Obesity has become a severe health challenge worldwide and contributes to a decline in both quality of life and life expectancy(Reference Bluher1). With the changes in modern lifestyles, factors such as unhealthy eating habits and lack of exercise have led to a rapid increase in obesity(Reference Caballero2). Previous studies have shown that the obesity epidemic is closely associated with the prevalence and severity of non-alcoholic fatty liver disease(Reference Fan, Kim and Wong3,Reference Hagström, Simon and Roelstraete4) The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing at a rate of 1 % per year, afflicting 30 % of the worldwide population(Reference Henry, Paik and Younossi5).
Several studies have shown that obesity is positively associated with NAFLD(6–Reference Machado and Cortez-Pinto8). Although NAFLD is typically identified in individuals with obesity, up to 7–20 % are characterised as having lean non-alcoholic steatohepatitis(Reference Zou, Yeo and Nguyen9). The relationship between obesity and NAFLD is also influenced by a combination of factors, such as genetics, metabolic abnormalities, lifestyle and other potential comorbidities(Reference Machado and Cortez-Pinto8).
The relationships between obesity and other liver diseases are still complex and diverse. A positive correlation between obesity and the aggravation of viral hepatitis has been reported(Reference Wang, Yan and Wang10,Reference Nickbakhsh, Leitch and Smith11) . In contrast, several studies have suggested an inverse correlation between obesity and virus activity and the success rate of antiviral treatment(Reference Wu, Koch and Bachmann12–Reference Chiang and Huang14). Additionally, the relationship between obesity and autoimmune liver disease is ambiguous(Reference Xu, Wu and Feng15–Reference Floreani, Cazzagon and Franceschet17). The results from prior studies are inconsistent, and these differences may be attributed to limited sample sizes, residual confounding and reverse causation bias. These studies have explored correlation rather than causal relationship and lacked evidence from randomised controlled trials. It remains controversial whether causal effects exist between obesity and the development of liver disease.
As genetic variants are fixed at conception and not influenced by disease status, Mendelian randomisation (MR) has the potential to use genetic variants as instrumental variables to evaluate the causal relationship between exposure and lifelong differences in disease outcomes(Reference Sekula, Del Greco and Pattaro18). Public large-scale genome-wide association study (GWAS) data can be used to explore the effects of life-long perturbations in risk factors and rare diseases, which require large sample sizes and long-term follow-up for sufficient occurrence of endpoints in a randomised controlled trial(Reference Larsson, Butterworth and Burgess19).
BMI, as a commonly used index to measure the degree of obesity(6), mainly considers the relationship between height and weight and cannot fully capture individual differences in visceral fat, which is closely related to liver disease and function(Reference Cen, Fan and Ding20,Reference Khan, Chong and Le21) . The waist-to-hip ratio adjusted for BMI (WHRadjBMI) is a surrogate for abdominal fat and is less influenced by muscle and bone mass than BMI(Reference Hsuan, Lin and Lee22). The waist–hip ratio may be superior to BMI in predicting liver-related outcomes(Reference Åberg, Färkkilä and Salomaa23,Reference Pang, Kartsonaki and Guo24) .
This study used both BMI and WHRadjBMI, as instrumental variables to explore the causal relationships between obesity and liver disease and function. Through comprehensive analysis and integration of existing research findings, we hope to deepen the understanding of obesity, and in the occurrence and development of liver diseases, and provide more targeted strategies for the prevention, diagnosis and treatment of related liver diseases, thereby improving public health.
Method
Study design and selection of SNP
The two-sample MR method was used to explore the causal relationships between exposures (BMI and WHRadjBMI) and outcomes (liver diseases and liver function) (Fig. 1). Suitable SNP should meet three criteria. First, SNP associated with BMI at a significance threshold of P < 5 × 10–8 were selected. Second, the independence among the selected SNP was assessed by pairwise-linkage disequilibrium. SNP were clumped at r2 < 0·01 via a 10 000 kb window. Third, we calculated the F-statistic to assess the strength of individual SNP. SNP with F-statistics >10 are considered sufficient to eliminate potential bias(Reference Pierce, Ahsan and Vanderweele25).
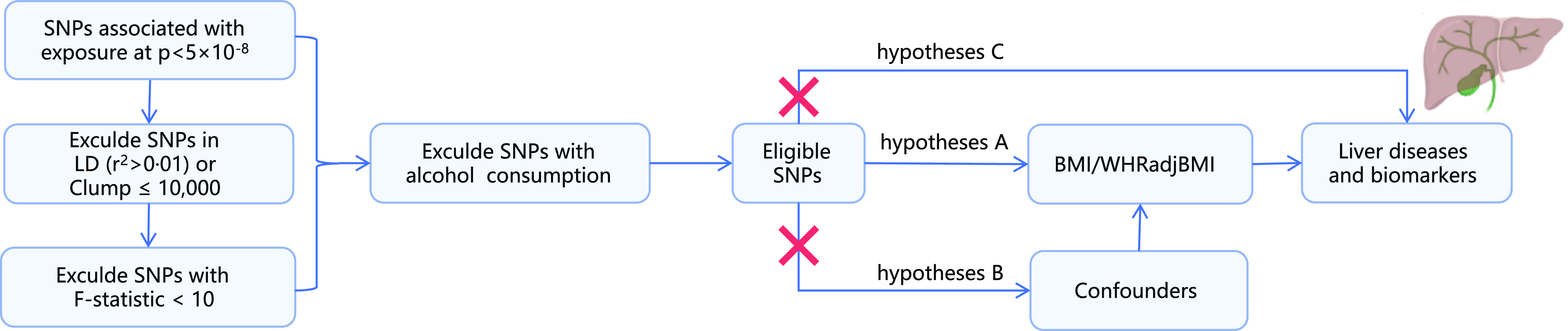
Fig. 1. Study design overview. The MR study should have three core three hypotheses: (1) genetic variants (SNP) are strongly related to the exposure; (2) SNP are independent of known confounders and (3) SNP cannot directly affect the outcome but only through the exposure of interest. LD, linkage disequilibrium; SNP, single-nucleotide polymorphisms; WHRadjBMI: waist-to-hip ratio adjusted for BMI.
Data sources
GWAS summary statistics for BMI (n 461 460) were obtained from the UK Biobank study, which assessed the relationships between BMI and SNP. WHRadjBMI data (SNP = 4 238 887) were obtained from an open GWAS dataset. Eight liver disease-related datasets and nine liver biomarker-related datasets were obtained from the open GWAS database(26). Liver disease outcomes were NAFLD, fibrosis and cirrhosis of liver, autoimmune hepatitis, viral hepatitis, hepatic failure, liver cell carcinoma, secondary malignant neoplasm of liver and primary biliary cholangitis. Liver function outcomes were alanine aminotransferase, aspartate aminotransferase, bilirubin, blood plate count, HDL, LDL, very LDL, apolipoprotein B and fasting blood insulin.
Detailed information on GWAS of liver diseases and related biomarkers, including the number of participants and adjusted covariates, is presented in online Supplementary Table 1 and online Supplementary Table 2, respectively.
Multivariable Mendelian randomisation
Multivariable MR can be applied for multiple genetic variants and independent exposures in an instrumental-variable analysis to determine the direct causal effect of each risk factor included in the model(Reference Burgess and Thompson27). Multivariate MR was used to reveal the relationships between multiple exposure variables (BMI and WHRadjBMI) and liver diseases.
Mendelian randomisation analysis
The inverse-variance weighted (IVW) method under a random-effects model was used for the primary analysis. Sensitivity analyses, including the MR pleiotropy residual sum and outlier test (MR-PRESSO)(Reference Kintu, Soremekun and Kamiza28) and the MR-Egger method(Reference Bowden, Davey Smith and Burgess29), were conducted to check if the associations were consistent and to adjust for any horizontal pleiotropy.
If significant heterogeneity was detected, weighted median analysis was used to estimate the MR effect size. If significant pleiotropy was detected, MR-Egger was used to estimate the MR effect size. The Cochrane’s Q-value can indicate heterogeneity among selected instrumental variables, and the P-value should be greater than 0·05(Reference Brion, Shakhbazov and Visscher30). Additionally, leave-one-out sensitivity analysis was used to check whether the overall estimates were affected by an individual SNP. The funnel plot was used to focus on whether the points on the left and right sides of the IVW line were roughly symmetric. These SNP that affected overall estimates were removed, and effect estimates were recalculated. To account for multiple comparisons of in BMI with liver disease outcomes, Bonferroni-corrected thresholds of P < 0·05 were used for liver disease outcomes. All tests were two-sided and performed via the TwoSampleMR (0·5·5), MR-PRESSO (1·0), multivariable Mendelian randomisation (0·3) packages in the R software (version 4.0.3).
Results
SNP validation
In summary, 458 SNP in the BMI database exhibited significant genome-wide differences, and all F-statistics were greater than 10. Alcohol is a major cause of liver disease(Reference Devarbhavi, Asrani and Arab31). SNP of alcohol were the confounders of the studies. In contrast, thirty-one of the 458 traits associated with alcohol consumption were excluded for traits with alcohol consumption. Finally, 427 SNP were included. 218 SNP in the WHRadjBMI database fulfilled the inclusion criteria and did not meet the exclusion criteria (Fig. 1).
Univariable Mendelian randomisation analysis of the associations between BMI or WHRadjBMI and the risk of liver disease
The IVW analysis revealed that the genetically predicted BMI was associated with four of the eight liver diseases, including non-alcoholic fatty liver disease (OR = 2·12; 95 % CI, 1·49, 3·02; P = 2·9 × 10–5), liver cell carcinoma (OR = 1·00; 95 % CI, 1·000, 1·001; P = 0·006), fibrosis and cirrhosis of the liver (OR = 1·78; 95 % CI, 1·18, 2·68; P = 0·006) and autoimmune hepatitis (OR = 1·47; 95 % CI, 1·055, 2·041; P = 0·023). BMI was positively associated with liver cell carcinoma (P = 5·6 × 10–3). However, the OR is 1·000 (1·000–1·001), which means that morbidity in obese people and non-obese people is almost equal. Therefore, the positive correlation is meaningless. In contrast, no associations were observed for hepatic failure, viral hepatitis, secondary malignant neoplasm of the liver or primary biliary cholangitis. No evidence of directional heterogeneity or pleiotropy was detected (Table 1).
Table 1. Associations between genetically predicted obesity and liver diseases in sensitivity analyses using the weighted-median and MR-Egger methods (OR and 95 % CI)

HCC: hepatocellular carcinoma; NAFLD: non-alcoholic fatty liver disease; PBC: Primary biliary cholangitis; IVW: inverse-variance weighted; WHRadjBMI: waist-to-hip ratio adjusted for BMI.
WHRadjBMI was associated with three of the eight liver diseases, including non-alcoholic fatty liver disease (OR = 1·89; 95 % CI, 1·31, 2·7; P = 7·5 × 10–4), fibrosis and cirrhosis of liver (OR = 2·33; 95 % CI, 1·54, 3·52; P = 5·7 × 10–5) and autoimmune hepatitis (OR = 1·84; 95 % CI, 1·26, 2·68; P = 5·7 × 10–5) (Table 1).
The leave-one-out sensitivity analysis and the funnel plot of the association between BMI, WHRadjBMI and liver diseases are shown in online Supplementary Fig. S1–S2 and Fig. S5–S6, respectively.
Univariable Mendelian randomisation analysis of the associations between BMI or WHRadjBMI and the risk of liver-function biomarkers
The IVW analysis revealed that a genetically predicted BMI increase was positively associated with ApoB (OR = 1·19; 95 % CI, 1·11, 1·28; P = 1·4 × 10–6). Because significant heterogeneity was detected in some exposures, weighted median analysis was used to estimate the MR effect size. The weighted median analysis suggested that BMI had positive causal relationships with higher serum levels of very LDL (OR = 1·06; 95 % CI, 1·01, 1·11; P = 0·01), platelet count (OR = 0·953; 95 % CI, 0·929, 0·976; P = 1·2 × 10–4) and fasting blood insulin (OR = 1·15; 95 % CI, 1·09, 1·23; P < 0·001). Genetically predicted BMI increase was a negative association with HDL level (OR = 0·81; 95 % CI, 0·75, 0·87; P = 4·1 × 10–8). The IVW and MR-Egger analyses revealed similar estimates but of low precision. No evidence of directional pleiotropy was detected in the majority of biomarkers except for very LDL (Table 2).
Table 2. Associations between genetically predicted obesity and liver biomarkers in sensitivity analyses using the weighted-median and MR-Egger methods (OR and 95 % CI)
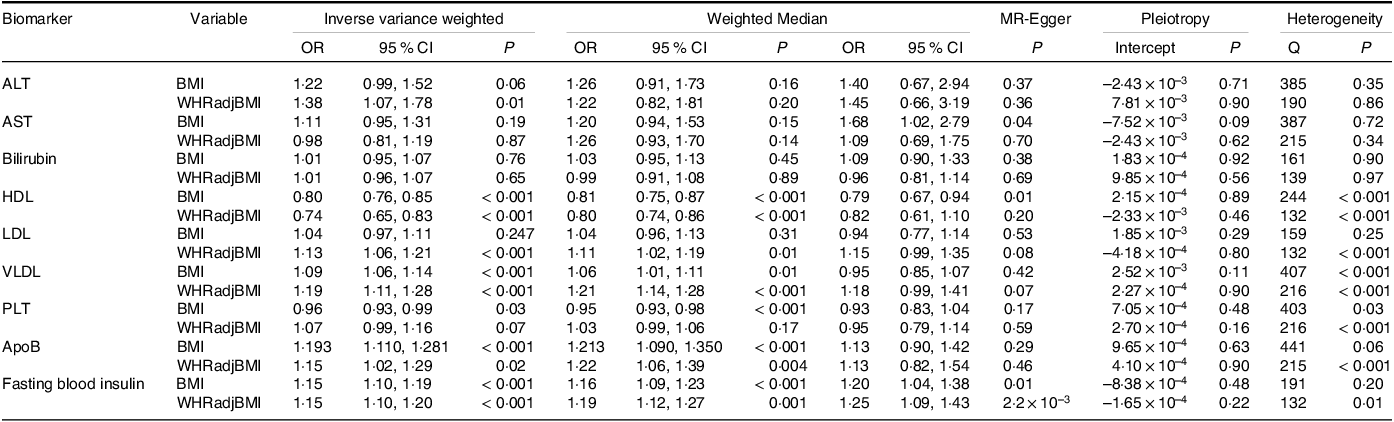
ALT: alanine aminotransferase; AST: aspartate aminotransferase; Apo B: apolipoprotein B; MVMR: multivariable Mendelian randomisation; IVW: inverse-variance weighted; VLDL: very LDL; WHRadjBMI: waist-to-hip ratio adjusted for BMI; PLT: platelet count.
The IVW analysis revealed that genetically predicted higher WHRadjBMI was associated with alanine aminotransferase (OR = 1·38; 95 % CI, 1·07, 1·78; P = 0·01). Weighted median analysis was used to estimate the MR effect size when heterogeneity was detected in exposures. The weighted median analysis implied that a genetically predicted higher WHRadjBMI was associated with higher serum level of few biomarkers, including LDL (OR = 1·10; 95 % CI, 1·02, 1·20; P = 0·01), very LDL (OR = 1·21; 95 % CI, 1·44, 1·29; P = 0·01), ApoB (OR = 1·22; 95 % CI, 1·06, 1·39; P = 0·01) and fasting blood insulin (OR = 1·19; 95 % CI, 1·12, 1·27; P = 0·001). A higher WHRadjBMI was also associated with lower serum levels of some biomarkers, including HDL (OR = 0·80; 95 % CI, 0·74, 0·86; P = 4·0 × 10–9) and no evidence of directional pleiotropy was detected (Table 2).
The leave-one-out sensitivity analysis and the funnel plot of the association between BMI, WHRadjBMI and liver-function biomarkers are shown in online Supplementary Fig. S3–S4 and Figs. S7–S8 respectively.
Multivariable Mendelian randomisation: direct causal effects of obesity on liver disease and function
As shown in Figs. 2 and 3, genetically high BMI and WHRadjBMI were both associated with increased risk of NAFLD, liver fibrosis and several liver-function biomarkers (alanine aminotransferase, HDL, fasting blood insulin). Moreover, a genetically high WHR was associated with increased LDL, very LDL and ApoB when BMI was accounted for. A genetically high BMI was associated with a decreased platelet count when WHRadjBMI was accounted for. However, they were no longer related to liver cell carcinoma or autoimmune hepatitis.
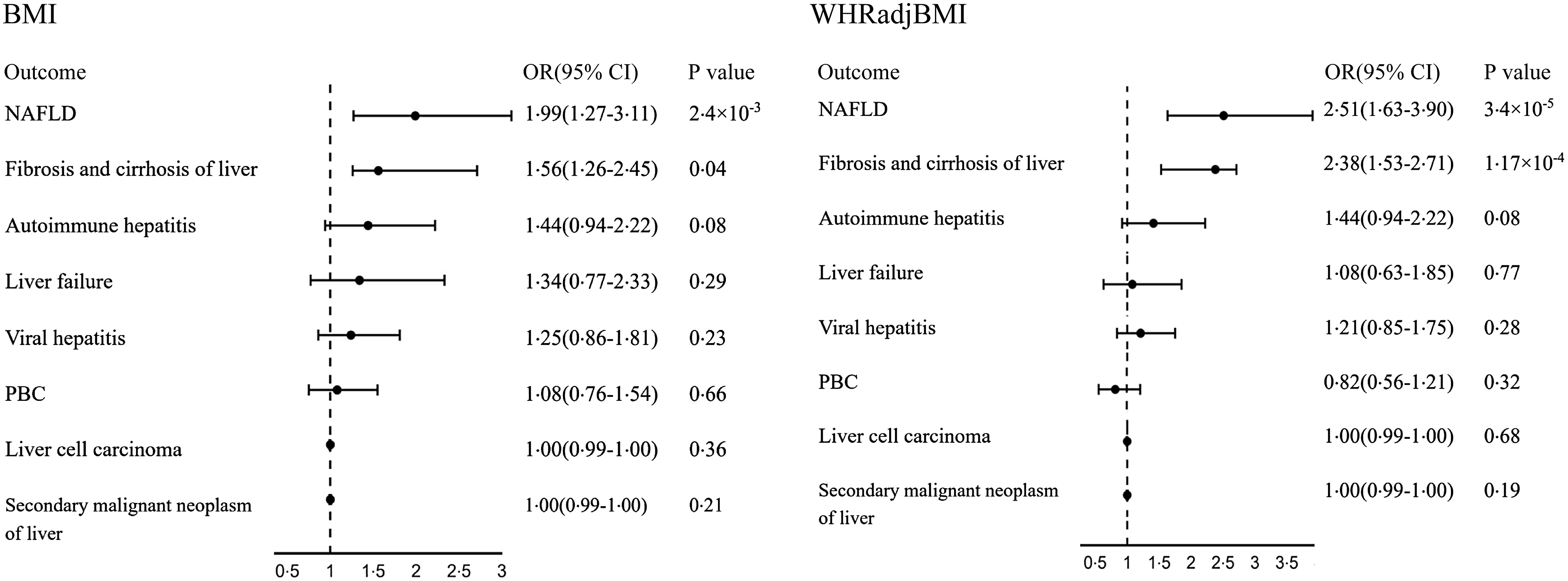
Fig. 2. Multivariable MR estimated the direct causal effects of BMI and WHRadjBMI on liver disease while accounting for each other. WHRadjBMI: waist-to-hip ratio adjusted for BMI; NAFLD: non-alcoholic fatty liver disease; PBC: primary biliary cholangitis.
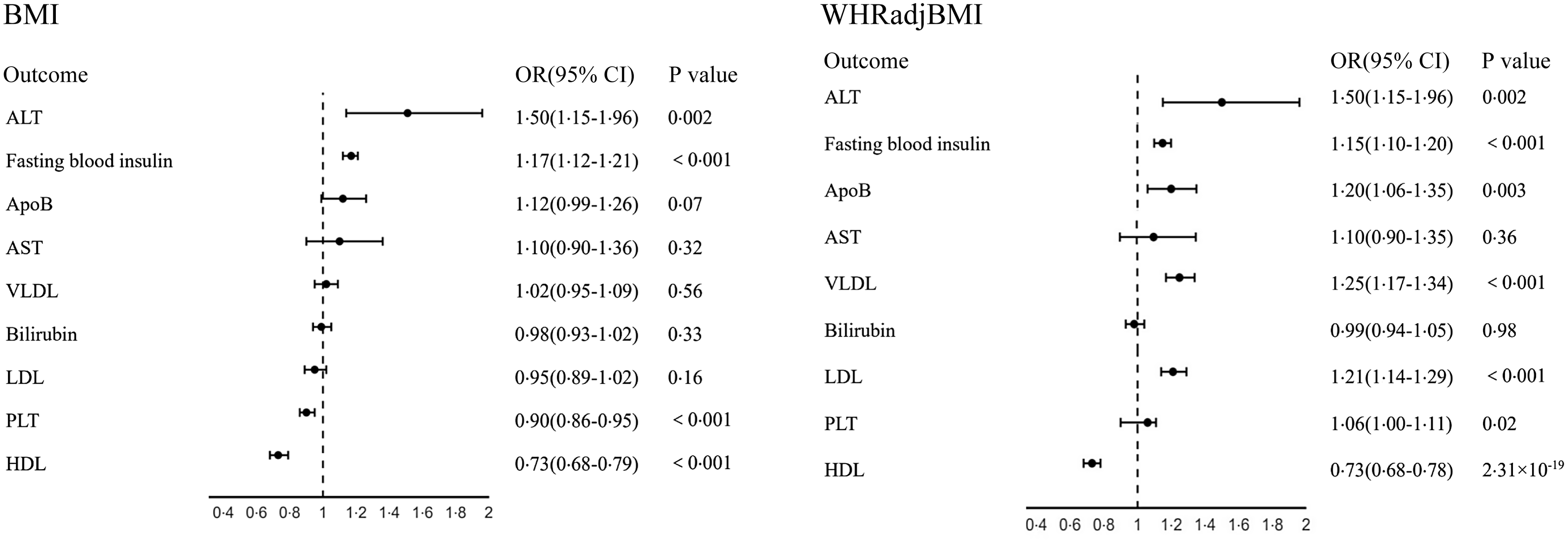
Fig. 3. Multivariable MR estimated the direct causal effects of BMI and WHRadjBMI on liver function, while accounting for each other. ALT: alanine aminotransferase; AST: aspartate aminotransferase; Apo B: apolipoprotein B; VLDL: very LD:; WHRadjBMI: waist-to-hip ratio adjusted for BMI; PLT: platelet count.
Discussion
Using a genetic approach, our study suggested a causal relationship between obesity and some liver diseases, as well as aberrant liver function and lipid metabolism markers. According to univariable and multivariable MR analyses, higher BMI and WHRadjBMI were positively associated with the increased risk of NAFLD and liver fibrosis. A genetically higher BMI and WHRadjBMI were associated with impaired liver and lipid metabolism function.
Our study suggested that total and abdominal fat are both risk factors for NAFLD and liver cirrhosis, which is consistent with several studies(Reference Kuang, Sheng and Hu7,Reference Younossi, Stepanova and Ong32,Reference Feng, Wang and Zhao33) . Unlike observational studies, MR analysis can reduce bias from confounding factors and reveal a causal relationship between obesity and liver disease. The waist-to-hip ratio adjusted for the BMI can measure abdominal obesity(Reference Hansen, Sobreira and Weber34). A high WHRadjBMI implies a high burden of abdominal fat and is associated with fibrosis severity in obese and non-obese NAFLD patients(Reference Jang and Kim35,Reference Li, Guo and Zhang36) . Our results suggested that the WHR is a marker for diagnosing NAFLD and fibrosis patients. Without active intervention, NAFLD may even progress to non-alcoholic steatohepatitis, which has been the fastest-growing and second-leading indication for liver transplantation in the past 20 years(Reference Cotter and Charlton37,Reference Lassailly, Caiazzo and Buob38) . For these patients, active treatment is especially necessary. Our research confirmed that obesity was also a real cause of liver cirrhosis in the European population. Two large studies from the UK have also demonstrated that obesity increases the incidence of liver cirrhosis and liver-related mortality(Reference Hart, Morrison and Batty39,Reference Liu, Balkwill and Reeves40) .
A positive correlation between obesity and the aggravation of viral hepatitis has been reported(Reference Wang, Yan and Wang10,Reference Nickbakhsh, Leitch and Smith11) . In contrast, several studies have suggested that an inverse correlation between obesity and virus activity and the success rate of antiviral treatment(Reference Wu, Koch and Bachmann12–Reference Chiang and Huang14). Unlike those studies, multivariable MR analysis did not find a causal relationship between obesity and viral hepatitis, autoimmune liver disease or hepatocellular carcinoma. Metabolic risk factors rather than obesity seem to independently facilitate hepatocarcinogenesis(Reference Huang and Liu13). Compared with virus infection or immune system disorders, abnormal lipid metabolism may not be the core trigger of these liver diseases(Reference Hirschfield, Beuers and Corpechot41–Reference Kuipery, Gehring and Isogawa44). The endemicity of hepatitis virus infection varies according to regional hygienic standards and lifestyles(Reference Cooke, Flower and Cunningham45). This may also be attributed to the influence of confounding factors and ethnic differences, which may require further exploration in larger cohorts.
BMI is a commonly used index to assess the degree of obesity and reflects total body fat mass(6). The WHRadjBMI is a surrogate for abdominal fat and is less influenced by muscle and bone mass than BMI(Reference Hsuan, Lin and Lee22). The waist–hip ratio may be superior to BMI in predicting liver-related outcomes(Reference Åberg, Färkkilä and Salomaa23,Reference Pang, Kartsonaki and Guo24,Reference Haufs and Zöllner46) . Our results revealed that a high WHRadjBMI rather than BMI was associated with higher levels of biomarkers of lipid metabolism, indicating that a high WHRadjBMI can better and more sensitively reflect the presence of lipid accumulation and metabolic disorders. Our research also explored a positive association between obesity and elevated serum insulin levels. These findings suggested that elevated BMI and WHR may be risk factors for insulin resistance. Obesity can interfere with the normal regulation of lipid metabolism through various pathways, such as influencing hormone secretion, altering the function and activity of adipose tissue and causing insulin resistance. Insulin resistance is the most likely link between obesity and obesity-related metabolic disorders, where obesity leads to insulin resistance, and insulin stimulates the degradation of apolipoprotein B-100 while inhibiting the secretion of very LDLs from the liver(Reference Choi and Ginsberg47,Reference Sparks, Sparks and Adeli48) .
In the diagnosis of liver fibrosis, the clinical application of liver biopsy is limited, owing to its inherent limitations, including its invasive nature, difficult sampling operation, high time consumption, false negatives, subjectivity and low degree of patient acceptance(Reference Friedman and Pinzani49). Our study found that high BMI leads to abnormally elevated alanine aminotransferase and abnormally reduced platelet count, which is consistent with several previous non-invasive diagnostic models. In 2007, Paul et al. published the NAFLD Fibrosis Score (NFS), which for the first time included BMI to assess the risk of non-alcoholic steatohepatitis progressing to liver fibrosis(Reference Angulo, Hui and Marchesini50). In the future, we need to break through the traditional definition, establish a new model to predict the reversal of liver fibrosis with BMI and verify it in prospective studies to provide a reference index for the clinical monitoring of the reversal tendency of liver fibrosis after antiviral treatment. MR studies can effectively mitigate the influence of individual differences and confounding factors, thereby enabling a more accurate and reliable evaluation of the effects of intervention measures(Reference Sekula, Del Greco and Pattaro18).
The strengths of this study were as follows: first, we used BMI and WHR to evaluate total body fat and abdominal fat. We used large GWAS summary datasets to explore the causal associations between two indices and liver diseases. Second, we tested the direct effects of the two indices on liver disease and function via multivariable MR. However, there were several limitations: most of the GWAS data we used were from individuals of European ancestry, and the results may not be extrapolated to those of different races. Sarcopenia is a disorder characterised by loss of muscle mass, strength and function(Reference Tarantino, Sinatti and Citro51). Sarcopenia is an independent predictor of mortality in NAFLD and cirrhosis(Reference Iwaki, Kobayashi and Nogami52). An index that can accurately measure skeletal muscle mass and sarcopenia is not available in GWAS. Therefore, we only evaluated the causal association of fat mass.
BMI and WHR, which are widely used and simple indicators, are extensively applied in clinical practice. Our study suggested a causal relationship between obesity and liver fibrosis, NAFLD, as well as aberrant lipid metabolism markers. Neither BMI nor WHRadjBMI had a causal relationship with viral hepatitis, primary biliary cholangitis and secondary tumour of liver. A higher WHRadjBMI rather than BMI was associated with higher levels of biomarkers of lipid accumulation and metabolic disorders. These findings indicated that the waist–hip ratio may be superior to BMI in predicting liver metabolic disorders.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82170541).
Conceptualization: W. A.; Funding acquisition: H. W., L. H., F. X.; Investigation: W. A., J. L., Z. Y.; Methodology: Z. Y., Y. M., M. L.; Project administration: H. B., F. X.; Software: W. A., A. S.; Supervision: H. W., H. W.; Validation: H. W.; Writing—original draft: W. A., J. L.; Writing—review and editing: F. X., H. W.
The authors have no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452400237X








