The effects of dietary fish oil or n-3 PUFA on T-cell function are considered to be primarily anti-inflammatory. The basis for the claimed anti-inflammatory effects of n-3 PUFA on T-cell function is that in most studies they decrease T-cell proliferation and the secretion of IL-2(Reference Endres, Meydani, Ghorbani, Schindler and Dinarello1–Reference Zhang, Kim, Zhou, Wang, Ly, McMurray and Chapkin5). Dietary fish oil and n-3 PUFA also decrease the secretion of the pro-inflammatory cytokine TNF-α by murine splenocytes stimulated with concanavalin A (ConA)(Reference Sun, Krishnan, Zaman, Lawrence, Bhattacharya and Fernandes6, Reference Petursdottir and Hardardottir7). In contrast, the effects of dietary fish oil or n-3 PUFA on the secretion of the T helper (Th) 1-type cytokine interferon (IFN)-γ are inconsistent with results showing decreased secretion(Reference Gallai, Sarchielli, Trequattrini, Franceschini, Floridi, Firenze, Alberti, Di Benedetto and Stragliotto8, Reference Ly, Smith, Chapkin and McMurray9), no effect(Reference Fes, Anderson, Zhang and Fritsche10–Reference Yaqoob and Calder12) or increased secretion(Reference Gorjão, Verlengia and Lima13, Reference Trebble, Wootton, Miles, Mullee, Arden, Ballinger, Stroud, Burdge and Calder14) of this cytokine by human lymphocytes or murine splenocytes stimulated with ConA or anti-CD3/anti-CD28.
The effects of dietary fish oil on the secretion of Th2 or anti-inflammatory cytokines are also inconsistent. Dietary fish oil decreased ConA-induced IL-10 secretion by total splenocytes in a previous study from our laboratory(Reference Petursdottir and Hardardottir7) but had no effect in another study(Reference Yaqoob and Calder12). It increased ConA-induced secretion of IL-4 in one study(Reference Venkatraman and Chu15) but had no effect in several other studies(Reference Yaqoob and Calder12, Reference Albers, Bol, Bleumink, Willems, Blonk and Pieters16–Reference Wu, Chiang, Chang and Lin19). In the study by Venkatraman & Chu(Reference Venkatraman and Chu15) the cells were stimulated for 48 h whereas in two of the latter studies they were only stimulated for 24 h(Reference Wallace, Miles, Evans, Stock, Yaqoob and Calder17, Reference Wu, Chiang, Chang and Lin19). Dietary fish oil also did not affect IL-4 secretion by purified CD4+ or CD8+T cells activated with various stimuli, including anti-CD3/anti-CD28(Reference Arrington, Chapkin, Switzer, Morris and McMurray20).
The reasons for the varied results obtained in the studies discussed above may include the different cell types used, the different means and duration of stimulation, the use of fetal calf serum in the cell cultures, the duration of feeding, the control group and the amount or type of fish oil or n-3 PUFA used. The first objective of the present study was to determine the effects of dietary fish oil on the secretion of Th1, Th2, anti- and pro-inflammatory cytokines by total splenocytes and isolated T cells stimulated with well-defined T-cell agonists, using homologous serum and optimal duration of stimulation. Our hypothesis was that dietary fish oil directed the immune response towards a Th2 type as has been proposed before(Reference Zhang, Smith, Chapkin and McMurray21).
The mechanism by which dietary n-3 PUFA may affect proliferation and IL-2 secretion has been studied and may include both a direct effect on the T cells and an indirect effect through the accessory cells(Reference Chapkin, Arrington, Apanasovich, Carroll and McMurray22). Direct effects of n-3 PUFA on T cells may be through their effects on lipid rafts(Reference Fan, McMurray, Ly and Chapkin23, Reference Fan, Ly, Barhoumi, McMurray and Chapkin24), the inhibitory protein cytotoxic T-lymphocyte antigen 4(Reference Ly, Smith, Switzer, Chapkin and McMurray25) or their effects on activation-induced cell death(Reference Switzer, McMurray, Morris and Chapkin11). Indirect effects of n-3 PUFA on T cells through accessory cells are indicated by results showing that dietary fish oil decreases T-cell proliferation following stimulation with anti-CD3/anti-CD28 in co-cultures containing pooled T cells from mice fed n-3 or n-6 PUFA and accessory cells from mice receiving dietary n-3 PUFA(Reference Chapkin, Arrington, Apanasovich, Carroll and McMurray22). Effects of dietary n-3 PUFA on accessory cells include decreased antigen presentation activity in rat dendritic cells(Reference Sanderson, MacPherson, Jenkins and Calder26), inhibition of the ability of splenic accessory cells to present antigen to murine helper T-cell clones(Reference Fujikawa, Yamashita, Yamazaki, Sugiyama, Suzuki and Hamazaki27) and decreased level of expression of molecules involved in accessory cell function, including major histocompatibility complex class II molecules, on human monocytes and rat dendritic cells(Reference Sanderson, MacPherson, Jenkins and Calder26, Reference Hughes, Pinder, Piper, Johnson and Lund28).
The second objective of the present study was to determine whether the effects of dietary fish oil on cytokine secretion by splenocytes were through direct or indirect effects on the T cells and to investigate the mechanism by which dietary fish oil may exert its effects on the secretion of Th2-type cytokines.
Experimental methods
Animals and diets
All experimental procedures using laboratory animals complied with the National Research Council's Guide for the Care and Use of Laboratory Animals(29). Female BalbC mice, aged 6–7 weeks and weighing 18–20 g (Taconic Europe AS, Ejby, Denmark), were randomly divided into two groups of ten mice each. Mice were housed five per cage at 25°C with a 12 h light–dark cycle. Experimental diets were designed according to AIN-93 guidelines(Reference Reeves, Nielsen and Fahey30) with modification in fat content. The experimental diets were based on a nutritionally complete diet made for the addition of 200 g fat/kg (ICN Pharmaceuticals, Aurora, OH, USA) as previously described(Reference Petursdottir, Olafsdottir and Hardardottir31). The fish oil diet contained 180 g menhaden fish oil/kg and 20 g maize oil/kg (ICN Pharmaceuticals, Asse-Relegem, Belgium). The maize oil diet contained 200 g maize oil/kg. These amounts of fish and maize oil were used in our previous studies(Reference Petursdottir and Hardardottir7, Reference Petursdottir, Olafsdottir and Hardardottir31) and by others(Reference Fes, Anderson, Zhang and Fritsche10, Reference Wallace, Miles, Evans, Stock, Yaqoob and Calder17, Reference Wu, Chiang, Chang and Lin19) at the time when these studies were initiated. The antioxidant tert-butylhydroquinone (ICN Biomedicals, Aurora, OH, USA) (1·2 mmol/l) was added to the oils to prevent their deterioration(Reference Fritsche and Johnston32). Diets were prepared in bulk and daily portions packed in zip-lock bags, flushed with N2, sealed and stored at − 20°C. Water and food were provided ad libitum. The mice were fed the experimental diets for 5–6 weeks. The length of feeding did not affect the effect of dietary fish oil on the parameters measured. An equal number of animals from each dietary group was killed at each time point.
Fatty acid analysis
Fat was extracted from the diets with CHCl3–methanol (1:2, v/v) after which the fatty acids were methylated for 45 min at 110°C using 14 % boron-trifluoride-methanol (Merck, Hohenbrunn, Germany). The fatty acid methyl esters were analysed using a gas chromatograph (Hewlett-Packard HP 7673; Hewlett-Packard, Palo Alto, CA, USA) equipped with a Chrompack CP-Wax 52CB polyethylene glycol column (25 m × 0·32 mm internal diameter × 0·2 μm film thickness) with H2 at 110 kPa as the carrier gas. Table 1 shows the fatty acid composition of the diets.
Table 1 Fatty acid composition of the diets (g/100 g total fatty acids)*
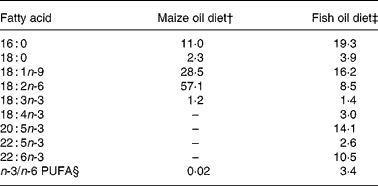
* Only selected fatty acids are listed.
† Diet contained 200 g maize oil/kg.
‡ Diet contained 180 g menhaden fish oil/kg and 20 g maize oil/kg.
§ Σ18 : 3;18 : 4; 20 : 5; 22 : 5, 22 : 6/18 : 2.
Isolation and activation of splenocytes
Mice were anaesthetised with isoflurane (Abbot Scandinavia, Solna, Sweden) and blood collected by axillary bleeding. Serum from two to three mice in the same dietary group was pooled and heat inactivated at 56°C for 40 min and used as homologous serum in cell cultures. The mice were killed by cervical dislocation. Spleens were removed aseptically post-mortem, cut and passed through a wire mesh to obtain a single cell suspension. The cells were treated with lysing buffer (0·15 m-NH4Cl, 1 mm-KHCO3, 0·1 mm-Na2EDTA) to lyse erythrocytes. For cytokine measurements, 5 × 109 cells/l were cultured in 0·2 ml/well on a ninety-six-well plate with 5 % homologous serum at 37°C in an atmosphere of 5 % CO2. The concentration of cells in the culture has been used by others(Reference Zhang, Kim, Zhou, Wang, Ly, McMurray and Chapkin5, Reference Zhang, Smith, Chapkin and McMurray21) and was selected after careful optimisation of the cell-culture conditions. The cells were stimulated with plate-bound hamster anti-mouse CD3ɛ monoclonal antibody (1 mg/l) with or without soluble purified hamster anti-mouse CD28 monoclonal antibody (5 mg/l) (BD Biosciences, San Jose, CA, USA) for 48 h or for 6, 12, 24, 34 and 48 h for the time-course. Cells were also incubated with neutralising monoclonal antibodies against CD80 (5 mg/l) or CD86 (10 mg/l) (eBioscience, San Diego, CA, USA). The concentration of neutralising antibodies against CD80 and CD86 used was double the concentration needed to achieve total neutralisation, as determined by flow cytometry.
Isolation and activation of T cells, CD11b positive cells and cells depleted of T cells
CD90 (Thy1.2) positive (CD90+) T cells and CD11b+ cells (mainly macrophages) were isolated from total spleen preparations using magnetic activated cell sorting (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). T cells were depleted from the cell preparation (to achieve a preparation of accessory cells) by using a threefold higher concentration of anti-CD90 than was used for positive selection and the cells were passed through two columns. After isolation, T cells and accessory cells were cultured at 3 × 109 cells/l alone or in co-culture at a ratio of 1:1. T cells (2 × 109 cells/l) were also co-cultured with CD11b+ cells (2·1, 4·2 or 8·4 × 108 cells/l). The isolated cells were activated as described above. The purity of the isolated cells was assessed using flow cytometry. In the CD90+ fraction, CD3 positive cells were 93·1 (sem 1·5) % (n 4) of all viably gated events (based on forward and side scatter) whereas in the CD90 depleted fraction 3·4 (sem 1·1) % (n 4) of the cells were CD3 positive. In the CD11b+ fraction, 97·3 (sem 0·5) % (n 4) of the cells were CD11b positive.
Cytokine assays
After incubation the culture plates were centrifuged and cell-culture supernatant fractions collected and stored at − 70°C. TNF-α and IL-10 were measured in the supernatant fractions using Duo Set ELISA kits (R&D Systems, Minneapolis, MN, USA). IL-4 and IFN-γ levels were measured using antibody pairs and standards from BD Biosciences (San Jose, CA, USA) in a sandwich ELISA set up in the laboratory, according to the manufacturer's protocol. Trimethylbenzidine (R&D Systems) was used as a chromogen in all assays. The absorbance was read at 450 nm in a microplate reader (SpectraMax 250; Molecular Devices, Sunnyvale, CA, USA). The detection limit for the ELISA assays was 20–40 ng/l.
Flow cytometric analysis of CD80 and CD86 on total splenocytes
Cells were pre-incubated with anti-mouse CD16/32 antibody (2 μg/106 cells) (Mouse Fc Block; BD Biosciences, San Jose, CA, USA), stained with phycoerythrin-labelled anti-mouse CD80 or anti-mouse CD86 (eBioscience, San Diego, CA, USA) and analysed on a Becton Dickinson FACScalibur flow cytometer equipped with an Ar ion laser (BD Biosciences, San Jose, CA, USA). Appropriate isotypic controls were used to set the quadrants and evaluate background staining. A total of 25 000 events were collected and the data analysed using FCS express V3 (de Novo Software, Los Angeles, CA, USA).
Statistical analysis
Differences between dietary groups were analysed by an unpaired Student's t test using the StatsDirect statistical program (version 2.3.3; StatsDirect Ltd, Altrincham, Cheshire, UK). Results are expressed as mean values with their standard errors. Differences were determined to be significant when P < 0·05 (two-tailed).
Results
Body weights, spleen weights and cell yield
There were no differences in the body weights or relative weight gains of mice fed the different diets. Mice fed the fish oil diet had heavier spleens (171 (sem 6) mg) than those fed the maize oil diet (120 (sem 4) mg; P < 0·0001). Spleens from mice fed the fish oil diet also had a greater cell count (98 (sem 4) × 106 cells/spleen) than spleens from mice fed the maize oil diet (71 (sem 4) × 106 cells/spleen; P < 0·0001).
Interferon-γ, TNF-α, IL-10 and IL-4 secretion by total splenocytes stimulated with anti-CD3 or anti-CD3/anti-CD28
When stimulated with anti-CD3 or anti-CD3/anti-CD28, splenocytes from mice fed the fish oil diet secreted significantly less IFN-γ and TNF-α than splenocytes from mice fed the maize oil diet (Figs. 1 (a) and (b)). However, IL-10 secretion by splenocytes from mice fed the fish oil diet was similar to the IL-10 secretion by splenocytes from mice fed the maize oil diet (Fig. 1 (c)). When stimulated with anti-CD3 or anti-CD3/anti-CD28, splenocytes from mice fed the fish oil diet secreted significantly more IL-4 than splenocytes from mice fed the maize oil diet (Fig. 1 (d)).
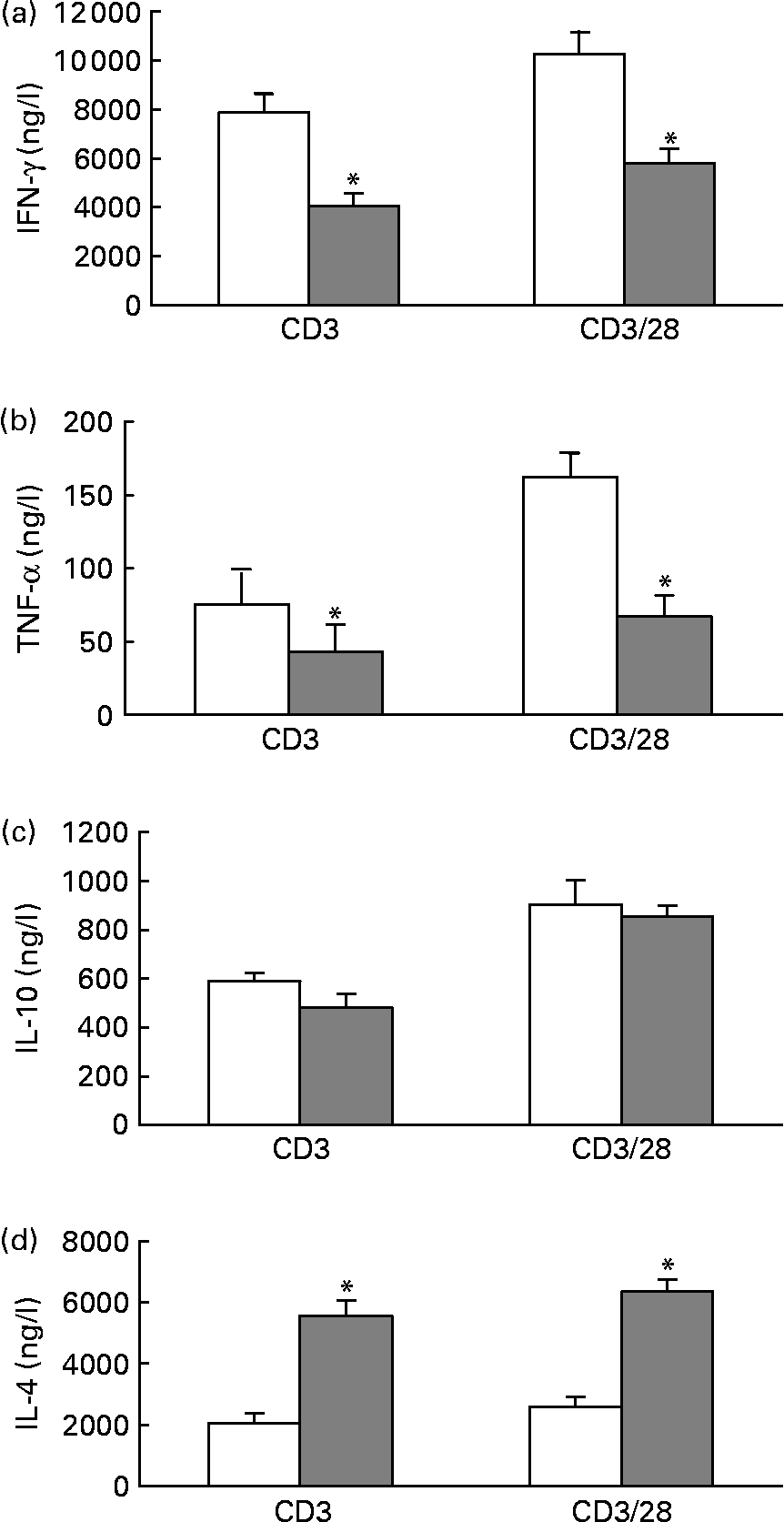
Fig. 1 Anti-CD3 and anti-CD3/anti-CD28-induced interferon (IFN)-γ (a), TNF-α (b), IL-10 (c) and IL-4 (d) secretion by total splenocytes from mice fed the maize oil diet (□) or the fish oil diet (▓). Splenocytes (5 × 109 cells/l) were stimulated with anti-CD3 (1 mg/l) or anti-CD3 (1 mg/l) and anti-CD28 (5 mg/l) for 48 h. Values are means (n 10), with standard errors represented by vertical bars. * Mean value was significantly different from that of cells from the maize oil-fed mice (P < 0·05).
Time-course for anti-CD3/anti-CD28-induced interferon-γ and IL-4 secretion by total splenocytes
The concentration of IFN-γ and IL-4 in the medium from total splenocytes stimulated with anti-CD3/anti-CD28 had increased at 24 h and continued to increase, not reaching a plateau at 48 h (Fig. 2). After stimulation for 24 and 48 h, splenocytes from mice fed the fish oil diet had secreted significantly less IFN-γ, compared with splenocytes from mice fed the maize oil diet (Fig. 2 (a)). In contrast, splenocytes from mice fed the fish oil diet had secreted more IL-4 than splenocytes from mice fed the maize oil diet after 48 h of stimulation but not after 24 h of stimulation (Fig. 2 (b)).
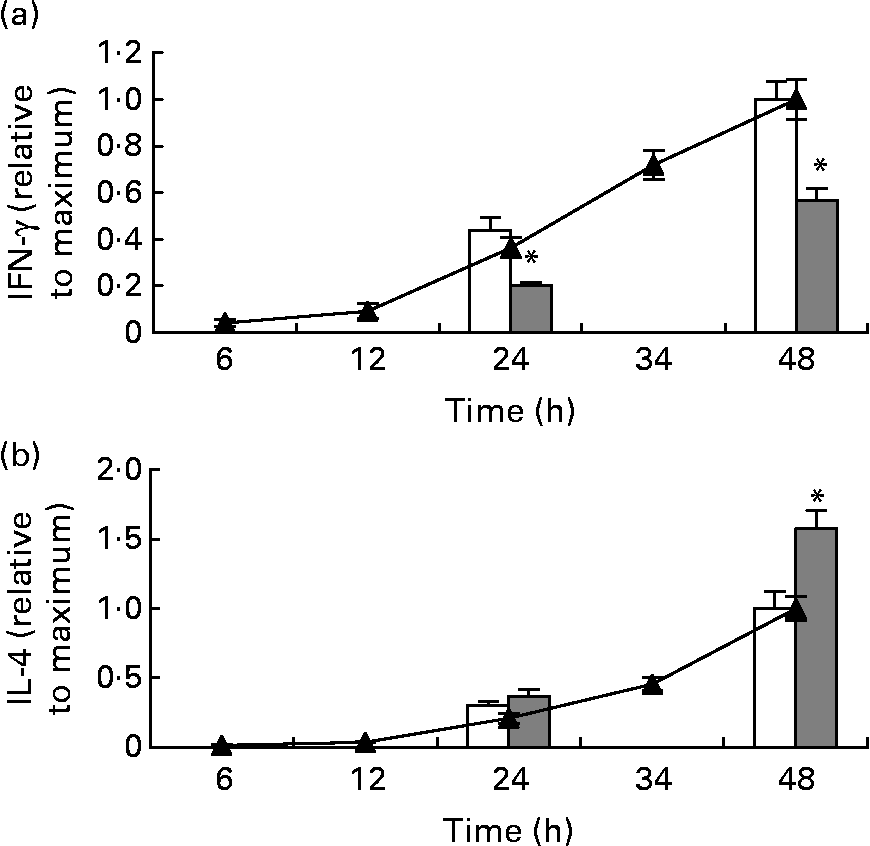
Fig. 2 Time-course for anti-CD3/anti-CD28-induced interferon (IFN)-γ (a) and IL-4 (b) secretion by total splenocytes from mice receiving standard laboratory chow (–▲–) and secretion of IFN-γ and IL-4 by splenocytes from mice fed the maize oil diet (□) or the fish oil diet (▓) at 24 and 48 h. Values are means (n 3 for the time-course and n 10 for the dietary experiment), with standard errors represented by vertical bars. * Mean value was significantly different from that of cells from the maize oil-fed mice (P < 0·05).
The neutralising antibody against IFN-γ did not affect anti-CD3/anti-CD28-induced IL-4 secretion by splenocytes from mice fed the standard chow (data not shown).
Interferon-γ, TNF-α, IL-10 and IL-4 secretion by isolated T cells
When stimulated with anti-CD3/anti-CD28, isolated T cells from mice fed the fish oil diet secreted significantly less IFN-γ and TNF-α than isolated T cells from mice fed the maize oil diet (Figs. 3 (a) and (b)). There was not a statistically significant difference in IL-10 or IL-4 secretion by isolated T cells from mice fed the different diets (Figs. 3 (c) and (d)).
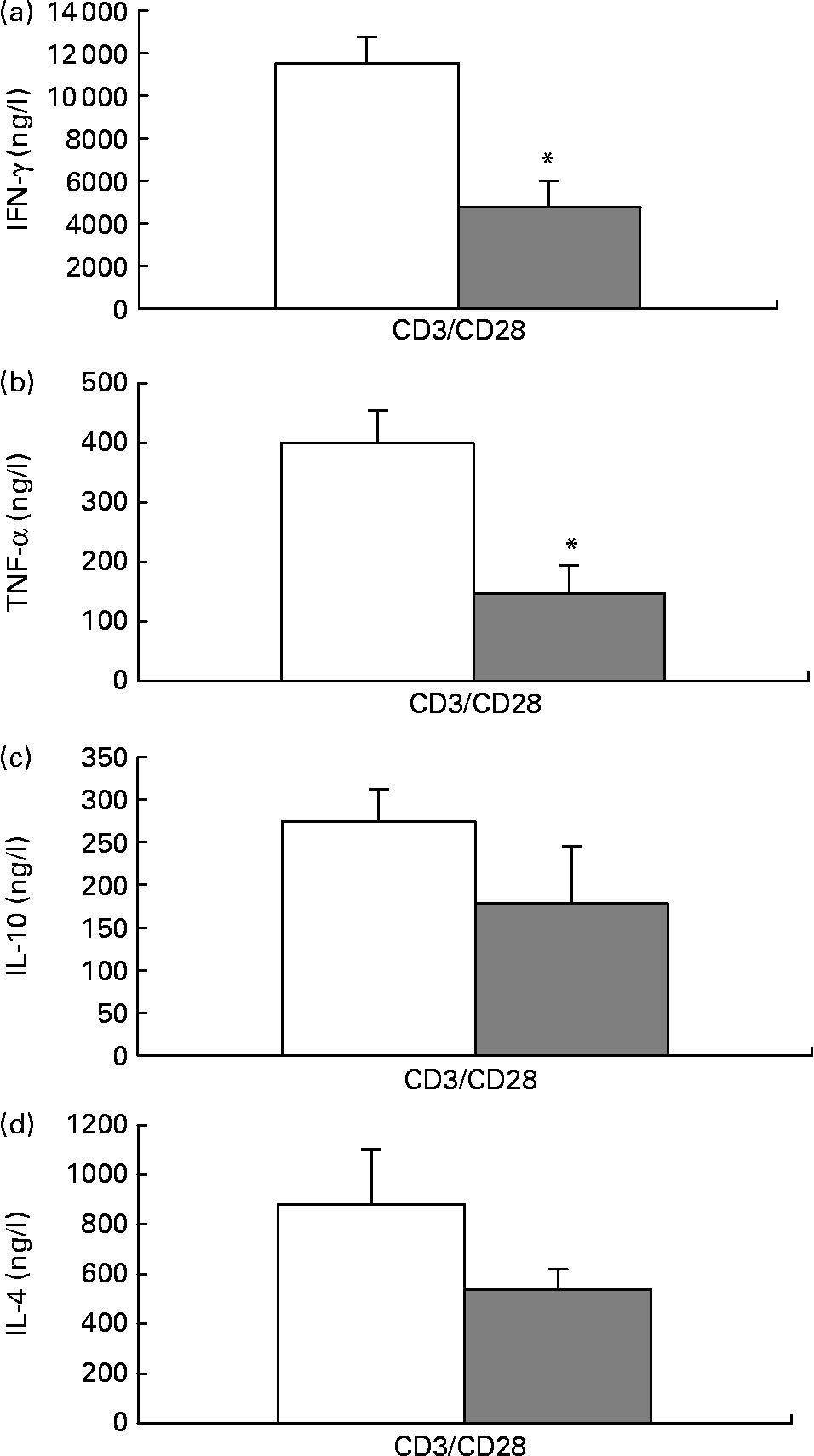
Fig. 3 Anti-CD3/anti-CD28-induced interferon (IFN)-γ (a), TNF-α (b), IL-10 (c) and IL-4 (d) secretion by isolated splenic T cells from mice fed the maize oil diet (□) or the fish oil diet (▓). T cells expressing CD90 (3 × 109 cells/l) were stimulated with anti-CD3 (1 mg/l) and anti-CD28 (5 mg/l) for 48 h. Values are means (n 10), with standard errors represented by vertical bars. *Mean value was significantly different from that of cells from the maize oil-fed mice (P < 0·05).
IL-4 secretion by T cells cultured with or without accessory cells
To investigate if CD11b+ cells (mainly macrophages) mediated the increased IL-4 secretion by total splenocytes from mice fed the fish oil diet, T cells were cultured with an increasing number of CD11b+ splenocytes from the same mouse and stimulated with anti-CD3/anti-CD28. When T cells were co-cultured with the highest concentration of isolated CD11b+ splenocytes (8·4 × 108 cells/l), there was significantly more IL-4 in the medium from co-cultures from mice fed the fish oil diet than in the medium from co-cultures from mice fed the maize oil diet (Fig. 4). Isolated T cells were also co-cultured with splenocytes depleted of T cells (3 × 109 cells/l), a cell fraction that includes all accessory cells, not only CD11b+ cells. When T cells from mice fed the fish oil diet were cultured with accessory cells from mice fed the fish oil diet they secreted more IL-4 than T cells from mice fed the maize oil diet co-cultured with accessory cells from mice fed the maize oil diet (Fig. 4). Accessory cells (cells depleted of T cells) cultured alone secreted almost no IL-4 after stimulation with anti-CD3/anti-CD28 (Fig. 4).
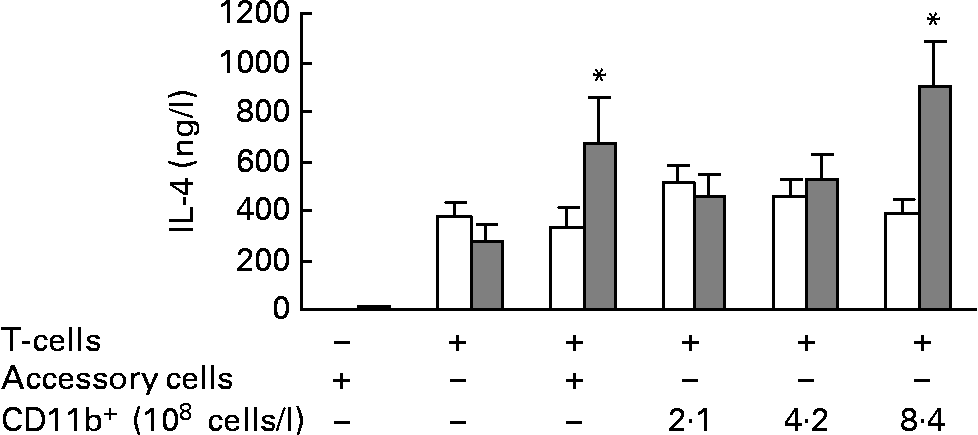
Fig. 4 IL-4 secretion by isolated splenic T cells cultured with (+) or without ( − ) accessory cells or CD11b+ splenocytes from mice fed the maize oil diet (□) or the fish oil diet (▓). Accessory cells (splenocytes depleted of cells expressing CD90) (3 × 109 cells/l) were stimulated with anti-CD3 (1 mg/l) and anti-CD28 (5 mg/l) for 48 h. T cells (3 × 109 cells/l) were co-cultured with or without accessory cells (3 × 109 cells/l) or with increasing number of cells expressing CD11b+(2·1, 4·2, or 8·4 × 108 cells/l) and stimulated with anti-CD3 (1 mg/l) and anti-CD28 (5 mg/l) for 48 h. Values are means (n 10), with standard errors represented by vertical bars. * Mean value was significantly different from that of cells from the maize oil-fed mice (P < 0·05).
The effects of neutralising antibodies against CD80 and CD86 on IL-4 secretion by total splenocytes stimulated with anti-CD3/anti-CD28
The neutralising antibody against CD80 did not affect IL-4 secretion by total splenocytes from mice fed the fish oil or the maize oil diet (data not shown). However, the neutralising antibody against CD86 decreased IL-4 secretion by total splenocytes from mice fed the fish oil diet (441 (sem 80) ng/l with neutralising antibody v. 518 (sem 76) ng/l without neutralising antibody) without affecting IL-4 secretion by splenocytes from mice fed the maize oil diet (241 (sem 51) ng/l with neutralising antibody v. 236 (sem 43) ng/l without neutralising antibody). However, neutralising CD86 did not eliminate the difference in IL-4 secretion by splenocytes from mice fed the different diets.
CD80 and CD86 expression on total splenocytes
Spleens from mice fed the fish oil diet had a similar proportion of cells expressing CD80 (21·4 (sem 1·7) %) to spleens from mice fed the maize oil diet (21·9 (sem 1·3) %). Similarly, spleens from mice fed the fish oil diet had a similar proportion of cells expressing CD86 (45·6 (sem 2·7) %) to spleens from mice fed the maize oil diet (43·7 (sem 2·1) %). Splenocytes from mice fed the fish oil diet had similar levels of expression of CD80 and CD86 to splenocytes from mice fed the maize oil diet (data not shown).
Discussion
The results from the present study demonstrate that dietary fish oil directs the immune response in splenocytes, stimulated with T cell agonists, towards a Th2-type response. This effect was shown by a decreased secretion of Th1-type cytokines as well as by an increased secretion of the Th2-type cytokine IL-4. The effect of dietary fish oil on IL-4 secretion was dependent on accessory cells, more specifically CD11b+ cells.
The present results showing decreased secretion of the Th1 and pro-inflammatory cytokines IFN-γ and TNF-α by splenocytes from mice fed the fish oil diet are in agreement with other studies showing decreased secretion of these cytokines by murine splenocytes from mice fed fish oil or n-3 PUFA(Reference Ly, Smith, Chapkin and McMurray9, Reference Wallace, Miles, Evans, Stock, Yaqoob and Calder17, Reference Khan, Yessoufou, Kim and Hichami33). Decreased secretion of IFN-γ and TNF-α by splenocytes from mice fed the fish oil diet compared with that by splenocytes from mice fed the maize oil diet may be by a direct effect on the T cells, as secretion of these cytokines was decreased in cultures of isolated T cells. However, a complementary indirect influence of the accessory cells in the cultures of total splenocytes cannot be excluded. On the other hand, increased IL-4 secretion by splenocytes from mice fed the fish oil diet in the present study is not in agreement with results from previous studies showing either a decrease or no effect of fish oil or n-3 PUFA on IL-4 secretion by splenocytes(Reference Yaqoob and Calder12, Reference Albers, Bol, Bleumink, Willems, Blonk and Pieters16–Reference Wu, Chiang, Chang and Lin19). In these studies the splenocytes were stimulated with ConA but in the present study they were stimulated with anti-CD3/anti-CD28. When we used ConA to stimulate the splenocytes we did not see a difference in IL-4 secretion between cells from mice fed the different diets (data not shown), indicating that the effect of dietary fish oil on IL-4 secretion is dependent on the stimulus.
The increased IL-4 secretion by splenocytes from mice fed the fish oil diet, along with the decreased IFN-γ secretion, indicates a polarisation of their immune response towards a Th2-type. As IL-12 has been shown to promote a Th1-type immune response(Reference Nakamura, Lee, Nam, Podack, Bottomly and Flavell34) we attempted to examine whether IL-12 secretion was altered by the fish oil diet, but this cytokine was not detected in the cell-culture supernatant fractions in the present study and is therefore not likely to play a role in the effect of dietary fish oil on cytokine secretion. We also investigated whether IFN-γ could affect IL-4 secretion, as IFN-γ secretion by splenocytes from mice fed the fish oil diet was decreased at an earlier time-point than IL-4 secretion was increased. Neutralising IFN-γ did not affect the secretion of IL-4 by splenocytes from mice fed the standard laboratory chow, making it unlikely that the effect of dietary fish oil on IL-4 secretion is dependent on its effect on the secretion of IFN-γ.
The increase in IL-4 secretion by total splenocytes from mice fed the fish oil diet was dependent on the accessory cells (i.e. cells other than T cells), as dietary fish oil did not increase IL-4 secretion by isolated T cells. In an attempt to identify the accessory cells that mediated the increased IL-4 secretion, we isolated CD11b+ cells and demonstrated that when these cells were added to the T cells, co-cultures of these cells from mice fed the fish oil diet secreted more IL-4 than co-cultures of these cells from mice fed the maize oil diet. These results indicate that CD11b+ cells from mice fed the fish oil diet either function differently from those from mice fed the maize oil diet or that they include a higher proportion of a subpopulation of cells that induce IL-4 secretion or secrete IL-4. Although the accessory cells did not secrete detectable levels of IL-4 when cultured without T cells it cannot be ruled out that the activated T cells stimulated the accessory cells to secrete IL-4. Others have shown that T cells pre-stimulated with a peptide and re-stimulated with anti-CD3/anti-CD28 stimulated basophils to secrete IL-4(Reference Oh, Shen, Le Gros and Min35). However, whether T cells that have only been stimulated with anti-CD3/anti-CD28 can activate accessory cells to secrete IL-4 is unknown.
We considered whether the co-stimulatory molecules CD80 and/or CD86 on the accessory cells could play a role in the increased IL-4 secretion by total splenocytes from mice fed the fish oil diet. It did not seem likely that ligation of CD80 or CD86 with CD28 could mediate the increased IL-4 secretion, as stimulation with anti-CD28, in addition to anti-CD3, did not increase IL-4 secretion compared with that when the cells were stimulated with anti-CD3 alone. Therefore, any effect of CD80 or CD86 on IL-4 secretion would have to be through other ligands, such as cytotoxic T-lymphocyte antigen 4 or programmed death ligand 1. However, blocking CD80 and CD86 did not eliminate the difference in IL-4 secretion between splenocytes from mice fed the different diets, indicating that interaction between these co-stimulatory molecules with their ligands are not important in the effects of fish oil on IL-4 secretion.
In summary, the results from the present study demonstrate that dietary fish oil induces a Th2 polarised cytokine response when splenocytes are activated with T cell agonists. The effect of dietary fish oil on IL-4 secretion by splenocytes from mice fed the fish oil diet was dependent on CD11b+ accessory cells.
Acknowledgements
The authors have no conflicts of interest. The present study was supported by grants from the Research Fund and the Graduate Research Fund (D. H. P.) from the Icelandic Centre for Research and from the University of Iceland Research Fund. D. H. P. and I. H. both contributed to the conception and design of the work, analysis and interpretation of the data and to drafting the manuscript as well as revising it.







