Case report
A 20-year-old male with a history of hypoplastic left heart syndrome who underwent lateral tunnel Fontan palliation with right iliofemoral vein occlusion presented with intra-atrial re-entry tachycardia. He was on chronic aspirin and clopidogrel for thrombus prophylaxis which were not held for the procedure. He presented for mapping and ablation of the atrial tachycardia. Vascular access was obtained through the left femoral vein and left femoral artery. His Fontan circuit pressure was elevated at 16–18 mmHg.
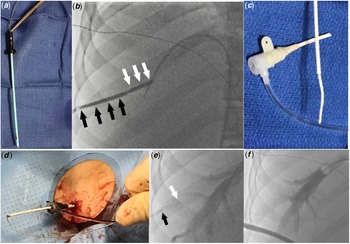
We attempted to perform transbaffle puncture through the left femoral vein; however, we were unable to position the transseptal needle at a safe location. Therefore, the decision was made to proceed with transhepatic access. From the left femoral vein access a 7-Fr wedge catheter (Arrow, Morrisville, NC) was placed in an adequately sized right hepatic vein. Under biplane fluoroscopy guidance a 15 cm 22-gauge Chiba needle (Cook Medical, Bloomington, IN) was advanced toward the wedge catheter. After accessing the hepatic vein, the access wire was exchanged for a 0.035" Amplatzer Super Stiff guidewire (Boston Scientific, Marlborough, MA). Upon advancing the guidewire into the Fontan circuit, the guidewire spontaneously crossed through a small baffle leak into the left atrium. An 8.5-Fr steerable Agilis sheath (St Jude Medical, St Paul, MN) was advanced over the guidewire across the baffle leak. Through the Agilis sheath, the mapping and ablation procedures were successfully completed. To mitigate the risk of bleeding from the transhepatic tract, we decided to close it. The Agilis sheath was pulled back inside the Fontan baffle over a guidewire and was exchanged for a 9-Fr 10 cm Pinnacle sheath (Terumo Medical, Somerset, NJ), and the dilator and wire were removed. The tip of the 9-Fr dilator was cut to match the length of the sheath. A 0.035” short access wire was inserted inside the dilator. The wire was secured within the dilator using a metallic hemostat, keeping the wire tip flush with the cut tip of the dilator (Fig 1a). This modification was created to stiffen the dilator and fill its lumen, so that gelatin “torpedoes” could be pushed with the dilator through the sheath. A 2 x 6 x 0.7 cm Surgifoam (Ferrosan Medical Devices, Soeborg, Denmark) gelatin sponge was chosen. The sponge was compressed by rolling a 5cc syringe over the gelatin sponge which was then cut into strips. The strips were turned into “torpedoes” by tightly rolling them. Two “torpedoes” were loaded inside a cut 5-Fr sheath. The 9-Fr sheath was pulled back inside the transhepatic tract to ensure that the gelatin “torpedoes” could be precisely placed within the parenchymal tract rather than within hepatic vein. An angiogram through the sidearm of the 9-Fr sheath confirmed the location of the sheath tip to be within the hepatic parenchyma (Fig 1b). Two “torpedoes” were loaded inside a cut 5-Fr sheath (Fig 1c) and pushed inside the 9-Fr sheath with the help of the stiff end of the 0.035” access wire (Fig 1d). The cut 5-Fr sheath was removed and the torpedoes were deployed sequentially inside the tract by pushing them with the modified 9-Fr dilator while pulling the sheath back (Fig 1e). An angiogram in the right hepatic vein showed no residual contrast flow inside the hepatic tract (Fig 1f) and no blood was seen at the skin access site. The next day an abdominal ultrasound showed no hemoperitoneum or intrahepatic haematoma.

Figure 1. (a) The dilator of the 9-Fr sheath was cut to match the length of the sheath and the access wire was clipped to the dilator, with the wire end flush with the end of the dilator. (b) Angiogram through the 9-Fr sheath showing the sheath tip is inside the transhepatic tract (black arrows delineate the sheath inside the tract, white arrows delineate the distal transhepatic tract with no sheath inside it). (c). Loading gelatin sponge “torpedoes” inside the cut 5-Fr sheath. (d). Loading gelatin “torpedoes” inside the 9-Fr sheath. (e). Angiogram through the wedge catheter after deploying the first gelatin “torpedo” shows no contrast filling the distal part of the transhepatic tract (white arrow delineates the end of the sheath, black arrow delineates the end of the dilator). (f). Final angiogram in the right hepatic vein after deploying the second torpedo showing no contrast filling the hepatic tract.
Discussion
Transhepatic venous access may be very useful when conventional access sites are untenable, such as in cases with occluded iliofemoral veins or interrupted inferior caval vein. Reference Ebeid1–Reference Shim, Lloyd, Cho, Moorehead and Beekman5
Transbaffle puncture is commonly needed to treat arrhythmias in patients with Fontan palliation. Transhepatic access provides a trajectory perpendicular to the baffle, allowing a more stable position of the transseptal needle when force is needed to overcome the thickness and stiffness of the baffle. Reference Singh, Neuzil and Skoka4,Reference Piorkowski, Eitel and Rolf6
We used fluoroscopic guidance to obtain transhepatic access. Alternatively, ultrasound guidance could be used. Reference Ebeid1,Reference Shim, Lloyd, Cho, Moorehead and Beekman5 Placing the wedge catheter in the hepatic vein via a femoral vein served four purposes. First, we used it as a target during transhepatic needle advancement. Second, it allowed us to judge the distance of the sheath from the accessed vein during closure. Third, an angiogram through it confirmed successful closure of the tract. Fourth and probably most important, it could be used for "bailout" if hemostasis could not be achieved: the wedge catheter could be quickly inflated at the junction of the accessed hepatic vein and the parenchymal tract until hemostasis was achieved (alternately, the wedge catheter could be exchanged over a wire for an angioplasty balloon to be used for balloon occlusion to help achieving hemostasis).
Although the need for closure of a small transhepatic tract is debatable, it is widely accepted to close the tract if a large introducer is placed, especially in patients with increased central venous pressure or bleeding diathesis. Reference Ebeid1
In the field of cardiac catheterisation, the transhepatic tract is usually closed using coils or occlusion devices. Reference Ebeid1,Reference Singh, Neuzil and Skoka4 Gelatin sponge was reported in only one case following atrial septal defect closure using an 8-Fr sheath. Reference Haddad, Maleux, Bonnet and Malekzadeh-Milani3 On the other hand, gelatin sponge “torpedoes” have been commonly used to close transhepatic portal vein tracts for sheaths up to 6-Fr. Reference Saad and Madoff7–Reference Medsinge, Zajko, Orons, Amesur and Santos9 To the best of our knowledge, our case represents the largest transhepatic venous tract that was closed successfully with gelatin sponge “torpedoes.” The Agilis steerable sheath has an inner and outer diameter of 8.5 and 11-Fr, respectively. Reference Piorkowski, Eitel and Rolf6 Hence, we used a 9-Fr sheath in its place to deliver the gelatin “torpedoes,” anticipating that this sheath would have a similar outer diameter of approximately 11-Fr.
The gelatin sponge is able to absorb blood and provide a scaffold for clot forming. Therefore, it has been used primarily to achieve local hemostasis at the oozing surgical sites. Other uses are embolising arterial vessels and occlude transparenchymal tracts after direct organ access, such as liver, spleen, and lung accesses. Reference Medsinge, Zajko, Orons, Amesur and Santos9,Reference Özer and Köstü10 Gelatin sponge has many advantages over coils or occlusion devices for closure of transhepatic tracts. First, relatively small caliber “torpedoes,” such as the ones we packed into the cut 5-Fr sheath, can be delivered within larger caliber tracts because the gelatin sponge expands when it gets in contact with blood. In addition, gelatin sponge gets absorbed within a few weeks and does not create artefacts with certain imaging modalities such as CT or MRI, it is relatively inexpensive, easy to use, and can be traversed with a needle for future transhepatic access. Importantly, if a gelatin “torpedo” is delivered too close to the hepatic capsule and protrudes into the abdominal wall and past the skin, it can be simply trimmed with scissors; in contrast, coils or nitinol-based devices are problematic if they are positioned across the abdominal wall, and cannot be left to protrude through the skin and would instead require removal, potentially resulting in intra-abdominal bleeding. Gelatin sponge material can be used as a slurry or made into “torpedoes.” To employ a slurry, the gelatin sponge is cut into small pieces and mixed with contrast and then injected inside the tract. We favoured the use of dry “torpedoes” which we expected to expand and take the shape of the tract providing more stability. Reference Haddad, Maleux, Bonnet and Malekzadeh-Milani3,Reference Uller, Muller-Wille and Grothues8,Reference Medsinge, Zajko, Orons, Amesur and Santos9,Reference Özer and Köstü10 We considered this important in our patient with multiple risk factors for bleeding, including the use of a large-bore sheath, increased central venous pressure, and being on dual antiplatelet therapy.
Conclusion
The use of gelatin sponge may be safe and effective for closing large transhepatic tracts and we suggest the use of a balloon wedge catheter within the accessed hepatic vein as an additional safety measure, when feasible. Due to many advantages over the use of metallic implants, gelatin sponge should be considered the preferred method for closure of transhepatic tracts
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
Dr Justino is a consultant for Abiomed, Abbott, Edwards Lifesciences, Medtronic, Pediastent, and Janssen Research and Development, is a co-founder of PolyVascular, and received grant support from W. L. Gore, none of which are relevant to the topic of this manuscript.
Ethical standards
No specific ethical approval from Institutional Reviews Boards is necessary for this type of publication. The authors assure that all patient data provided in this case report are anonymised.