto, Kinoshita, Sezaki, Mitani and Watabe2002). In our study, the eggs were preserved only in 96% ethanol. The smaller egg diameter (0.98 mm, see Table 1) can most likely be explained by a slight shrinkage due to storage in ethanol (Fowler and Smith, Reference Fowler and Smith1983). Combined morphological and molecular approaches from our study confirm the presence of early life stages of B. mollis in the north central Indian Ocean.
Low global records of Beryx mollis
Adults of B. mollis have been observed in the northwest Arabian Sea (off Somalian waters) and near the coast of Indonesia (Figure 1A). These observations imply that (i) B. mollis adults have migrated towards the north central Indian Ocean from the Arabian sea; or (ii) were already present in these areas but never detected. Although in some situations it would be reasonable to suggest one of the hypotheses as to be more likely, this might not be the case for B. mollis. As mentioned above there is a misidentification of B. mollis as B. splendens historically due to their similar morphological features (Yoshino et al., Reference Yoshino, Kon and Miura1999; Akimoto et al., Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006), but also due to that adult B. mollis can migrate into several regions across long distances. That would also explain the trans-oceanic migration and distribution of the species (Akimoto et al., Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006). Nonetheless, it was observed that the B. mollis sequences in this study showed a high identity (>99%) with two individuals described as B. cf. splendens uploaded by Akimoto et al. (Reference Akimoto, Itoi, Sezaki, Borsa and Watabe2006) in the GenBank database (accession numbers GU673458 and GU673459). However, these authors already highlighted the uncertainty of the sequence identity, stating it is likely that these sequences are indeed B. mollis. This evidence reinforces not only the high similarity in the morphological characteristics between B. mollis and B. splendens but also the importance of having a robust and accurate database, especially in poorly researched areas like the north central Indian Ocean.
Although trawling for demersal fish was conducted on our survey, this was only carried out in specific regions to avoid damage to benthic organisms, corals reefs, etc. This could be a contributing factor for not recording adult Beryx spp. in the survey catches from this region. The presence of both egg and larvae in the study region does, however, indicate the likelihood of the presence of adult B. mollis in the region.
Future research focus
The present study shows that the productive areas southwest of Sri Lanka are an important spawning ground of B. mollis. Future studies should be focused on strengthening combined morphological and molecular approaches to reveal spatial distribution, abundance and species diversity of ichthyoplankton in the region. For such studies to be successful, it is necessary to establish appropriate sampling strategies of fish eggs and larvae using available research vessels and ships of opportunity. Further details of survey, sampling and management strategies in the Indian Ocean ecosystem have been documented in a recent study (Dalpadado et al., Reference Dalpadado, Roxy, Arrigo, van Dijken, Chierici, Ostrowski, Skern-Mauritzen, Bakke, Richardson and Sperfeld2023).
Acknowledgments
This paper uses samples and data collected through the scientific surveys with the R/V Dr. Fridtjof Nansen as part of the collaboration between the EAF-Nansen Programme of the Food and Agricultural Organization (FAO) and the government of Sri Lanka. The authors thank the scientists and the crew of the survey for their enormous help in sample collection. The support given by the National Aquatic Resources Research and Development Agency (NARA), Sri Lanka, and the Institute of Marine Research (IMR), Norway is highly appreciated.
Author contributions
In situ data collection and database management: Sudheera S. Gunasekara, R. P. Prabath K. Jayasinghe, Padmini Dalpadado. DNA Barcoding: Alejandro Mateos-Rivera. Formal analysis: Yasmin C. Aluwihare, Alejandro Mateos-Rivera, Magnus Reeve, Padmini Dalpadado. Writing: All authors contributed to the writing of this manuscript.
Financial support
Financial support for this research work was provided by NARA through counter funds for Sri Lanka-Norway bilateral project (15670 – Technical Assistance to Improve Management of the Fish Resources of Sri Lanka, Phase II LKA-3183, LKA-19/0001).
Competing interest
None.
Ethical standards
These studies were requested and given permission by the Sri Lankan and Norwegian governments under the bilateral research agreement in the Sri Lanka and Norway. Hence, any other specific permission was not required. The animals (ichthyoplankton) used in this work were collected from the sea (their natural environment) in accordance with the national and international regulations compiled by the two countries. No animals were kept in experimental conditions in this study.
Data availability
All data generated in this study are included in the published article.






