The prevalence of obesity has increased worldwide to epidemic proportions. Weight gain occurs when energy intake exceeds energy expenditure for a prolonged period of time. Therefore, novel foods that promote satiety and thereby reduce energy intake may be promising tools in weight management. A potential candidate ingredient for such foods is oligofructose, a fructan obtained as a partial enzymatic hydrolysate from chicory root inulin. It is fermented in the colon, and it is especially known for its prebiotic effects and associated physiological effects(Reference Delzenne and Kok1–Reference Roberfroid3).
The hypothesis that oligofructose might have beneficial effects on energy intake is based upon rat studies(Reference Delzenne, Cani and Daubioul4, Reference Delzenne, Cani and Neyrinck5). In rats, energy intake was decreased over time in the animals fed with oligofructose compared with the animals fed with a control diet. This resulted in a decrease in epididymal fat mass and visceral adipose tissue at the end of the treatment(Reference Cani, Dewever and Delzenne6, Reference Cani, Neyrinck and Maton7). In addition, glucagon-like peptide 1 (GLP-1) amide and proglucagon mRNA concentrations were found to be higher in the oligofructose-fed rats(Reference Cani, Dewever and Delzenne6, Reference Cani, Neyrinck and Maton7). GLP-1 is released from enteroendocrine L-cells in response to nutrient ingestion. Fermentation of oligofructose into SCFA in the gut has been shown to promote enteroendocrine L-cell differentiation in the proximal colon by up-regulation of the differentiation factors (neurogenin 3 and NeuroD), thereby contributing to a higher endogenous GLP-1 production(Reference Cani, Hoste and Guiot8). GLP-1 was found to be essential in the control of food intake by oligofructose, since the beneficial effects of oligofructose were totally prevented in the presence of a GLP-1 receptor antagonist(Reference Cani, Knauf and Iglesias9). In addition, GLP-1 receptor knockout mice (GLP-1R− / −) were completely insensitive to the actions of oligofructose(Reference Cani, Knauf and Iglesias9).
Although animal studies suggest that oligofructose may be a promising tool in the nutritional approach in controlling obesity, only a few studies have investigated the effect of oligofructose in human subjects. For instance, plasma GLP-1 concentrations significantly increased after oligofructose feeding of 20 g/d for 7 d in patients with gastro-oesophageal reflux disease(Reference Piche, des Varannes and Sacher-Huvelin10). It also has been demonstrated that inulin-type fructans, added in food as fat replacement, were able to lower energy intake(Reference Archer, Johnson and Devereux11). In a pilot study by Cani et al. (Reference Cani, Joly and Horsmans12), a 2-week treatment with 16 g/d oligofructose has been shown to promote satiety following breakfast and dinner, and to reduce hunger and prospective food consumption following dinner. Total energy intake per d was 5 % lower during the oligofructose treatment than during the control treatment(Reference Cani, Joly and Horsmans12). These investigators(Reference Cani, Lecourt and Dewulf13) also showed that a different type of inulin consumption of 16 g/d led to increased plasma levels of GLP-1 and peptide YY3-36 (PYY). Recently, a 12-week treatment with 21 g/d oligofructose has been shown to increase the area under the curve (AUC) for the anorexigenic hormone PYY and to decrease the AUC for the orexigenic hormone ghrelin(Reference Parnell and Reimer14). Self-reported energy intake was significantly lower in the oligofructose group, and there was a reduction in body weight of about 1 kg over 12 weeks(Reference Parnell and Reimer14).
However, information on the minimal dosage of oligofructose necessary for establishing beneficial effects is still lacking. Therefore, we performed a placebo-controlled cross-over study to examine the effect of 10 and 16 g/d oligofructose for 13 d on the appetite profile, GLP-1 and PYY concentrations and energy intake in normal-weight and overweight men and women.
Subjects and methods
Subjects
A total of thirty-one healthy subjects (ten men and twenty-one women) aged 20–60 years with a BMI of 23–28 kg/m2 were recruited by advertisements in local newspapers and on notice boards at the university. The subjects underwent a screening, and all were in good health, non-smokers, not using medication (except oral contraception) and moderate alcohol users. None of the subjects had a food allergy, gained or lost more than 5 kg in 3 months before the study, or were cognitive dietary restrained (F1>9) as assessed by a validated Dutch translation of the Three-Factor Eating Questionnaire(Reference Stunkard and Messick15). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Central Committee on Human Research and by the Medical Ethics Committee of the University of Maastricht (Maastricht, The Netherlands). Written informed consent was obtained from all subjects.
Study design
The study had a randomised, placebo-controlled cross-over design. It consisted of three 13 d supplementation periods, wherein oligofructose (Fructalose® L92, 10 or 16 g; Sensus, Roosendaal, The Netherlands) or placebo (maltodextrin, 16 g) supplements were consumed daily, separated by a 2-week washout period. Maltodextrin was selected as a placebo following previous studies on the effect of oligofructose(Reference Cani, Joly and Horsmans12, Reference Parnell and Reimer14). Furthermore, maltodextrin has a similar taste and appearance as oligofructose. The supplements were provided in one-shot fruit drinks of 100 ml. Daily supplements were divided into two equal portions of either 5 or 8 g, each to be consumed at home during breakfast and lunch. To determine compliance, the subjects kept the empty bottles and handed them in on later visits to the university. Potential adverse effects including flatulence, bloated feeling, abdominal rumbling and abdominal pain were monitored daily using a diary. The subjects were asked not to gain or lose weight consciously and to avoid pre- and probiotic foods as indicated on a provided list of food products. The subjects reported to the university six times at 08.00 hours after an overnight fast on days 0 and 13 of each intervention interval. They were asked to abstain from strenuous physical activity and alcohol, and not to eat or drink from 22.00 hours the night before each test day. Energy intake and appetite profile ratings were determined on days 0 and 13. GLP-1 and PYY concentrations were determined on day 13.
Study protocol
On each test day, the subject's body weight was measured with minimal clothing in the fasting state. The subjects received a standardised breakfast at 08.30 hours consisting of 20 % of the subject's individual daily energy requirements. Subject-specific daily energy requirements were derived from BMR, which was calculated from the equation of Harris–Benedict(Reference Harris and Benedict16). BMR was multiplied by an activity index of 1·5, as indicated for a sedentary day(Reference Westerterp and Kester17). Breakfast consisted of brown bread with cheese and marmalade, and fruit yogurt (21, 62 and 17 En % from protein, carbohydrate and fat, respectively). At 12.30 hours, the subjects received a standardised lunch, which provided 40 % of the subject's individual daily energy requirements. The lunch consisted of brown bread with egg and tuna sandwich salad, tomato soup and grape juice (18, 55 and 27 En % from protein, carbohydrate and fat, respectively). Food and energy intake were assessed on days 0 and 13 via an ad libitum dinner at 17.00 hours, which consisted of a homogeneous hot pasta meal. The dinner was weighed before and after eating. The subjects were instructed to eat till they were comfortably full. The lasagna (1350 g) provided 5 kJ/g (31, 45 and 24 En % from protein, carbohydrate and fat, respectively). Appetite profile ratings were measured sixteen times with regular intervals between 08.30 and 20.00 hours on days 0 and 13. On day 13, nine blood samples were collected at 08.20, 09.00, 09.30, 10.30, 12.20, 13.00, 13.30, 14.30 and 15.30 hours, respectively, in order to obtain GLP-1 and PYY concentrations over the day.
Appetite profile
Appetite profile ratings were evaluated using anchored 100 mm visual analogue scales (VAS)(Reference Flint, Raben and Blundell18, Reference Stubbs, Hughes and Johnstone19). Hunger, fullness, satiety, thirst, desire to eat and prospective food consumption were measured. The scale was anchored from ‘not at all’ on the left to ‘extremely’ on the right. The participants were instructed to rate their feelings by marking the scale with a vertical line at a point that was most appropriate at that time. The distance from the left end of the scale to this vertical line on the scale was measured in mm; changes from baseline were calculated by subtracting the baseline score from the score at a certain time point. On each test day, these questionnaires were completed at 08.30, 09.00, 09.30, 10.30, 11.30, 12.30, 13.00, 13.30, 14.30, 15.30, 16.30, 17.00, 17.30, 18.00, 19.00 and 20.00 hours.
Blood sampling
On days 0 and 13, a catheter was placed into the antecubital vein for blood sampling. Blood samples were collected into tubes containing EDTA, 10 μl dipeptidyl peptidase IV per ml blood and 50 μl aprotonin per ml blood for measurements of PYY concentrations. For GLP-1 measurements, blood was collected into EDTA tubes containing 10 μl dipeptidyl peptidase IV per ml blood. Plasma was obtained by centrifugation (4°C, 1000 g, 10 min) and stored at − 80°C until analysed. PYY3-36 concentrations were measured with a specific and sensitive RIA (Linco Research, Inc., St Charles, MO, USA). Plasma-active GLP-1 concentrations were measured by enzyme-linked immunoradiometric assay (EGLP-35K; Linco Research, Inc.).
Statistical analysis
Data are presented as mean changes from baseline with their standard errors, unless otherwise indicated. AUC of changes from baseline over time was calculated by using the trapezoid method. A Student's t test (two-tailed distribution) was carried out to determine the possible differences between the conditions. ANOVA repeated measures was carried out with a repeated covariance structure to determine possible differences in appetite ratings and energy intake between conditions and time × treatment interactions. Significance was defined as P < 0·05. All the statistical analyses were executed with SPSS version 16.0 for Macintosh OS X (SPSS, Inc., Chicago, IL, USA).
Results
Subject characteristics
Of the thirty-one subjects, two dropped out because of personal reasons, and twenty-nine subjects (nine men and twenty women) completed the study. The baseline characteristics of these subjects are presented in Table 1. As expected, body weight and height were significantly different between men and women. Body weight did not change over time, and there were no significant differences in body weight between the conditions. Moreover, no sex differences were present with respect to changes in measured variables, so the whole group was analysed together.
Table 1 Subject characteristics
(Mean values with their standard errors)
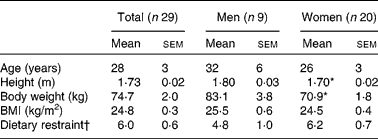
* Mean values were significantly different from those of men (P < 0·01, by ANOVA).
† Dietary restraint, factor 1 of the Three-Factor Eating Questionnaire(Reference Stunkard and Messick15).
Appetite profile
On day 0, the AUC for appetite profile ratings was not significantly different between the three conditions. No time × treatment differences were present with respect to the appetite profile ratings.
Peptide YY3-36 and glucagon-like peptide 1
Fasting PYY concentrations, but not fasting GLP-1 concentrations, were significantly lower on day 13 compared with those on day 0 in all conditions (49·9 (sem 4·4) v. 71·2 (sem 3·9) pg/ml for placebo; 46·9 (sem 4·6) v. 71·9 (sem 3·6) pg/ml for 10 g/d oligofructose and 53·8 (sem 4·7) v. 74·1 (sem 3·9) pg/ml for 16 g/d oligofructose; P < 0·01).
After the meals, PYY concentrations significantly increased in all conditions (Fig. 1(a) and (b), P < 0·05). In the morning until lunch, AUC0–230 min for PYY was significantly higher with 16 g/d oligofructose than with 10 g/d oligofructose and placebo (409 (sem 35) v. 222 (sem 19) and 211 (sem 20) pg/ml × h, P < 0·01). This gradually diminished over the day, resulting in a trend towards a larger total AUC for PYY with 16 g/d oligofructose than with 10 g/d oligofructose or placebo (P = 0·065).

Fig. 1 Mean values for (a) plasma peptide YY3-36 (PYY; time × treatment interaction P = 0·065) and (b) active glucagon-like peptide 1 (GLP-1; time × treatment interaction P = 0·041) concentrations during day 13 as absolute concentrations (pg/ml, pmol/l) with breakfast consumed at 10 min and lunch at 250 min, (c) area under the curve (AUC) for PYY3-36 and (d) AUC for active GLP-1 (pg/mml × h, pmol/l × h). 16 g/d oligofructose (![]() ,
, ![]() ), 10 g/d oligofructose (
), 10 g/d oligofructose (![]() ,
, ![]() ) and placebo (
) and placebo (![]() ,
, ![]() ). Data were analysed by ANOVA with repeated measures. * Mean values were significantly different between 16 g/d oligofructose and 10 g/d oligofructose (P < 0·05). † Mean values were significantly different between 16 g/d oligofructose and placebo (P < 0·05). ‡ Mean values were significantly different between 10 g/d oligofructose and placebo (P < 0·05). § Mean values were significantly different between 10 g/d oligofructose and 16 g/d oligofructose and placebo (P < 0·05).
). Data were analysed by ANOVA with repeated measures. * Mean values were significantly different between 16 g/d oligofructose and 10 g/d oligofructose (P < 0·05). † Mean values were significantly different between 16 g/d oligofructose and placebo (P < 0·05). ‡ Mean values were significantly different between 10 g/d oligofructose and placebo (P < 0·05). § Mean values were significantly different between 10 g/d oligofructose and 16 g/d oligofructose and placebo (P < 0·05).
After the meals, GLP-1 concentrations also increased significantly in all conditions (Fig. 1(c) and (d), P < 0·05). AUC for GLP-1 was significantly higher with 16 g/d oligofructose compared with 10 g/d oligofructose (P < 0·05). In addition, AUC for GLP-1 with 10 g/d oligofructose was significantly lower compared with placebo (P < 0·05).
Compliance and side effects
Compliance was high, as shown by the empty packages returned at the end of each treatment. Incidentally, some subjects returned a full package, indicating a missed dose. The subjects also reported this in their diary, showing that missing doses remained below 5 %. Only a small proportion of these missing doses was on the day before the measurements, and no doses were missed on the day of the measurements.
Adverse effects, such as flatulence, abdominal rumbling, bloated feeling and cramps, were significantly higher for 10 and 16 g/d oligofructose compared with placebo on several days during the treatment, respectively (P < 0·05, data not shown). However, none of the subjects complained about side effects, and there were no significant time × treatment interactions. Also, side effects did not influence compliance, since compliance was similar in the placebo treatment as with 10 and 16 g/d oligofructose.
Energy intake
On day 0, energy intake during the ad libitum dinner was similar for all the conditions (2788 (sem 251), 3127 (sem 267) and 3217 (sem 321) kJ for placebo, 10 and 16 g/d oligofructose, respectively). Subsequently, energy intake was significantly lower on day 13 after the 16 g/d oligofructose treatment (11 %, P < 0·05) compared with 10 g/d oligofructose (2801 (sem 301) v. 3177 (sem 276) kJ, P < 0·05; Fig. 2), but not compared with placebo (2979 (sem 276) kJ).

Fig. 2 Changes in energy intake (kJ) between days 0 and 13 after placebo (□), 10 g/d oligofructose (![]() ) and 16 g/d oligofructose (
) and 16 g/d oligofructose (![]() ) treatment. Data were analysed by ANOVA with repeated measures. * Mean values were significantly different from baseline (P < 0·05). † Mean values were significantly different between the conditions for time × treatment interaction (P = 0·068).
) treatment. Data were analysed by ANOVA with repeated measures. * Mean values were significantly different from baseline (P < 0·05). † Mean values were significantly different between the conditions for time × treatment interaction (P = 0·068).
Discussion
In the present study, the effect of oligofructose supplementation on appetite profiles, satiety hormone concentrations and energy intake in human subjects was examined. Energy intake decreased over time with 16 g/d oligofructose, whereas consumption of 10 g/d oligofructose did not change energy intake. There were no significant associations of the oligofructose-induced reduction in energy intake with appetite profile ratings.
Reduced energy intake on day 13, with 16 g/d oligofructose was underscored by higher AUC0–230 min of PYY hormone concentrations with 16 g/d oligofructose compared with 10 g/d oligofructose and placebo, and higher AUCtotal of GLP-1 hormone concentrations with 16 g/d oligofructose compared with 10 g/d oligofructose, although not significantly different from placebo. Based on rat studies(Reference Cani, Dewever and Delzenne6, Reference Cani, Neyrinck and Maton7, Reference Cani, Knauf and Iglesias9), it is suggested that oligofructose intake results in increased GLP-1 and PYY concentrations mediated via the SCFA, which are produced by the fermentation of oligofructose. Previous studies in human subjects showed an increased AUC for PYY with 21 g/d oligofructose, whereas GLP-1 did not change(Reference Parnell and Reimer14). Moreover, fasting PYY concentrations on day 13 compared with day 0 were decreased in all conditions, suggesting an effect not specifically due to oligofructose consumption. However, the subjects may have experienced a different feedback of SCFA from the colon due to the consumption of a different macronutrient and particularly fibre composition in general, resulting in an overall reduction of PYY.
The results on appetite profile ratings suggest that oligofructose does not suppress appetite. This is not consistent with previous findings(Reference Cani, Joly and Horsmans12). On the other hand, Archer et al. (Reference Archer, Johnson and Devereux11) could not find an effect for inulin on appetite at a lower dosage, but they did find a decrease in energy intake. However, differences in study design, such as standardised meals v. ad libitum and free-choice meals, and differences in population size and type of inulin used complicate comparisons between studies. In addition, measuring subjective appetite ratings with VAS can result in high variability(Reference Flint, Raben and Blundell18, Reference Stubbs, Hughes and Johnstone19). Probably, oligofructose does not affect appetite feelings sufficiently to be detected with the use of VAS.
Observations on side effects as reported in the literature are not consistent. Several measurement methods are used, such as diaries, questionnaires with four-point scales(Reference Peters, Boers and Haddeman20) or VAS questionnaires(Reference Parnell and Reimer14). In addition, some studies report about side effects without explaining the measurement procedures(Reference Cani, Joly and Horsmans12) or do not report about side effects at all(Reference Archer, Johnson and Devereux11). This makes it difficult to compare results between studies. We combined the VAS questionnaires with diaries to analyse occurrence of side effects over time. Consumption of 10 and 16 g oligofructose daily for 2 weeks did result in minor gastrointestinal side effects. However, no subjects complained; compliance was not affected by side effects, and we did not observe a relation of side effects with energy intake.
The observed reduction in energy intake induced by 16 g/d oligofructose over a time interval of 13 d is in agreement with earlier findings(Reference Cani, Joly and Horsmans12, Reference Parnell and Reimer14). However, differences between 16 g/d oligofructose and placebo were not significant. Since the placebo is a polysaccharide, there might be an effect of 16 g/d maltodextrin itself on energy intake, through which the differences between placebo and 16 g/d oligofructose become too small in order to reach significance. Maltodextrin, in higher concentrations (62·9 g), has been shown to lower energy intake(Reference Yeomans, Gray and Conyers21).
Energy intake did not change with 10 g/d oligofructose. This suggests that only 16 g/d oligofructose is a minimal dosage to induce an effect of oligofructose on energy intake. The present results suggest that oligofructose-induced reduction in energy intake might be exerted via increased GLP-1 and PYY concentrations.
A limitation of the present study is the nature of the treatment. With such a minimal dosage, i.e. 16 g/d compared with 21 g/d in other studies, the study needs more sensitivity. A larger number of subjects and a longer period of time may have shown more robust results. However, we found for consumption of 16 g/d oligofructose a trend for a time × treatment effect over 13 d, as well as lower energy intakes on the final day of treatment. The latter was supported by higher AUC concentrations of GLP-1 and for PYY in the morning. Another limitation of the present study is that we did not measure SCFA. Although measuring SCFA in vivo in human subjects is challenging, combining SCFA measurements in both faeces and blood could contribute to explaining the mechanism behind the effect of oligofructose on energy intake.
In conclusion, 16 g/d and not 10 g/d oligofructose may be an effective dose to reduce energy intake in normal-weight and overweight men and women, underscored by elevated GLP-1 and PYY concentrations.
Acknowledgements
D. M. designed the study. S. P. M. V. collected and analysed the data and wrote the manuscript. K. R. W. contributed to the interpretation of the data and reviewed the manuscript. The study was executed under the supervision of K. R. W. All authors read and approved the final manuscript. The study was financially supported by Sensus. None of the authors had any conflict of interest.





