Introduction
Toxoplasma gondii (T. gondii), an apicomplexan parasite with a global distribution, can infect all kinds of warm-blooded vertebrates, including humans [Reference Dubey1]. Nearly one-third of the world's population is thought to be infected with this parasite [Reference Montoya and Liesenfeld2]. The prevalence rate of T. gondii in China is over 7% and this rate is constantly rising due to the rapid growth of the number of cats (pet and stray cats) [Reference Cong3–Reference Zhou5]. This parasite has three infective forms: the tachyzoite, the bradyzoite inside tissue cysts and the sporozoite in sporulated oocyst [Reference Ferreira and Borges6]. Humans are mainly infected through these ways: ingesting cysts from raw or uncooked meat, ingesting oocysts from food or water contaminated with cat faeces, and through vertical transmission from mother to foetus [Reference Montoya and Liesenfeld2]. Latent toxoplasmosis is mainly associated with neurodegenerative disorders and autoimmune diseases [Reference Majidiani7, Reference Sutterland8]. For individuals with the normal immune system, toxoplasmosis usually does not cause notably clinical symptoms and does not need to be treated. Benign and self-limited lymphadenopathy and lymphomonocytosis are found in nearly 20% of cases [Reference Yazar9]. Nevertheless, for immunocompromised individuals, T. gondii might be fatal, especially for patients with neoplasia, transplant recipients and patients with AIDS [Reference Cong3, Reference Ferreira and Borges6, Reference Yazar9]. Thus, more attention should be paid to these groups of individuals. Host inflammation responses may increase due to chronic infection with T. gondii, thus promoting mutations and enhancing cancer [Reference Thomas10].
Cancer is the leading and the second leading cause of death in economically developed countries and developing countries, respectively [Reference Jemal11]. It is estimated that deaths from cancer will continue to rise, and the number will reach 11 million by 2030 [Reference Cannon12]. Currently, clinical toxoplasmosis in cancer patients has gradually turned into an important public health concern [Reference Dubey13, Reference Jiang14]. Cancer patients who are seronegative for T. gondii infection could benefit from advice on precautionary measures, to avoid seroconversion that may induce active severe toxoplasmosis [Reference Imam15]. Therefore, potential associations between T. gondii infection and cancer have attracted a lot of attention [Reference Cong3, Reference Wang4, Reference Chintakuntlawar16–Reference Wang26]. Oral cancer is one of the most common malignant tumours. According to available statistical data, there were 300 000 new cases and 145 000 disease-related deaths in 2012 [Reference Ferlay27], Still, the association between T. gondii infection and oral cancer remains unexplored. The following study examined t the relationship between T. gondii infection and oral cancer in eastern China in order to identify associated potential risk factors and possible routes of infection for oral cancer patients.
Materials and methods
Study sites
The study was conducted at the Affiliated Hospital of Qingdao University, a large provincial comprehensive hospital located in Qingdao (35°35′–37°09′N, 119°30′–121°00′E), Shandong province, Eastern China.
Study design and sample collection
Here we investigated the T. gondii seroprevalence and identified potential risk factors of T. gondii infection as well as possible infection routes in oral cancer patients and control subjects in China from September 2013 to March 2017. Eight hundred and sixty-one oral cancer patients who presented to the Affiliated Hospital of Qingdao University were included in the study. In addition, 861 Control subjects were selected to match oral cancer patients by age, gender and residence.
Serum samples were randomly obtained from persons who participated in health screenings at the Affiliated Hospital of Qingdao University.
Approximately 5 ml of venous blood samples was drawn from participants who gave their consent to participate in this study. Blood samples were incubated at room temperature overnight to allow clotting and centrifuged at 3000 rpm for 10 min. The sera were collected in Eppendorf tubes and stored at 4 °C for 24–72 h and then kept at −20 °C until further testing.
Socio-demographic, clinical and behavioural data collection
Socio-demographic data including age, gender and area of residence were obtained from all participants. In addition, the following clinical data were collected from all patients: surgery history, blood transfusion history, chemotherapy history, differentiation degree and the TNM stage of cancer; and behavioural data including tobacco use, alcohol consumption, keeping cats at home, consumption of raw/undercooked meat, consumption of oyster, gardening or agricultural activities, exposure with soil apart from gardening or agricultural activities, source of drinking water and washing hands before meals. These variables were selected based on the previous literature. Data was obtained from the patients/guardians, medical examination records and informants. Patients were invited to provide veridical information and they were informed that data were used in a confidential manner.
Serological assay
Sera were analysed for the presence of IgG and IgM antibodies to T. gondii using the commercially available enzyme-linked immunosorbent assay (ELISA) (Demeditec Diagnostics GmbH, Germany) according to the manufacturer's instructions. Positive and negative serum controls were included in every plate. To avoid biases, the serology test was done using double blinded approach. Samples from oral cancer patients and control subjects were randomly mixed, and the person performing the test did not know the source of samples in advance.
Statistical analysis
The results were analysed with SPSS 18.0 software package. For the univariate analysis, χ2, test or Fisher's exact test provided a comparison of the categorical variables. The Mantel–Haenszel test was used to probe for any differences between patient and control groups. Multiple logistic regression models were used to adjust for potential confounders. Variables were included in the multiple logistic analysis if they had a P-value of equal or less than 0.25 in the univariate analysis [Reference Cong3]. Odds ratios (ORs) and the corresponding 95% confidence interval (CI) were calculated to identify independent risk factors for T. gondii infection. Results with a P-value of <0.05 were considered statistically significant.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. Patients were made aware of the aim of the study. Each individual provided written consent for their participation in the study. Control sera were collected from volunteers.
Results
The overall seroprevalence of T. gondii infection in oral cancer patients and control subjects were 21.72% (187/861) and 8.25% (71/861), respectively. Of these, IgG antibodies to T. gondii were found in 144 (16.72%) out of 861 oral cancer patients and in 71 (8.25%) out of 861 control subjects (P < 0.001). Fifty-four (6.27%) oral cancer patients and 9 (1.05%) controls were positive for IgM antibodies to T. gondii (P < 0.001). Among oral cancer patients, 133 (15.45%) were positive for IgG antibodies compared to 62 (7.20%) controls. 43 (4.99%) oral cancer patients and 0 (0%) controls were positive for IgM antibodies only, while 1.28% oral cancer patients and 1.05% controls were positive for both IgG and IgM antibodies. Detailed information is summarised in Table 1. Univariate analysis of socio-demographic and risk factors for oral cancer patients and controls identified some factors with a P-value of <0.25 that may be related to infection (Table 1). Three of these, i.e. blood transfusion history, keep cats at home, and consumption of oysters were found to be significantly associated with T. gondii infection in the multivariable logistic analysis (Table 2).
Table 1. Seroprevalence of T. gondii infection in oral cancer patients and control subjects in China
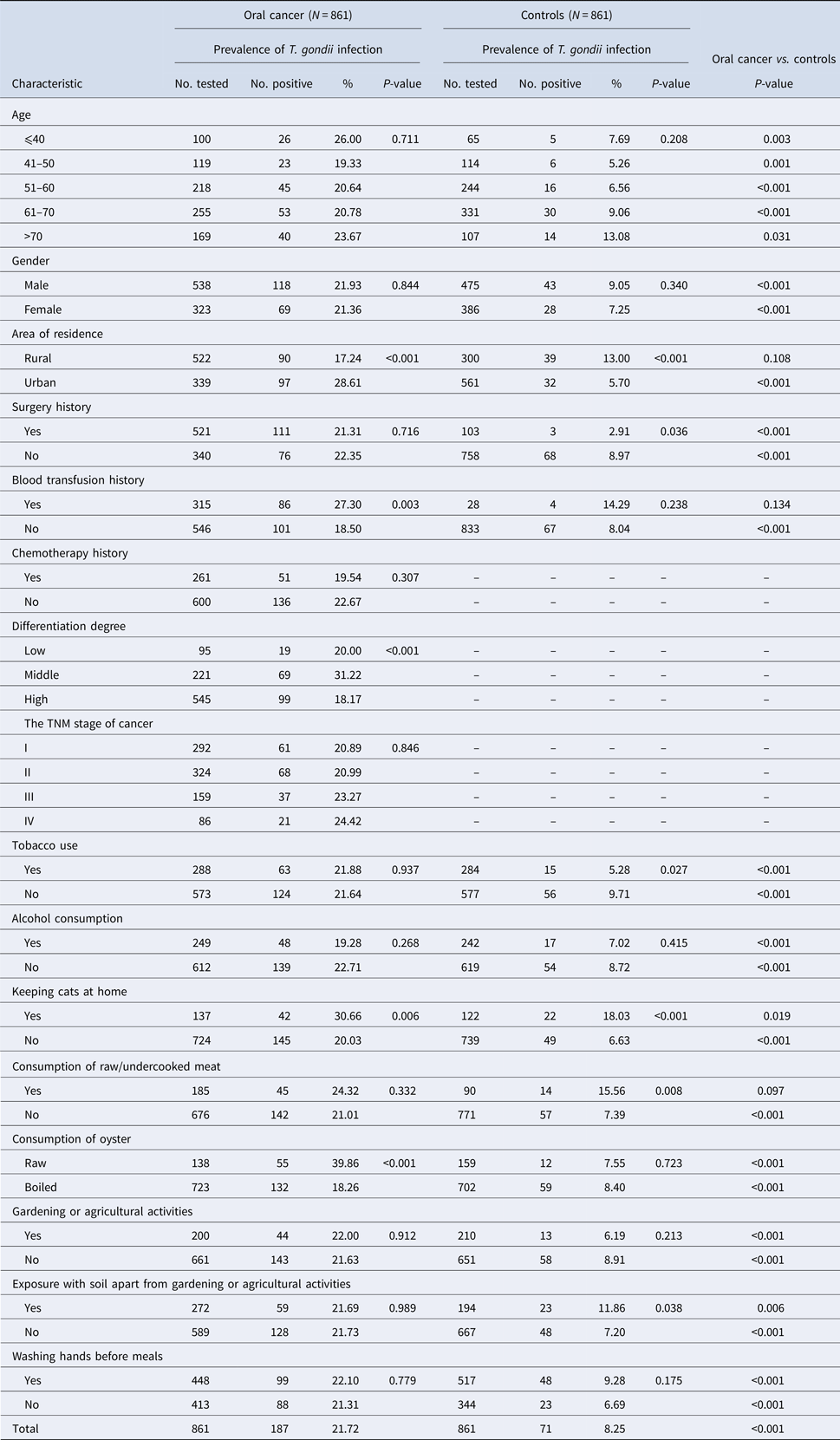
Table 2. Multiple logistic analysis of selected characteristics of oral cancer patients and their association with T. gondii infection
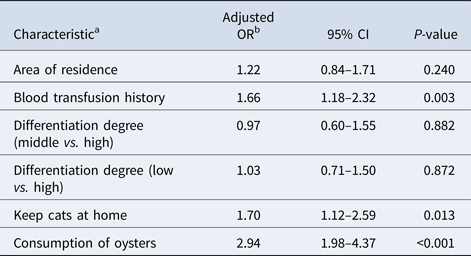
a The variables included were those with a P < 0.25 obtained in the univariate analysis.
b Adjusted by age.
Discussion
Oral cancer is considered to be the sixth most common malignancy worldwide [Reference Warnakulasuriya28]. Nearly 145 000 patients die from oral cancer each year [Reference Ferlay27]. It is generally recognised that the development of oral cancer is mainly caused by tobacco consumption including smokeless tobacco and heavy alcohol consumption [Reference Dhanuthai29]. Apart from these risk factors, human papilloma virus has been found to be associated with oral cancer [Reference Chaturvedi30, Reference D'Souza31]. Yet, the connection between oral cancer and parasitic infection remains unexplored. In this study, we first explored the relationship between oral cancer and T. gondii infection. Briefly, we found that oral cancer patients (21.72%, 187/861) have a significantly higher T. gondii seroprevalence compared to control subjects (8.25%, 71/861) (P < 0.001). Thus, our findings based on serological methods support a potential association between oral cancer and T. gondii infection.
After infection, T. gondii can disseminate into each organ of the infected host through blood circulation [Reference Harker32]. Previous studies have shown that intracellular tachyzoites can disseminate into the whole body through the blood circulation, while extracellular tachyzoites do not have this ability [Reference Unno33, Reference Alvarado-Esquivel34]. In this study, oral cancer patients with blood transfusion history had a significantly higher seroprevalence than control subjects (adjusted OR = 1.66, 95% CI: 1.18–2.32, P = 0.003). Although the current epidemiological knowledge suggests that T. gondii can be transmitted by blood transfusion in oral cancer patients, further studies should be conducted to explore the magnitude of the association between T. gondii infection and blood transfusion in oral cancer patients.
With respect to behavioural characteristics, the multivariable logistic analysis showed that keeping cats at home and fresh oyster consumption were associated with T. gondii seropositivity. As the definitive hosts for T. gondii, cats have a vital role in maintaining T. gondii in nature [Reference Dabritz and Conrad35]. In China, cats are prevalently kept as pets because they are very easy to take care of and they provide company thus enriching people lives. Still, little attention has been given to the fact that potentially pollute the environment with T. gondii [Reference Du36]. In this study, we found that keeping cats at home was highly associated with T. gondii seropositivity (adjusted OR = 1.70; 95% CI: 1.12–2.59; P = 0.013). Moreover, we found that fresh oyster consumption (adjusted OR = 2.94; 95% CI: 1.98–4.37; P < 0.001) was another potential risk factor for T. gondii infection in oral cancer patients, which was similar to the study from the United States [Reference Jones37]. Previous studies have shown that T. gondii oocysts can be washed into the sea through rainwash and runoff [Reference Dubey38, Reference Lindsay39], and oocysts may be stored in shellfish such as oysters, clams and mussels [Reference Dubey38–Reference Schott43]. Similarly, T. gondii oocysts have been found in oysters in China [Reference Cong44], and fresh oyster consumption is becoming ever more popular over recent years. This phenomenon may contribute to the higher T. gondii seroprevalence in the oral cancer patients with the habit of fresh oyster consumption. Therefore, knowledge of these risk factors can be of great help in prevention efforts. So, having in mind the public health, it is very important to publicise the knowledge of disease prevention.
Conclusion
In this study, we reported a serological evidence of an association between T. gondii infection and oral cancer patients. Moreover, blood transfusion history, keeping cats at home and consumption of raw oysters were identified as risk factors for T. gondii positivity. This information may be used to guide future research and control policies. Beyond that, further studies are necessary to elucidate the role of T. gondii in oral cancer patients.
Acknowledgements
This study was supported by the Shandong Provincial Natural Science Foundation, China (ZR2017PC004, ZR2016HQ18), and National Natural Science Foundations of China (31702383).
Declaration of interest
The authors declare that they have no competing interests.





