Introduction
Motivation is an integral component of human experience. Children spontaneously explore novel items, and adults autonomously engage in new hobbies, even in the absence of clear extrinsic reinforcers. Thus, not all actions are driven by tangible external stimuli or outcomes, known as ‘extrinsic’ motivation, but are driven by more internal drivers, known as ‘intrinsic’ motivation, where the activity is perceived as its own outcome.
Intrinsically motivated behaviors are computationally similar to extrinsically motivated behaviors, in that they strive to maximize goal attainment and minimize punishment, represented mathematically as value and effort cost functions, respectively (Gottlieb, Lopes, & Oudeyer, Reference Gottlieb, Lopes, Oudeyer, Kim, Reeve and Bong2016). However, subjective internal value functions are difficult to characterize, and our understanding of how they are computed and integrated is limited (Gottlieb et al., Reference Gottlieb, Lopes, Oudeyer, Kim, Reeve and Bong2016).
Dysfunction in intrinsic motivation represents an important transdiagnostic facet of psychiatric symptomology, which is often classified as distinct psychological constructs, such as apathy in neurological disorders, anhedonia in depression, and negative symptoms in schizophrenia. Each of these symptom domains may be underpinned by a shared dysfunction of intrinsic motivation, and interventions targeting intrinsic motivation have the potential to improve treatment outcomes for affected individuals.
However, due to a lack of clear consensus, the contribution of intrinsic motivation to psychiatric disorders remains poorly understood. This review aims to provide an overview of the conceptualization, measurement, and neurobiology of intrinsic motivation, providing a framework for understanding the potential contributions to psychopathology and its treatment.
Historical conceptualizations of intrinsic motivation
During the early 20th century, prominent descriptions of motivation were at odds with each other. Woodworth (Reference Woodworth1918) suggested that intrinsic motivation governed activities perpetuated by their own ‘native drive’, whereas Thorndike (Reference Thorndike1911) and Watson (Reference Watson1913) argued that external stimuli governed behavior. Also centered on internal drives, Hull's (Reference Hull1943) ‘drive theory’ posited that all behaviors were performed to seek or avoid primary biological states, including hunger or pain. However, the drive theory could not explain many behavioral anomalies, such as hungry rats withstanding painful electric shocks to explore a novel environment (Nissen, Reference Nissen1930), or rhesus monkeys performing a puzzle task for no biological reason or external reinforcer (Harlow, Reference Harlow1950). By narrowly presuming that biological states drive all behavior, drive theory failed to account for instances in which an organism prioritizes higher-order cognitive drives over physiological ones.
The shortcomings of drive theory led to the emergence of alternate theories of intrinsic motivation. Some argued that homeostatic maintenance of optimal biological or cognitive states (Hebb, Reference Hebb1955; McClelland & Clark, Reference McClelland, Clark, Mcclelland, Atkinson, Clark and Lowell1953; McClelland, Atkinson, Clark, & Lowell, Reference McClelland, Atkinson, Clark, Lowell and Haber1967), or mitigation of incongruency or uncertainty (Festinger, Reference Festinger1957; Kagan, Reference Kagan1972), drove behavior. However, these theories emphasized external stimuli or cognitive representations of external goal states as key drivers of behavior. In the mid-to-late 20th century, several models underscored the importance of novelty-seeking, interest, and autonomy in driving intrinsic motivation. Novelty-seeking was suggested to energize approach behavior via curiosity and exploration that leads to skill mastery, information attainment, or learning (Kaplan & Oudeyer, Reference Kaplan and Oudeyer2007). Interest and enjoyment in an activity might boost intrinsic motivation by engendering ‘flow’, a prolonged state of focus and enjoyment during task engagement that stretches one's skillset (Csikszentmihalyi, Reference Csikszentmihalyi1975; Nakamura & Csikszentmihalyi, Reference Nakamura, Csikszentmihalyi, Lopez and Snyder2009). Finally, self-determination theory (Deci & Ryan, Reference Deci and Ryan1980) proposed that human needs for competence, achievement, and autonomy drive intrinsic motivation, aligning with observations that intrinsic motivation stems from an internal perceived autonomy during task engagement (DeCharms, Reference DeCharms1968; Lamal, Reference Lamal2003). These models highlight the role of achievement and perceived autonomy (DeCharms, Reference DeCharms1968) in driving intrinsic motivation, coinciding with current computational frameworks of intrinsic reward (Chew, Blain, Dolan, & Rutledge, Reference Chew, Blain, Dolan and Rutledge2021; Murayama, Matsumoto, Izuma, & Matsumoto, Reference Murayama, Matsumoto, Izuma and Matsumoto2010).
The introduction of external goals: a shift to extrinsic motivation
While intrinsic motivation has been proposed to be divorced from external reinforcers, our understanding of motivation has been led largely by using external reinforcers as conceptual and experimental tools. Here, we briefly review historical perspectives on external drivers of motivated behavior, outlining prominent goal- and action-focused models of extrinsic motivation.
Early psychological models of extrinsic motivation suggested that ‘will’ and ‘intention’ fostered goal achievement, emphasizing the influence of goal expectation on action and control (Lewin, Reference Lewin and Rapaport1951; Tolman, Reference Tolman1932). Within this framework, environmental features, as well as an individual's internal state or memory, determine their actions when pursuing a goal, or, more specifically, the cognitive representation of a goal (Kagan, Reference Kagan1972). This requires multiple cognitive representations to be developed, maintained, and updated, with a particular reliance on external stimuli and learning (Deci, Koestner, & Ryan, Reference Deci, Koestner and Ryan1999; Kagan, Reference Kagan1972; Kagan & Moss, Reference Kagan and Moss1983).
Alongside psychological model development, economic models of motivation emerged. These models propose that extrinsic goals, or incentives, elicit motivated behavior via a cost-benefit analysis, where motivated choice occurs when benefits outweigh costs. More recently, behavioral economics has considered how individual personality traits, biases, and irrationalities influence motivated behavior (Strombach, Strang, Park, & Kenning, Reference Strombach, Strang, Park and Kenning2016). A recent model (Strombach et al., Reference Strombach, Strang, Park and Kenning2016) incorporates various factors into the classical cost-benefit analysis, including traditional intrinsic (e.g. satisfaction) and extrinsic drivers (e.g. money), with negative influences from costs (e.g. effort, pain), which are merged into a single dynamic, subjective and state-dependent factor that drives motivated behavior. Though this approach is powerful, the explicit focus on incentives provides limited explanatory power for various paradoxical behaviors, including rodents overcoming the high cost to self-stimulate certain brain regions (e.g. nucleus accumbens; Nac) or extrinsic reinforcers' dampening effect on intrinsic motivation.
In reinforcement learning models of decision-making, an organism, or agent, learns which actions maximize total reward. This process has been formalized within computational sciences and modern artificial intelligence systems (Sutton & Barto, Reference Sutton and Barto1981; Witten, Reference Witten1977), where learning and decision-making depend on an extrinsic outcome. One theory suggests that motivated action is driven solely by a need to reduce reward prediction errors (RPEs; Kaplan and Oudeyer, Reference Kaplan and Oudeyer2007), or the mismatch between expectation and outcome (Montague, Dayan, & Sejnowski, Reference Montague, Dayan and Sejnowski1996; Schultz et al., Reference Schultz, Dayan and Montague1997). RPEs can also be conceptualized as valuation signals for novel outcomes or unexpected stimuli. RPE-based learning then drives motivated behavior, or action choice, but even if the agent displays intact encoding of action or outcome value, motivated behavior can be dampened by reduced novelty. This highlights the role of novelty, expectation and prediction in learning per se, rather than choice valuation.
In action-focused models of motivation, incentives can trigger approach or avoidance behavior by signaling a potential goal state (Berridge, Robinson, & Aldridge, Reference Berridge, Robinson and Aldridge2009). Incentive motivation thus relies on expectancy, probability, and value of outcomes, which are thought to dictate behavioral choice and decision-making. While greater reliance on stimulus-outcome rather than stimulus-response contingencies has led some to describe incentive motivation as proactive (Beckmann & Heckhausen, Reference Beckmann, Heckhausen, Heckhausen and Heckhausen2018), others have characterized it as reactive due to the central role of learning from past experience (Bolles, Reference Bolles1972). Reliance on an expected outcome was central to behaviorism (Watson, Reference Watson1913, Reference Watson1930) and operant conditioning (Skinner, Reference Skinner1938), which assume that actions are driven by a reinforcer, and instrumental value is assigned to the behavior itself. Stimulus-response pairs dominate behaviorism and modern theories of habitual behavior (Gläscher, Daw, Dayan, & O'Doherty, Reference Gläscher, Daw, Dayan and O'Doherty2010), where the dependency on previously reinforced actions ultimately governs motivated choice (de Wit et al., Reference de Wit, Standing, DeVito, Robinson, Ridderinkhof, Robbins and Sahakian2011; Gillan, Robbins, Sahakian, van den Heuvel, & van Wingen, Reference Gillan, Robbins, Sahakian, van den Heuvel and van Wingen2016; Voon et al., Reference Voon, Derbyshire, Rück, Irvine, Worbe, Enander and Bullmore2014). However, this renders behaviors as repetitive, insensitive to punishment and divorced from goals (Robbins, Gillan, Smith, de Wit, & Ersche, Reference Robbins, Gillan, Smith, de Wit and Ersche2012). Therefore, these action-focused models of motivated behavior almost entirely discount intrinsic motivation since extrinsic motivators usurp control of behavior.
Several limitations of extrinsic motivation models must be considered when attempting to characterize intrinsic motivation. First, for cost-benefit analysis and reinforcement learning, an internal representation of the outcome must first be learned, which requires previous experience of the goal. However, intrinsic motivation can occur for novel outcomes, or behaviors that are uncertain or ambiguous. Second, motivation can occur for activities that may already be fully predictable, marking a significant limitation for reinforcement-learning models of motivation, which assume that reward prediction errors drive learning for motivated action. Third, these frameworks cannot fully explain spontaneous novelty seeking or exploratory behavior, in which no external reward is expected and no cost is overcome (Deci et al., Reference Deci, Koestner and Ryan1999; Marsden, Ma, Deci, Ryan, & Chiu, Reference Marsden, Ma, Deci, Ryan and Chiu2014).
Separating and integrating intrinsic and extrinsic motivation
A key question is whether intrinsic and extrinsic motivation can, or should, be experimentally or theoretically separated. There is some evidence that they are dissociable constructs at the neural level. The most compelling support comes from case reports of patients with basal ganglia lesions who developed ‘psychic akinesia’, a syndrome characterized by difficulty with self-generated action initiation but no difficulty in performing complex cognitive or motor tasks when prompted (Laplane, Baulac, Widlocher, & Dubois, Reference Laplane, Baulac, Widlocher and Dubois1984; Lugaresi, Montagna, Morreale, & Gallassi, Reference Lugaresi, Montagna, Morreale and Gallassi1990). In patients with alien hand syndrome, medial prefrontal and anterior cingulate cortex (ACC) lesions lead to a loss of intentional motor control, whereas (pre)-supplementary motor area lesions lead to impairments in implementing motor intentions (Brugger, Galovic, Weder, & Kägi, Reference Brugger, Galovic, Weder and Kägi2015; Nachev, Kennard, & Husain, Reference Nachev, Kennard and Husain2008). Preclinical findings further show that photostimulation of GABAergic amygdala projections modulates extrinsic motivation without affecting intrinsically motivated behavior (Seo et al., Reference Seo, Funderburk, Bhatti, Motard, Newbold, Girven and Bruchas2016). Together, these findings suggest that intrinsic and extrinsic motivation reflect different cortico-striatal-limbic circuits.
Behavioral research primarily supports the view that intrinsic and extrinsic motivation are partially distinct, interacting processes. For example, if the motivation for intrinsic and extrinsic goals were independent constructs, they might demonstrate an additive or subtractive effect on each other (Woodworth, Reference Woodworth1921). Indeed, the expectation (Liu & Hou, Reference Liu and Hou2017) and experience (Badami, VaezMousavi, Wulf, & Namazizadeh, Reference Badami, VaezMousavi, Wulf and Namazizadeh2011) of an extrinsic reinforcer can increase intrinsic motivation. However, reports of the ‘undermining effect’, in which an external reinforcer reduces intrinsic motivation (Cerasoli, Nicklin, & Ford, Reference Cerasoli, Nicklin and Ford2014; Deci, Reference Deci1971; Deci, Benware, & Landy, Reference Deci, Benware and Landy1974; Lepper & Greene, Reference Lepper and Greene1978; Lepper, Greene, & Nisbett, Reference Lepper, Greene and Nisbett1973) have sparked debate over how extrinsic reinforcers affect internally-motived behaviors (Cameron & Pierce, Reference Cameron and Pierce2002; Lamal, Reference Lamal2003; Lepper, Keavney, & Drake, Reference Lepper, Keavney and Drake1996). One explanation for the undermining effect suggests that the presence of an external reinforcer shifts one's perception of the locus of control over the behavior from internal to external (Deci & Ryan, Reference Deci and Ryan1980). This implicates a key role of agency, or the belief of action ownership, in intrinsic motivation. While controversial, mounting evidence supports this account of the undermining effect, where various extrinsic motivators (e.g. food, social observation; Ryan, Reference Ryan1982) decrease intrinsic motivation when their delivery is contingent on task-performance.
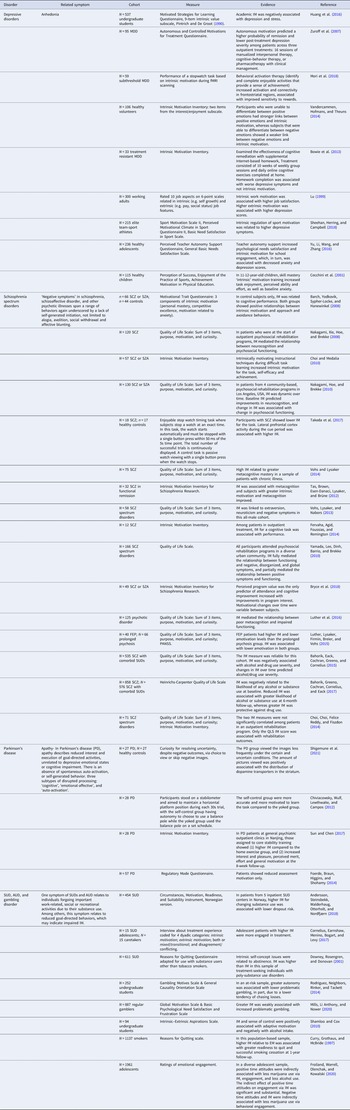
A useful framework for parsing motivated action into intrinsic and extrinsic is the Rubicon model of action phases (Heckhausen & Heckhausen, Reference Heckhausen and Heckhausen2018; Heckhausen, Reference Heckhausen1989). Within this framework, pre-decisional option deliberation occurs, which is followed by choice intention formation and planning, volitional action, outcome achievement, and evaluation (Fig. 1). Husain and Roiser (Reference Husain and Roiser2018) recently proposed a complementary model to deconstruct apathy and anhedonia into underlying cognitive processes, including option generation, anticipation, action initiation, prediction, consumption and learning. This parcellation broadly reflects the five main stages of the Rubicon model: (1) pre-decisional deliberation (option generation); (2) intention formation, planning, initiation (anticipation); (3) volitional action (action initiation, prediction), (4) outcome achievement (consumption); and (5) evaluation (learning; Figure 1). Within these overlapping frameworks, the initial pre-decisional deliberation/option generation phase represents the point at which intrinsic and extrinsic facets of motivation diverge, as early drivers of behavior can be intrinsic (e.g. enjoyment, interest, exploration) or extrinsic (e.g. social reward). The differences between these early drivers highlight a key distinction between intrinsic and extrinsic motivation, in which the former is a fundamentally proactive process and the latter reactive.

Fig. 1. Schematic framework for parsing motivated action. Motivated decision-making and action is parsed into separate phases of choice, action and outcome valuation, combining and building upon separate frameworks including the Rubicon model of action phases, well-established computational mechanisms and a recent cognitive framework describing anhedonia and apathy. During choice valuation, pre-decisional deliberation includes option generation, a cost-benefit analysis and option selection. Intrinsic and extrinsic motivation diverges during this early choice valuation phase. Once choice valuation has been computed and an option selected, planning and anticipation occurs. During action valuation, volitional action is initiated and action sustainment or acceleration is maintained. During outcome valuation, outcome achievement and consumption ensue, followed by evaluation based on learning via prediction error (PE) updating. Created with BioRender.com.
If a behavior were intrinsically motivated, the pre-decisional deliberation phase might be determined by biological drives, the need to restore homeostasis (Hebb, Reference Hebb1949; Hull, Reference Hull1943), or a state of incongruency resolution (Festinger, Reference Festinger1957; Kagan, Reference Kagan1972) as described by early theories of intrinsic motivation. In contemporary frameworks, novelty-seeking, exploration, or interest in learning or achievement would render subsequent actions as intrinsically motivated. If a behavior were extrinsically motivated, this pre-decisional deliberation phase would represent the cost-benefit analysis in economic models, prediction-error minimization in reinforcement learning, or effort-reward trade-off computation. Under incentive motivation and behaviorist theories, the pre-decisional deliberation phase would be triggered by conditioned stimuli making conscious deliberation unnecessary and inefficient.
A combination of intrinsic and extrinsic factors likely enters into the pre-decisional deliberation phase to guide motivated behavior (Fig. 1). Although intrinsic and extrinsic motivation are conceptually distinct processes, attempts to formally define them have identified several mechanisms by which they interact, leading to questions about their dissociability. Since they can interact in an additive or subtractive fashion, they may indeed be separate, independent drivers of behavior that are amalgamated during a pre-decisional deliberation phase of behavioral choice.
Measuring intrinsic motivation
Human behavior
Early attempts to quantify intrinsic motivation were largely based on behavioral observation, wherein intrinsic motivation was measured as free choice of an activity in the absence of an external stimulus or performance rating (Butler & Nisan, Reference Butler and Nisan1986; Daniel & Esser, Reference Daniel and Esser1980; Liu & Hou, Reference Liu and Hou2017). These studies also implemented self-report measures of participants' interest or enjoyment in an activity. While such measures do capture intrinsic motivation as inherent task enjoyment, they are limited by their qualitative and indirect nature, as well as by variability in participant insight. However, more objective measures are difficult to develop due to the inherently unobservable nature of intrinsic motivation.
Since spontaneous novelty-seeking and exploratory behavior reflect intrinsic motivation, one candidate objective measure may be the explore-exploit paradigm (Gittins & Jones, Reference Gittins and Jones1979; Robbins, Reference Robbins1952). In explore-exploit foraging tasks, participants must choose among various options and either exploit a previously reinforced choice or explore a novel alternative option. An individual's tendency to either explore an environment or exploit their pre-existing knowledge is influenced by perseverance (Von Culin et al., Reference Von Culin, Tsukayama and Duckworth2014), which acts as an indicator of confidence in the absence of immediate reward. Healthy adults flexibly employ a mix of exploitative and exploratory choices, where striatal and prefrontal dopamine signaling is proposed to drive exploration and exploitation, respectively (Badre, Doll, Long,, & Frank,, Reference Badre, Doll, Long and Frank2012; Daw, O'Doherty, Dayan, Seymour, & Dolan, Reference Daw, O'Doherty, Dayan, Seymour and Dolan2006; Mansouri, Koechlin, Rosa, & Buckley, Reference Mansouri, Koechlin, Rosa and Buckley2017). While these tasks capture one's willingness to trade-off exploratory v. exploitative behaviors, they do not measure free-choice exploratory behavior in the absence of explicit reinforcers, which would be most consistent with intrinsic motivation.
Paradigms that allow an individual to choose to explore an environment without extrinsic reinforcers, or to engage in a previously enjoyable or interesting activity, would more closely index intrinsic motivation. Additionally, outcomes that relate to achievement or autonomy, without socially rewarding feedback or monetary outcomes, would also putatively engage intrinsic motivation. Task parameters related to exploration, enjoyment, achievement, and autonomy can each be modulated and computationally modeled to determine their effects on free choice or behavioral activation vigor.
Current computational approaches depend on modeling decision-making, outcome learning, or action-outcome associations to drive our understanding of motivation. Traditional decision-making models often rely on softmax functions to compute values of available actions (Wilson & Collins, Reference Wilson and Collins2019), where action selection is based on the ‘policy’ of the best outcome. Computationally, an action selection process computes the probability of an action occurring in any state and the expected reward. A policy is developed based on the assumption that motivated actions are performed to increase the probability of rewards and decrease the probability of punishment. Yet, in everyday life, our actions can be motivated by an arbitrary cue that may signal an internal rewarding state. For example, a standard algorithm solving for motivated action assumes that all actions have equal probability, yet this discounts the unknown drivers and evaluators of internal rewards. Hence, they act as limiting factors to the applicability of decision-making models in studies of intrinsic motivation.
Neuroimaging
Functional neuroimaging [e.g., functional magnetic resonance imaging (fMRI), electroencephalography (EEG)] offers a measurement modality that may be particularly apt for the study of internally driven processes like intrinsic motivation. Research using fMRI has characterized the neural correlates of various internal processes that lack clear behavioral indicators (e.g. rumination, emotion regulation, pain perception; Zhou et al., Reference Zhou, Li, Zhao, Xu, Zheng, Fu and Becker2020; Wagner, N'Diaye, Ethofer, and Vuilleumier, Reference Wagner, N'Diaye, Ethofer and Vuilleumier2011), yet few studies have assessed the neural correlates of intrinsic motivation in humans, which likely reflects the limitations in its behavioral measurement. Studies have largely assessed intrinsic motivation via comparisons with neural responses to extrinsic reinforcers during fMRI, which can be correlated with self-reported intrinsic motivation (Bengtsson, Lau, & Passingham, Reference Bengtsson, Lau and Passingham2009; Chew et al., Reference Chew, Blain, Dolan and Rutledge2021; Linke et al., Reference Linke, Kirsch, King, Gass, Hennerici, Bongers and Wessa2010). Despite the relative paucity of neuroimaging studies that clearly separate intrinsic v. extrinsic motivation, existing work provides preliminary insight into the neural circuitry of intrinsic motivation.
First, extrinsic reinforcers have elicited amygdala, ACC, ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), and ventral striatal (VS) or Nac activity in healthy subjects that was associated with higher self-reported extrinsic motivation but lower self-reported intrinsic motivation (Linke et al., Reference Linke, Kirsch, King, Gass, Hennerici, Bongers and Wessa2010). This could suggest that intrinsic motivation relates to a lower sensitivity of these regions to extrinsic reinforcers, general deactivation of these regions, or that the dampening impact of extrinsic reinforcers on intrinsic motivation is subserved by these regions. Others report that intrinsic motivation (operationalized as the amount of free-time spent on a puzzle-task, which did not relate to task enjoyment, interest, or accuracy), was associated with deactivation in the amygdala, dorsal ACC, dorsomedial striatum, and insula during puzzle-task onset (Marsden et al., Reference Marsden, Ma, Deci, Ryan and Chiu2014). This is another piece of evidence linking neural deactivation to intrinsic motivation; however, since these tasks were not related to traditional ‘intrinsic motivators’ like task enjoyment, findings may relate to boredom-reduction behavior that might be more related to punishment avoidance rather than intrinsic motivation per se.
Bengtsson et al. (Reference Bengtsson, Lau and Passingham2009) operationalized intrinsic motivation as task-performance with and without explicit experimental observation during fMRI scanning, which boosted self-reported intrinsic motivation. The authors found greater neural activation of ACC, OFC, and lateral prefrontal cortex during task-performance errors when participants were observed (Bengtsson et al., Reference Bengtsson, Lau and Passingham2009). While implicating a similar network of brain regions as prior studies, these findings cannot be divorced from error-related neural activation modulated by task salience (e.g. observed v. not).
In contrast, Murayama et al. (Reference Murayama, Matsumoto, Izuma and Matsumoto2010) provide a more optimal operationalization of intrinsic motivation, in which participants performed a task that was previously rated as inherently interesting, and successful task performance served as the intrinsic reward. During fMRI scanning, feedback for both extrinsic (monetary feedback) and intrinsic (accuracy feedback) rewards elicited VS activation. Participants then had the option to perform the same task without feedback, and intrinsic motivation was operationalized as time spent on the second version of the task. During the second session, VS activation was only diminished for extrinsic rewards, which could reflect reduced VS habituation to intrinsic rewards (Murayama et al., Reference Murayama, Matsumoto, Izuma and Matsumoto2010, Reference Murayama, Matsumoto, Izuma, Sugiura, Ryan, Deci and Matsumoto2015). Additionally, greater reductions in neural responses to extrinsic reinforcers were related to lower intrinsic motivation (i.e. task engagement time outside of the scanner), suggesting that neural habituation to extrinsic reinforcers may relate to lower intrinsic motivation. A recent computational neuroimaging study modeled intrinsic rewards as successful spatial-motor task performance without experienced errors, which was divorced from learning (Chew et al., Reference Chew, Blain, Dolan and Rutledge2021). This modeling of intrinsic rewards was akin to the accuracy feedback operationalization of Murayama et al. (Reference Murayama, Matsumoto, Izuma and Matsumoto2010). Both extrinsic (monetary) reward and intrinsic performance-based rewards (successful task completion) recruited vmPFC activation, which related to subjective happiness (Chew et al., Reference Chew, Blain, Dolan and Rutledge2021). Although limited in their ability to dissociate activation from task performance per se and explicit feedback related to achievement, these studies are the closest examples of objective measures of intrinsic motivation, and they suggest that putative reward-processing regions (VS, vmPFC) encode intrinsic rewards.
Complementary studies have examined how curiosity, or the intrinsic motivation to learn, modulates neural responses and influences memory recall (Gruber, Gelman, & Ranganath, Reference Gruber, Gelman and Ranganath2014; Kang et al., Reference Kang, Hsu, Krajbich, Loewenstein, McClure, Wang and Camerer2009). High-curiosity states augment midbrain and v. activity (Gruber et al., Reference Gruber, Gelman and Ranganath2014), as well as bilateral caudate (Kang et al., Reference Kang, Hsu, Krajbich, Loewenstein, McClure, Wang and Camerer2009) and anterior insula (Lee & Reeve, Reference Lee and Reeve2017) responses, which may improve learning and memory. As these paradigms index intrinsic motivation independently from a rewarding outcome, they perhaps provide the strongest support for partially overlapping circuits of extrinsic and intrinsic motivation.
Dopamine
The brain's dopamine system supports a range of appetitive and aversive motivational processes, including behavioral activation, exertion of effort, and sustained task engagement (Diederen & Fletcher, Reference Diederen and Fletcher2020; Salamone, Yohn, López-Cruz, San Miguel, & Correa, Reference Salamone, Yohn, López-Cruz, San Miguel and Correa2016). The mesolimbic pathway, projecting from the ventral tegmental area (VTA) to limbic regions, including the Nac, amygdala, and hippocampus, facilitates reinforcement and associative learning by acting as a ‘Go’ signal for foraging or exploration (Huang, Lv, & Wu, Reference Huang, Lv and Wu2016). Although it has long been known that dopamine transmission subserves motivational processes, some evidence suggests that it is particularly important for intrinsic motivation. For example, mesolimbic dopamine contributes to exploration for the sake of interest (DeYoung, Reference DeYoung2013; Panksepp & Moskal, Reference Panksepp, Moskal and Elliot2008), and novel and unexpected stimuli elicit phasic dopamine spikes in rodents (Fiorillo, Reference Fiorillo2003; Hooks & Kalivas, Reference Hooks and Kalivas1994; Schultz, Reference Schultz1998). In patients with depression, deep-brain stimulation of dopaminergic brain regions including the Nac (Schlaepfer et al., Reference Schlaepfer, Cohen, Frick, Kosel, Brodesser, Axmacher and Sturm2007) and the mesolimbic dopamine projections from the VTA (Fenoy et al., Reference Fenoy, Schulz, Selvaraj, Burrows, Zunta-Soares, Durkin and Soares2018) increased subjective interest in, and motivational energy for, previously enjoyable activities (Schlaepfer et al., Reference Schlaepfer, Cohen, Frick, Kosel, Brodesser, Axmacher and Sturm2007). Dopamine has also been associated with intrinsically motivated flow states (de Manzano et al., Reference de Manzano, Cervenka, Jucaite, Hellenäs, Farde and Ullén2013; Gyurkovics et al., Reference Gyurkovics, Kotyuk, Katonai, Horvath, Vereczkei and Szekely2016).
However, since VTA dopamine spiking is reduced for expected events (Schultz, Reference Schultz1998), it may not be a strong candidate neural mechanism for intrinsic motivation, which can occur for predictable activities. Efforts to reconcile the role of dopamine in learning and motivation suggest that while phasic cell firing signals RPEs (Kim et al., Reference Kim, Malik, Mikhael, Bech, Tsutsui-Kimura, Sun and Uchida2020), phasic dopamine release and local modulation in key regions, such as the VS/NAc, relates to approach motivation (Berke, Reference Berke2018; Mohebi et al., Reference Mohebi, Pettibone, Hamid, Wong, Vinson, Patriarchi and Berke2019). Indeed, while VTA dopamine cell firing occurs during reward prediction, only NAc dopamine release covaries with reward availability and ramps up during approach and consumption of reward (Mohebi et al., Reference Mohebi, Pettibone, Hamid, Wong, Vinson, Patriarchi and Berke2019). Moreover, increasing dopamine in rodents increases their willingness to exert effort, and this has since been replicated across species, including via pharmacological manipulation in humans (Salamone, Correa, Farrar, & Mingote, Reference Salamone, Correa, Farrar and Mingote2007; Treadway & Zald, Reference Treadway and Zald2011). This suggests that, while VTA dopamine spiking underpins reward prediction and learning, it is local NAc dopamine release that encodes motivational drive.
Opioids, norepinephrine, and related neurotransmitter systems
Though a comprehensive account of the neurotransmitter systems subserving motivated behavior is beyond the scope of this review, we note that endogenous opioid and cannabinoid systems may uniquely modulate intrinsically motivated behavior. For example, mu- and delta-opioid receptor activation underlies the pleasurable effects of opioid and non-opioid drugs of abuse (Berrendero, Robledo, Trigo, Martín-García, & Maldonado, Reference Berrendero, Robledo, Trigo, Martín-García and Maldonado2010; Trigo, Martin-García, Berrendero, Robledo, & Maldonado, Reference Trigo, Martin-García, Berrendero, Robledo and Maldonado2010), as well as primary reinforcers (Hsu et al., Reference Hsu, Sanford, Meyers, Love, Hazlett, Wang and Zubieta2013; Kelley & Berridge, Reference Kelley and Berridge2002). Activation of mu-opioid receptors has also been shown to mediate motivational states following delta-9-tetrahydrocannabinol (THC) administration in rodents (Ghozland et al., Reference Ghozland, Matthes, Simonin, Filliol, Kieffer and Maldonado2002), likely via interactions with the mesolimbic dopamine system. Further evidence implicates antidepressant effects of endogenous opioids in both animals and humans (Peciña et al., Reference Peciña, Karp, Mathew, Todtenkopf, Ehrich and Zubieta2018), which many partly reflect improved intrinsic motivation (e.g. time mice spent swimming during the forced swim test; Kastin, Scollan, Ehrensing, Schally, and Coy, Reference Kastin, Scollan, Ehrensing, Schally and Coy1978). Additionally, the endocannabinoid system interacts with both endogenous opioid and dopaminergic systems to influence intrinsic motivation, such as social play (Trezza et al., Reference Trezza, Damsteegt, Manduca, Petrosino, Van Kerkhof, Pasterkamp and Vanderschuren2012; Trezza & Vanderschuren, Reference Trezza and Vanderschuren2008), and voluntary exercise, in rodents (Dubreucq, Koehl, Abrous, Marsicano, & Chaouloff, Reference Dubreucq, Koehl, Abrous, Marsicano and Chaouloff2010). Since these systems have been primarily examined in animal models, pharmacological manipulation in humans would be an important next step in delineating the contribution of opioid and endocannabinoid systems to intrinsic v. extrinsic motivation.
Intrinsic motivation and psychiatry: focus on anhedonia
Problems with motivation are observed across many neuropsychiatric disorders, and these often correspond to distinct symptoms (Table 1). This section focuses on anhedonia, a reduced ability to experience pleasure (Ribot, Reference Ribot1986), as a prevalent clinical manifestation of deficient intrinsic and extrinsic motivation.
Table 1. Explicit studies of ‘intrinsic motivation’ in neuropsychiatric disorders
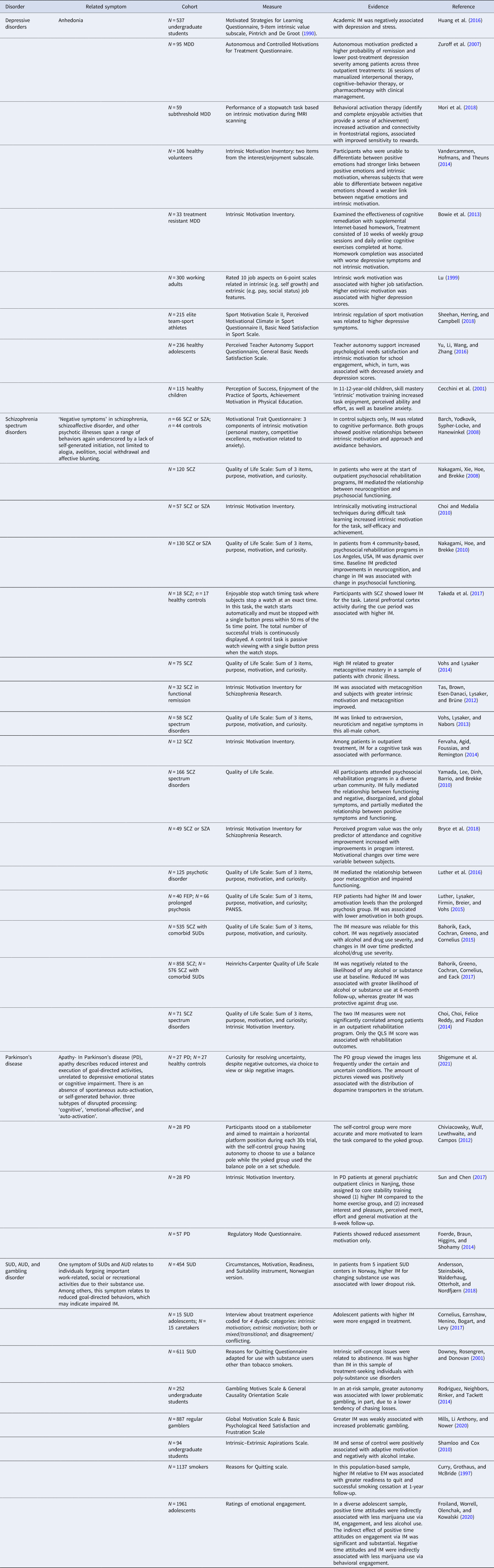
Note: Cohort abbreviations: AUD, alcohol use disorder; FEP, first-episode psychosis; MDD, major depressive disorder; PD, Parkinson's disease; SCZ, schizophrenia; SUDs, substance use disorders; SZA, schizoaffective disorder. Evidence abbreviations: EM, extrinsic motivation; IM, intrinsic motivation.
In the Diagnostic and Statistical Model of Mental Disorders, 5th edition (DSM-5), anhedonia serves as one of two cardinal symptoms of depressive disorders, where it is defined as the ‘loss of interest or pleasure in all, or almost all, activities’, (American Psychiatric Association, 2013). The second cardinal symptom relates to persistent depressed mood. Approximately one-third of individuals with depression report clinically significant anhedonia (Pelizza & Ferrari, Reference Pelizza and Ferrari2009), and these individuals are at-risk for poorer treatment outcomes, including nonresponse, relapse, and increased suicidality, relative to their non-anhedonic peers (Morris, Bylsma, & Rottenberg, Reference Morris, Bylsma and Rottenberg2009; Nierenberg et al., Reference Nierenberg, Keefe, Leslie, Alpert, Pava, Worthington and Fava1999).
Anhedonia remains an important clinical target that, by definition, implicates perturbations in intrinsically-motivated behavior, yet most empirical studies of anhedonia and motivation have investigated their relationship using extrinsic reinforcers. Findings broadly support theories of reward dysfunction in depression (reviewed by Sescousse, Caldú, Segura, and Dreher, Reference Sescousse, Caldú, Segura and Dreher2013; Roiser & Husain, 2018; Borsini, Wallis, Zunszain, Pariante, and Kempton, Reference Borsini, Wallis, Zunszain, Pariante and Kempton2020), where anhedonia has been associated with a reduced bias toward a monetary reward in individuals with depression (Liu et al., Reference Liu, Chan, Wang, Huang, Cheung, Gong and Gollan2011) and their first-degree relatives (Liu et al., Reference Liu, Roiser, Wang, Zhu, Huang, Neumann and Chan2016). Children who are at-risk for depression show reduced VS and anterior insula responses to monetary gains, implicating blunted reward sensitivity as an antecedent to anhedonia (Luking, Pagliaccio, Luby, & Barch, Reference Luking, Pagliaccio, Luby and Barch2016). Moreover, vmPFC responses during unexpected reward receipt may indirectly relate to anhedonia in depressed patients by modulating task motivation (Segarra et al., Reference Segarra, Metastasio, Ziauddeen, Spencer, Reinders, Dudas and Murray2016). Interestingly, reward sensitivity disturbances in depression might not extend to aberrant reward learning (Huys, Pizzagalli, Bogdan, & Dayan, Reference Huys, Pizzagalli, Bogdan and Dayan2013) where adults with moderate depression show intact VS RPE-signaling during probabilistic learning (Rutledge et al., Reference Rutledge, Moutoussis, Smittenaar, Zeidman, Taylor, Hrynkiewicz and Dolan2017). Nevertheless, there have been suggestions that perturbations in domains more related to intrinsic motivation, such as model-based future planning or effort initiation and invigoration, may be key in underlying anhedonia (Berwian et al., Reference Berwian, Wenzel, Collins, Seifritz, Stephan, Walter and Huys2020; Cooper, Arulpragasam, & Treadway, Reference Cooper, Arulpragasam and Treadway2018; Rutledge et al., Reference Rutledge, Moutoussis, Smittenaar, Zeidman, Taylor, Hrynkiewicz and Dolan2017). Finally, affect can also alter both the valence and evaluation of an activity, which can, in turn, modulate the likelihood of selecting a more inherently interesting task (Isen & Reeve, Reference Isen and Reeve2006). Anhedonic individuals have more pessimistic likelihood estimates and reduced positive affective forecasts relative to controls while also demonstrating greater reliance on negative emotion during future-oriented cognition (Marroquín & Nolen-Hoeksema, Reference Marroquín and Nolen-Hoeksema2015).
While few studies have implemented objective measures of intrinsic motivation in studying anhedonia, recent work links this symptom with difficulties with representations of future states during early stages of motivated behavior (Moutoussis et al., Reference Moutoussis, Rutledge, Prabhu, Hrynkiewicz, Lam, Ousdal, Guitart-Masip, Fonagy and Dolan2018). Since intrinsic motivation is driven more by proactive factors as opposed to the more reactive domain of extrinsic motivation, parsing future-oriented decision-making might provide novel insights not only into mechanisms of intrinsic motivation but also anhedonia. When considering the pre-decisional deliberation phase of motivated action (Fig. 1), the representation of a future state may be critical for distinguishing intrinsic v. extrinsic motivation. For example, disrupted representations of intrinsic reinforcers (e.g. autonomy, achievement, task enjoyment, novelty seeking), energy expenditure (Treadway, Cooper, & Miller, Reference Treadway, Cooper and Miller2019; Winch, Moberly, & Dickson, Reference Winch, Moberly and Dickson2014), or fatigue (Müller, Klein-Flügge, Manohar, Husain, & Apps, Reference Müller, Klein-Flügge, Manohar, Husain and Apps2021) might disrupt choice deliberation and interrupt ensuing stages of motivation. This could critically determine the capacity for self-generated, intrinsically-motivated actions (Husain & Roiser, Reference Husain and Roiser2018). However, relatively few studies have examined this distinction. One study developed a cognitive task that aimed to capture separate measures of self-generated (intrinsic) v. externally generated (extrinsic) motivation during the option-generation phase (Morris et al., Reference Morris, Norbury, Smith, Harrison, Voon and Murrough2020). This distinction linked self-generated option generation (intrinsic motivation) to anhedonia symptoms in healthy adults (Morris et al., Reference Morris, Norbury, Smith, Harrison, Voon and Murrough2020). However, this task still relies on extrinsic rewards, and there is a need for improved tasks that index both behavioral and neural correlates of intrinsic drivers of motivated behavior.
Summary and future directions
In this review, we summarize how intrinsic motivation has been conceptualized, measured, and related to neural function to elucidate its role in psychopathology. In contrast to extrinsic motivation, which has been rapidly incorporated into prominent cognitive, computational, and neurobiological models of human behavior, knowledge of intrinsic motivation remains limited due to evolving conceptualizations, imprecise measurement, and incomplete characterization of its biological correlates. We identify three potential areas of interest for future research.
First, additional objective measures of intrinsically motivation should be developed. This remains challenging experimentally since even the closest approximations of intrinsic motivation (Murayama et al., Reference Murayama, Matsumoto, Izuma and Matsumoto2010; Rutledge et al., Reference Rutledge, Moutoussis, Smittenaar, Zeidman, Taylor, Hrynkiewicz and Dolan2017) define the construct relative to extrinsic motivation, and other paradigms (e.g. exploration/exploitation tasks) rely on the presence of extrinsic reinforcers. Rather than defining motivated behavior as intrinsic or extrinsic, a more tractable approach might be to consider separate drivers of behavior that can be intrinsic or extrinsic. Future paradigms could index intrinsic motivation by characterizing the effects of intrinsic v. extrinsic reinforcers on motivation for an activity that is enjoyable. Such a design would enable more complex modeling of the effects of distinct reinforcers, and interactions between them, on motivated behavior, which would resolve inconsistencies surrounding the impact of extrinsic reinforcers on intrinsic motivation. For example, monetary incentives might reduce motivation only when a perceived agency is low, or when task enjoyment is high. These interactions might explain paradoxical observations like the undermining effect.
Second, computational models are needed to characterize intrinsic motivation. Computational models of motivation have been successfully implemented in studies of extrinsic motivation, yet few are appropriate for intrinsic motivation due to a focus on action-outcome associations. However, if the intrinsic reward were operationalized as a measurable outcome (e.g. completion of an enjoyable task), reinforcement-learning models could estimate how intrinsic reward value is represented. Advancements in the computational area could significantly improve understanding of the latent processes underlying (ab)normal decision-making, thereby identifying novel therapeutic targets.
Third, although evidence supports the bifurcation of intrinsic and extrinsic motivation at the psychological level, findings at the neural level are more equivocal. Given the overarching role of the mesolimbic dopamine system in learning, reward value estimation, and exploratory behavior, it is perhaps unsurprising that current evidence supports largely overlapping neural circuits for intrinsically and extrinsically motivated behavior. One potential avenue involves targeted pharmacological manipulations or neuromodulation of cortico-limbic circuits to determine if intrinsically and extrinsically motivated behaviors can be systematically modulated in humans. By elucidating the neural circuits of distinct motivational processes and their associations with specific symptom profiles, this approach would improve targeted interventions for highly heterogenous and debilitating disorders like depression.
Financial support
All authors report no financial disclosures. This work was supported by the National Institute of Mental Health (LSM, grant number K01MH120433) and the National Institute on Drug Abuse (MLW, T32DA022975).