INTRODUCTION
Understanding the habitat preferences of species within modified environments can provide valuable insights into the mechanisms facilitating invasion by non-native species (Maskell et al. Reference MASKELL, BULLOCK and SMART2006, Melero et al. Reference MELERO, PALAZON, REVILLA, MARTELO and GOSALBEZ2008, Thompson et al. Reference THOMPSON, HODGSON and RICH1995). Of particular importance are patterns of space use by both native and invasive species, and how these are influenced by the level of human-induced variation measured at different spatial scales (Holland et al. Reference HOLLAND, BERT and FAHRIG2013, Orrock et al. Reference ORROCK, PAGELS, MCSHEA and HARPER2000). Whilst past studies have tended to focus on the presence or absence of species at the level of entire land-uses (Maestas et al. Reference MAESTAS, KNIGHT and GILBERT2003, McKinney Reference MCKINNEY2008), few have provided a more detailed understanding of how the microhabitat structure of complex habitats, such as modified tropical forests, can affect species occurrence.
In this study, we investigate the determinants of trap-site detection probability of four non-volant rodent species (Muridae, hereafter murids), including three native and one non-native, in logged and unlogged tropical forests of northern Borneo. In recent years, the island of Borneo in South-East Asia has undergone some of the highest deforestation rates in the world (Achard et al. Reference ACHARD, EVA, STIBIG, MAYAUX, GALLEGO, RICHARDS and MALINGREAU2002, Sodhi et al. Reference SODHI, POSA, LEE and BICKFORD2010). These have reduced it to a patchwork of land-uses consisting mostly of logged secondary forests and fragmented agricultural landscapes, with very few expanses of old-growth forest remaining outside of protected areas (Bryan et al. Reference BRYAN, SHEARMAN, ASSNER, KNAPP, AORO and LOKES2013, McMorrow & Talip Reference MCMORROW and TALIP2001, Reynolds et al. Reference REYNOLDS, PAYNE, SINUN, MOSIGIL and WALSH2011). In this context, many studies have highlighted the value of logged forests to both the maintenance of key ecological processes and the conservation of a variety of taxa (Berry et al. Reference BERRY, PHILLIPS, LEWIS, HILL, EDWARDS, TAWATAO, AHMAD, MAGINTAN, KHEN, MARYATI, ONG and HAMER2010, Dunn Reference DUNN2004, Meijaard & Sheil Reference MEIJAARD and SHEIL2007, Reference MEIJAARD and SHEIL2008; Wearn et al. Reference WEARN, ROWCLIFFE, CARBONE, BERNARD and EWERS2013, Wilcove et al. Reference WILCOVE, GIAM, EDWARDS, FISHER and KOH2013). In contrast, few have assessed these changes in terms of whether or not they favour the spread of invasive species (Yaap et al. Reference YAAP, STRUEBIG, PAOLI and KOH2010).
Bornean murids vary widely in their morphology, body size, diet (Wells & Bagchi Reference WELLS and BAGCHI2005) and movement patterns (Wells et al. Reference WELLS, PFEIFFER, LAKIM and KALKO2006a), and can therefore be expected to show differences in their microhabitat requirements (Püttker et al. Reference PÜTTKER, PARDINI, MEYER-LUCHT and SOMMER2008). The fact that these have rarely been documented is surprising given recent records of non-native murids (mainly members of the Rattus rattus species complex) in primary and secondary tropical forests of northern Borneo (Wells et al. Reference WELLS, KOCK, LAKIM and PFEIFFER2006b). Recently, however, Wells et al. (Reference WELLS, LAKIM and O’HARA2014) showed that whilst native species were confined to forest habitats, non-native species such as members of the Rattus species complex had high probability of occurrence in urban habitats as well as areas of intermediate land-use intensity. Whilst the authors note that these species ‘are also likely to occasionally invade forested habitats’, their analysis does not reveal which attributes of forest stands favour their spread.
The first aim of this study was to investigate how specific components of microhabitat structure measured at the trap level influence the detection probability of three native (Maxomys surifer, Miller; Maxomys whiteheadi, Thomas and Leopoldamys sabanus, Thomas) and one non-native (Rattus rattus, Linnaeus) species. We expected possible differences between the three native species, thus reflecting a degree of partitioning in habitat use, and especially strong differences compared with the invading R. rattus. In addition, since selective logging practices may also affect the overall structure of tropical forests at small spatial scales relevant to murid species (August Reference AUGUST1983, Beck et al. Reference BECK, GAINES, HINES and NICHOLS2004), the second aim of this study was concerned with describing how the level of disturbance measured at the microhabitat level influences trap-site detection of the same native and non-native species. We hypothesized that native species would show a preference for less degraded sites whilst the reverse would be true for R. rattus.
METHODS
Study area
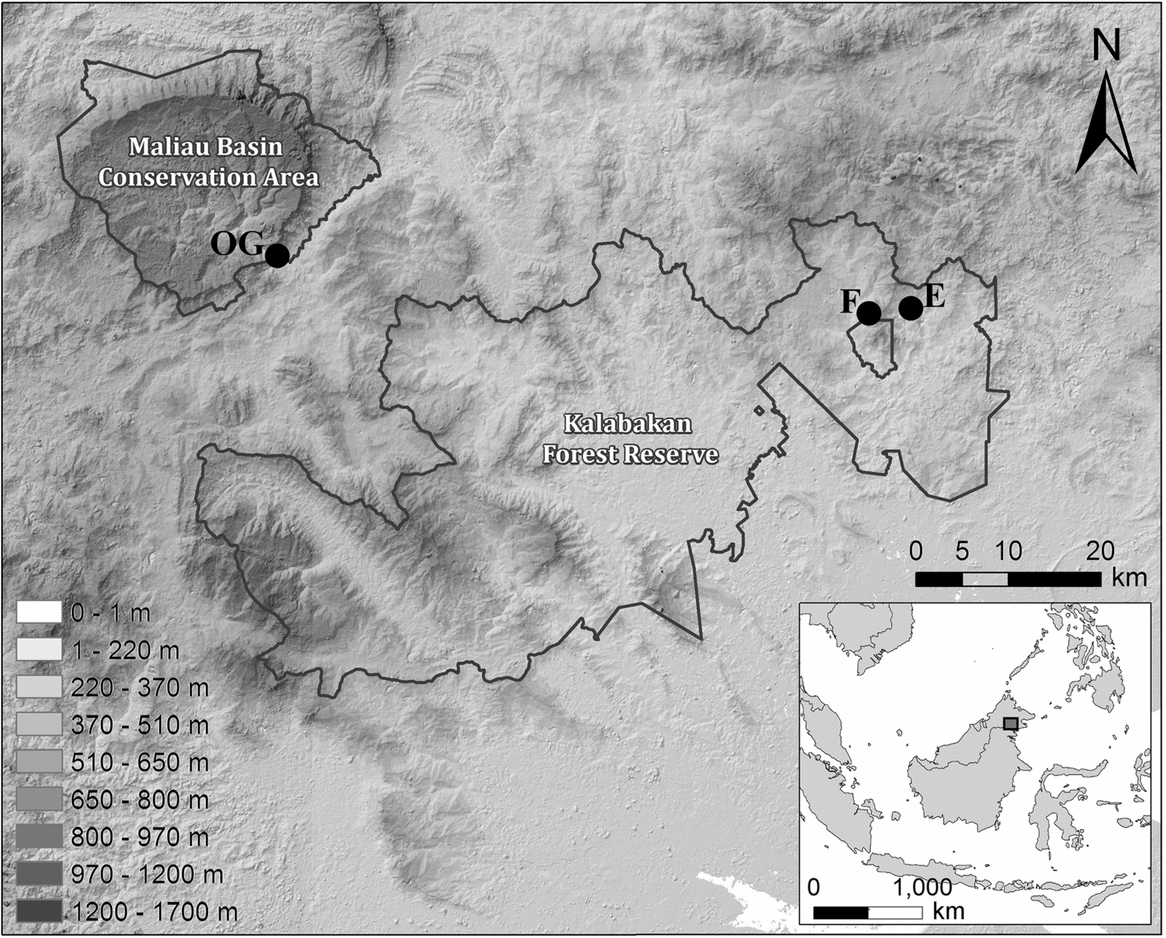
Fieldwork was carried out at the Maliau Basin Conservation Area (MBCA; 4°49′N, 116°54′E) and the Kalabakan Forest Reserve (KFR; 4°42′N, 117°34′E) in the state of Sabah, Malaysia (Figure 1), as part of the Stability of Altered Forest Ecosystems (SAFE) Project (Ewers et al. Reference EWERS, DIDHAM, FAHRIG, FERRAZ, HECTOR, HOLT, KAPOS, REYNOLDS, SINUN, SNADDON and TURNER2011). The 390-km2 MBCA was designated as a Class 1 Protection Forest Reserve by the Sabah State Assembly in 1997 due to its conservation importance. It remains unaffected by logging, with dominant tree species including Eusideroxylon zwageri, Shorea parvifolia and S. curtisii. The KFR comprises forest that has undergone multiple rounds of logging, beginning in 1978 and ongoing until the early 2000s. Logging activities have resulted in a heterogeneous forest landscape, which comprises a range of stand types. These vary from grassy open areas and low scrub vegetation to lightly logged forest on steep slopes and rocky escarpments.

Figure 1. Location of sampling sites in Malaysian Borneo, showing the unlogged control site within the MBCA (OG) and the repeatedly logged forest sites (E and F) within the Kalabakan Forest Reserve. All sites are spread out along a line of similar latitude. The figure legend shows altitude levels across the study sites.
Small-mammal sampling
We established two small-mammal trapping grids within the MBCA (OG1 and OG2 in unlogged forest) and two each within sampling blocks E and F of the SAFE experimental area (E1, E2, F1 and F2 in repeatedly logged forest), totalling six grids across three sampling areas (Figure 1). All grids in E and F, as well as grid OG2, were of a pre-determined size – 4 × 12 sampling locations, each separated by 23 m – whilst OG1 was a smaller subsample of this size (Table 1). Sampling locations were pre-determined in ArcGIS (Environmental Science Research Institute, Redlands, CA, USA) and marked out in the field using the resulting GPS coordinates. These defined the centre of a 10-m-radius circle within which two locally made wire-mesh traps (280 × 140 × 140 mm) were positioned. At each sampling location we attempted to maximize the distance between the two traps as much as possible (mean distance between pairs of traps at a trap station was 13.6 m, SD = 3.4). Traps were placed at heights varying from ground level to 0.5 m on fallen logs, and were equipped with a plastic cover to increase shelter and provide rain protection. In this study, trap site refers to the location of individual traps.
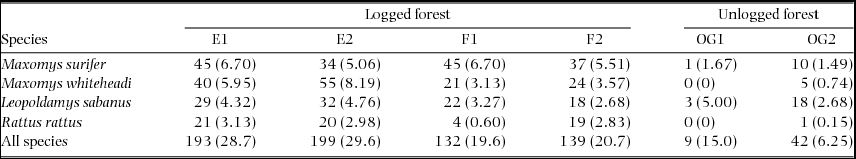
Table 1. Summary of small mammal live-trapping effort carried out across logged and unlogged forests between April and July 2011. We established two trapping grids within the Maliau Basin Conservation Area (OG1 and OG2 in unlogged forest) and two each within sampling blocks E and F of the SAFE experimental area (E1, E2, F1 and F2 in repeatedly logged forest), totalling six grids across three sampling areas. Trap sites refer to the location of individual traps (two per sampling location) and occasions refer to the number of consecutive sampling nights per grid. The total number of trap nights per grid represents the number of occasions multiplied by the number of trap sites.

Trapping was carried out between the months of April and July 2011. Each grid was sampled once for up to 7 consecutive days (referred to as occasions), with the location of traps remaining constant throughout that period. Traps were baited with oil palm fruits and checked daily (between 07h00 and 10h00). Animals caught for the first time in a trapping session were anaesthetized using diethylether (following Wells et al. Reference WELLS, KALKO, LAKIM and PFEIFFER2007), identified to species level, and injected with a unique passive-induced transponder tag (Francis Scientific Instruments, Cambridge) as part of a long-term mark-recapture study (O. R. Wearn, unpubl. data). All animals were released at their site of capture. Species identification was based on Payne & Francis (Reference PAYNE and FRANCIS2007), but detailed descriptions of each specimen were retained for future reference.
We did not euthanize any trapped individuals as voucher specimens, as this would affect the dynamics of our study populations, but we did take ear tissue samples (stored in 95% alcohol) from all individuals. MtDNA sequencing of the Rattus species we refer to in this study confirmed that it was a member of the Rattus rattus species complex (sensu Aplin et al. Reference APLIN, SUZUKI, CHINEN, CHESSER, TEN HAVE, DONNELIAN, AUSTIN, FROST, GONZALEZ, HERBRETEAU, CATZEFLIS, SOUBRIER, FANG, ROBINS, MATISOO-SMITH, BASTOS, MARYANTO, SINAGA, DENYS, VAN DEN BUSSCHE, CONROY, ROWE and COOPER2011, Pagès et al. Reference PAGÈS, CHAVAL, HERBRETEAU, WAENGSOTHORN, COSSON, HUGOT, MORAND and MICHAUX2010), based on both cytochrome b and CO1 gene sequences (M. Pagès, pers. comm.). In the case of M. surifer, it must be noted that identification of immature individuals based on morphology may be problematic in the presence of Maxomys rajah. Although we found these two species to co-occur at unlogged forest sites, only one immature individual was caught, and therefore we do not expect species misidentification to affect our results.
Microhabitat structure and disturbance
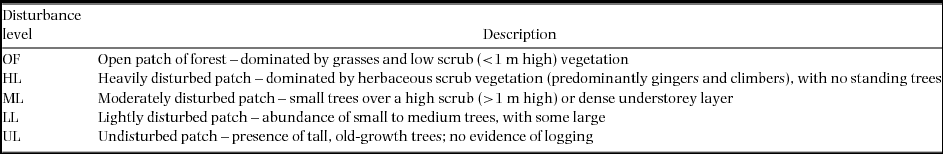
Quantitative and qualitative variables describing a number of forest attributes were collected at each trap site. These comprised structural and vegetation aspects of the environment measured within a 5-m radius centred on the trap. Six attributes were considered in this study: (1) percentage canopy closure as measured using a spherical densiometer (Lemmon Reference LEMMON1956); (2) a visual estimate of canopy height (after calibration using a 2-m pole); (3) a 1–4 index of ground vegetation cover (estimate describing what proportion of the ground area is covered in plants; 1 = 0–25% cover; 2 = 26–50% cover; 3 = 51–75% cover; 4 = 76–100%; Püttker et al. Reference PÜTTKER, PARDINI, MEYER-LUCHT and SOMMER2008); (4) a 1–4 index of understorey vegetation cover (estimate describing what proportion of the volume between 1 and 5 m was taken up by plants; 1 = 0–25% cover; 2 = 26–50% cover; 3 = 51–75% cover; 4 = 76–100%); (5) a count of the number of coarse woody debris items with diameter > 10 cm; and (6) leaf litter depth as measured with a needle ruler (averaged across four measurements taken at random within 1 m of the trap). Disturbance was also assessed within a 5-m radius centred on each trap site using a 5-level qualitative score. This index is based on a rapid assessment of the level of past or current anthropogenic disturbance and represents a gradient of small-scale intensity (OF – open patch of forest; HL – heavily disturbed patch; ML – moderately disturbed patch; LL – lightly disturbed patch; UL – undisturbed patch; see Appendix 1 for the specific criteria used for each level).
The design and layout of the SAFE sampling blocks aim to minimize the effect of potential large-scale confounding factors. All blocks are placed such that the majority of sampling points are located within a single altitudinal band between 400 and 500 m asl (Figure 1), and are oriented to control for differences in slope, latitude, longitude and distance to forest edges prior to the forest conversion (Ewers et al. Reference EWERS, DIDHAM, FAHRIG, FERRAZ, HECTOR, HOLT, KAPOS, REYNOLDS, SINUN, SNADDON and TURNER2011). These factors were therefore not considered in our analyses. Finally, although grids within a block were situated sufficiently far from each other to ensure they sampled different populations, the fact that no marked individual was ever caught at more than one grid (O. R. Wearn, unpubl. data) reinforces the independence of sampling grids.
Data analysis
Our analysis of trap-site detection was restricted to the three most commonly caught native species (Maxomys surifer, Maxomys whiteheadi and Leopoldamys sabanus) and one invasive species (Rattus rattus). For each of these, we pooled detection/non-detection data at the trap level across all six grids (E1, E2, F1, F2, OG1 and OG2), resulting in a total number of 500 sampling sites (sum of all trap sites across grids). We collapsed the data into a binary response variable describing species-specific detection (1) and non-detection (0) at each trap site. We took detection at a trap site as evidence that the species used the associated habitat. Importantly, when interpreting results, we use the expression ‘probability of detection’ to mean the unconditional probability of detection, as opposed to the probability of detection given presence, which is the conventional definition in occupancy analysis.
The influences of forest structure and patch disturbance on trap-site detection were modelled using logistic regression. In order to account for spatial auto-correlation, the term ‘grid’ was considered as a random intercept in a set of species-specific generalized linear mixed-effects models (GLMMs) with binomial error structures and logit link functions (Bolker et al. Reference BOLKER, BROOKS, CLARK, GEANGE, POULSEN, STEVENS and WHITE2009). For each species, the importance of the different microhabitat features was assessed by comparing models containing all possible combinations of the fixed effects describing canopy closure (z standardized), canopy height (z standardized), ground vegetation cover (GVC), understorey vegetation cover (UVC), number of coarse woody debris with diameter >10 cm (CWD; z standardized) and leaf litter depth (LL; z standardized). Pairwise comparisons showed low levels of collinearity between covariates describing microhabitat features. As a separate analysis, we also compared models with and without microhabitat disturbance as a fixed effect. In both cases, we used Akaike's Information Criterion (AIC) to carry out model selection (Akaike Reference AKAIKE1974) and averaged parameter estimates over models within 2 ΔAIC of the top model (Burnham & Anderson Reference BURNHAM and ANDERSON2002). In our results, the variance attributed to the random intercept is presented as the coefficient of variation (CV) of the random effect (random intercept value divided by its standard deviation) in the model with the lowest AIC, where a high value indicates higher relative variance in the intercept.
Analyses were carried out in R version 3.0.3. Models were constructed and compared using the lme4 package (Bolker et al. Reference BOLKER, BROOKS, CLARK, GEANGE, POULSEN, STEVENS and WHITE2009) and model averaging was carried out within package MuMIn (as per Grueber et al. Reference GRUEBER, NAKAGAWA, LAWS and JAMIESON2011). For fixed effects, we report transformed coefficient estimates and associated standard errors. P values were derived using an approximation of the Wald statistic, defined as the coefficient estimate divided by its standard error.
RESULTS
Trapping rates
In total, 504 detections of the four species were obtained over 3420 trap nights carried out across the two land-uses (Table 2). Overall trap success for these species was 14.7%. For M. surifer, the number of detections per trap night, or trapping rate, was highest in logged forest relative to unlogged forest. For both M. whiteheadi and R. rattus, trapping rates were considerably higher in logged forest, whilst those of L. sabanus were comparable across both land-uses (Table 2).
Table 2. Summary of detections per sampled grid for three native (M. surifer, M. whiteheadi and L. sabanus) and one non-native (R. rattus) murid species. Grids were established in repeatedly logged (E1, E2, F1 and F2) and unlogged (OG1 and OG2) forests of northern Borneo. Trapping rates (detections per 100 trap nights), calculated as the number of detections divided by the total number of trap nights (672 nights for all grids, except OG2 with 60) and multiplied by 100, are given in parentheses.

Influence of microhabitat structure on species-specific trap site detection
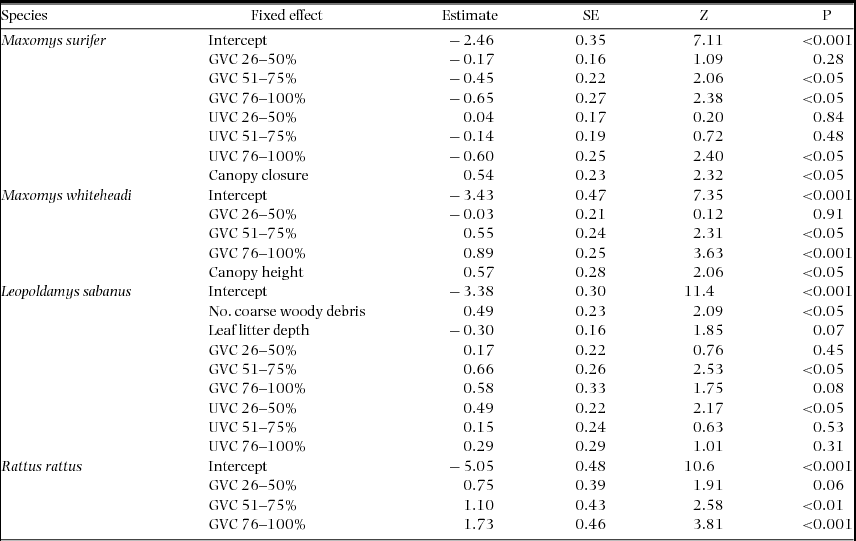
Overall, the effect and relative importance of each of the fixed variables describing forest microhabitat on probability of detection at the trap level varied considerably between species and between native and invasive rodents (Table 3, Appendix 2). Maxomys surifer was the only species to show a consistent negative response to GVC. Understorey vegetation cover – the forest stratum above the ground vegetation – was also retained as a predictor for this species, with both extreme levels of UVC (0–25% and 76–100% cover) having a significant negative effect on detection (Estimate + SE = −2.46 + 0.35, Z = 7.11, P < 0.001 and −0.60 + 0.25, Z = 2.40, P < 0.05, respectively). Finally, detection probability of M. surifer was also positively influenced by increased canopy closure (Estimate + SE = 0.538 + 0.232, Z = 2.32, P < 0.05).
Table 3. Model-averaged parameter estimates for forest structural components having a significant effect on the trap-site detection probability of three native (M. surifer, M. whiteheadi and L. sabanus) and one non-native (R. rattus) species of terrestrial murid in logged and unlogged forests of northern Borneo. Models were fitted in the glmer-function in the add-on library lme4 in the R software using a binomial error structure and a logit link function. The variable grid was fitted as a random intercept in all models. Estimates and their standard errors are measured on the logit scale (untransformed). Z represents an approximation of the Wald statistic (defined as Z = Estimate/SE) upon which the P value is based. Reference levels for ground-vegetation cover (GVC) and understorey-vegetation cover (UVC) (i.e. 0–25%) are confounded with the model-averaged intercept.

Increased detection probability of M. whiteheadi was associated with increased GVC and canopy height (Table 3). Ground vegetation cover also influenced detection of L. sabanus but with the difference that the highest level (75–100% cover) had a non-significant effect. In addition, the lowest level of UVC had a contrastingly negative effect on detection of this latter species relative to higher levels (Estimate + SE = −3.38 + 0.30, Z = 11.4, P < 0.001; Table 3). In contrast to all other species, the probability of detecting L. sabanus was influenced by the number of coarse woody debris pieces, which was found to have a positive effect on detection (Estimate + SE = 0.49 + 0.23, Z = 2.09, P < 0.05). Rattus rattus was the only species whose detection probability was influenced by only one of the microhabitat structure variables, namely ground vegetation cover. An increase in the latter had a positive effect on detection probability for this species (Table 3). Finally, the variance attributed to the random intercept (grid) was highest for L. sabanus (CV = 7.65). In contrast, it was much lower for M. surifer (CV = 0.95), R. rattus (CV = 1.19) and M. whiteheadi (CV = 0.92).
Influence of microhabitat disturbance on species-specific trap site detection
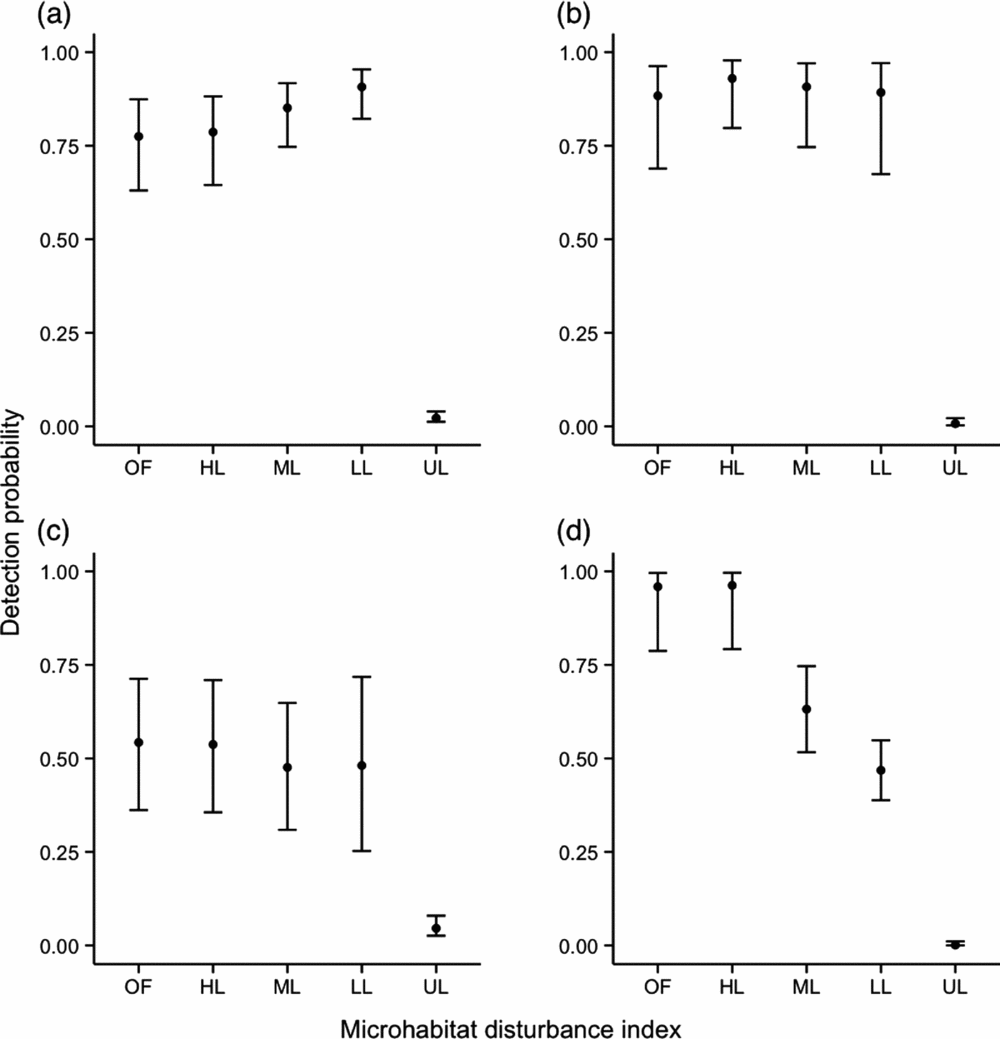
For M. surifer, M. whiteheadi and R. rattus, models that included a variable describing microhabitat disturbance were found to have lower AIC values, indicating its importance in determining the spatial patterns of these three species. Although M. surifer was more likely to be trapped at sites that had been logged, detection probability for this species tended to decrease with increased levels of disturbance (Figure 2). For M. whiteheadi, only trap sites representative of unlogged primary forest were found to have a significant negative effect on species detection at the trap level (Estimate + SE = −4.91 + 0.56, Z = −8.70, P < 0.001; Figure 2). In contrast, detection was significantly increased at logged sites. Probability of detection of R. rattus showed a consistent increase with patch disturbance, although it must be noted that the difference between the two highest levels of disturbance was not significant (Figure 2). Lowest detection probability for this species was associated with unlogged sites (Estimate + SE = −6.70 + 1.13, Z = −5.93, P < 0.001). Leopoldamys sabanus was the only species whose probability of detection was not associated with microhabitat disturbance. Coefficient estimates derived from the Detection~HQ model for this species are nevertheless shown in Figure 2.

Figure 2. Influence of microhabitat disturbance (OF – open patch of forest; HL – heavily disturbed patch; ML – moderately disturbed patch; LL – lightly disturbed patch; UL – unlogged patch; see Appendix 1) measured within a radius of 5 m around the trap site on detection probability of three native, Maxomys surifer (a), Maxomys whiteheadi (b), Leopoldamys sabanus (c), and one non-native, Rattus rattus (d), species of terrestrial murid in logged and unlogged forests of northern Borneo. Black dots are back-transformed (inverse logit) coefficient estimates derived from the model with microhabitat disturbance score as a fixed effect and grid as a random intercept. Error bars represent back-transformed 95% confidence intervals associated with these estimates.
Similarly to models investigating the influence of microhabitat structure on trap site detection probability, the variance attributed to the random effect grid was inconsistent across species. The model for M. surifer showed the highest variation in the random intercept (CV = 5.11), followed by L. sabanus (CV = 3.18). Models for M. whiteheadi and R. rattus showed similar levels of variance in their random intercept (CV = 2.38 and 2.08, respectively).
DISCUSSION
Influence of microhabitat structure
We investigated the microhabitat determinants of small-mammal detection probability at trap sites situated in logged and unlogged tropical forest. Each of the four species considered in this study showed contrasting preferences in terms of forest structural components. Rattus rattus was the only species whose probability of detection was dependent on only one variable, namely ground-vegetation cover, which suggests a less selective use of space. In addition, ground-vegetation cover has been shown to be higher in logged forests (especially Etlingera species (Zingiberaceae); Poulsen Reference POULSEN1996), thus making this land-use potentially more suitable for this invasive rodent (Bernard Reference BERNARD2004). Unlike ground vegetation cover, the influence of forest canopy, and in particular properties such as canopy height and closure, on the spatial patterns of small mammals is likely to be species dependent. For example, Beck et al. (Reference BECK, GAINES, HINES and NICHOLS2004) studied two species of Neotropical rodent and concluded that tree-fall-gap habitats had opposing effects on the measured fitness components of the species concerned. In our study, M. surifer appeared to be an example of a gap avoider in logged forests, as shown by the positive relationship between the probability of it being detected at a trap site and the percentage canopy closure. Heavily logged forest stands within the SAFE experimental area show a very discontinuous canopy cover owing to past selective logging practices and the existence of disused roads. In contrast, some stands contain occasional old-growth trees that significantly increase canopy height at specific locations (e.g. on steep slopes that cannot be accessed by logging operations; DeWalt et al. Reference DEWALT, MALIAKAL and DENSLOW2003). These may be favoured by certain species, such as M. whiteheadi in our study, and should be promoted in the management of logged forest concessions (Püttker et al. Reference PÜTTKER, PARDINI, MEYER-LUCHT and SOMMER2008).
Leopoldamys sabanus was the only species to be associated with the amount of coarse woody debris present in the vicinity of the trap. Deadwood items such as logs and fallen branches are known to be important for small-mammal movement as they provide uncluttered routes through the forest undergrowth (Wells et al. Reference WELLS, PFEIFFER, LAKIM and KALKO2006a) and also cover from predators. Interestingly, L. sabanus is the largest bodied of the four species (Payne & Francis Reference PAYNE and FRANCIS2007) and is known to travel significantly longer distances than most other sympatric terrestrial rodents (Nakagawa et al. Reference NAKAGAWA, MIGUCHI, SATO and NAKASHIZUKA2007, Wells et al. Reference WELLS, PFEIFFER, LAKIM and KALKO2006a). Preferential use of coarse woody debris by this species may explain its ability to travel further. However, it must be noted that L. sabanus is classified as semi-arboreal, being known to travel and build nests at heights of up to 10 m or more (Payne & Francis Reference PAYNE and FRANCIS2007). This fact corroborates well with the species’ preference for sites with higher levels of understorey vegetation cover, which could reflect increased availability of routes into the canopy.
Influence of microhabitat disturbance
For three of the species considered in this study (M. surifer, M. whiteheadi and R. rattus), probability of detection was higher at sites where the microhabitat showed evidence of past logging activities than in primary, unlogged forest. This finding is in agreement with previous studies showing that small terrestrial mammals are often highly adaptable to habitat disturbance (da Fonseca & Robinson Reference DA FONSECA and ROBINSON1990, Ochoa Reference OCHOA2000). In general, these habitats tend to have denser ground and understorey vegetation (Lambert & Adler Reference LAMBERT and ADLER2000), increased levels of coarse woody debris (Pfeiffer et al. unpubl. data), and reduced abundances of predators (Terborgh et al. Reference TERBORGH, LOPEZ, NUNEZ, RAO, SHAHABUDDIN, ORIHUELA, RIVEROS, ASCANIO, ADLER, LAMBERT and BALBAS2001), all of which are favourable to many small-mammal species. Highly disturbed logged forests, therefore, may be conducive to small-mammal conservation, at least for a subset of disturbance-tolerant species (Berry et al. Reference BERRY, PHILLIPS, LEWIS, HILL, EDWARDS, TAWATAO, AHMAD, MAGINTAN, KHEN, MARYATI, ONG and HAMER2010, Dunn Reference DUNN2004, Meijaard & Sheil Reference MEIJAARD and SHEIL2007, Reference MEIJAARD and SHEIL2008; Wearn et al. Reference WEARN, ROWCLIFFE, CARBONE, BERNARD and EWERS2013, Wilcove et al. Reference WILCOVE, GIAM, EDWARDS, FISHER and KOH2013). Conversely, small-mammal numbers may be constrained in unlogged forests by the prevalence of open ground and understorey levels (Lambert et al. Reference LAMBERT, MALCOLM and ZIMMERMAN2005) as well as reduced resources, although the latter hypothesis has not been rigorously tested.
The considerably higher trapping rates obtained for R. rattus at logged forest sites is testimony to its high potential to invade this land-use, and is in agreement with previous findings documenting higher occurrence of Rattus species in urban areas and areas of intermediate land-use intensity (Wells et al. Reference WELLS, LAKIM and O’HARA2014). Our study shows that this invasion has spread far from urban centres and into large tracts of logged forest contiguous with unlogged forest in Borneo. Nevertheless, within logged forest, we found reduced detection probability for the invasive R. rattus as evidence for disturbance decreased. This suggests that minimizing small-scale disturbance during logging could reduce the likelihood of invasion by R. rattus. This finding echoes Stokes et al. (Reference STOKES, BANKS, PECH and WILLIAMS2009a) who showed that disturbed microhabitats facilitated the invasion of introduced R. rattus, with varying consequences for native rodent species. Introduced Rattus species are known to compete with native species for access to resources (Gibson et al. Reference GIBSON, LYNAM, BRADSHAW, HE, BICKFORD, WOODRUFF, BUMRUNGSRI and LAURANCE2013, Stokes et al. Reference STOKES, BANKS, PECH and SPRATT2009b). Therefore, reducing the suitability of logged habitats for this species may represent an efficient way of preventing its spread whilst also offering overall better quality habitat for native species (Bernard et al. Reference BERNARD, FJELDSÅ and MOHAMED2009). Avoiding highly destructive logging practices through the implementation of reduced impact methods could be one way of achieving lower levels of small-scale habitat disturbance (Azevedo-Ramos et al. Reference AZEVEDO-RAMOS, DE CARVALHO and DO AMARAL2006, Gerwing & Uhl Reference GERWING and UHL2002, Putz et al. Reference PUTZ, SIST, FREDERICKSEN and DYKSTRA2008a).
Interestingly, the trap-site detection pattern of L. sabanus did not seem to be influenced by microhabitat disturbance. As mentioned above, this species is known to travel much greater distances than other smaller-bodied rodents (Wells et al. Reference WELLS, PFEIFFER, LAKIM and KALKO2006a). In doing so it may be more likely to travel through less suitable patches and therefore to be caught at a wider variety of microhabitats.
Study design considerations
Although grids, and land-uses, differed in the amount of sampling effort they received, we modelled detection at the individual trap-level, not pooled over each grid (or land-use). The number of binomial trials between grids, and land-uses, therefore differs, but this does not affect the magnitude of our parameter estimates, only their precision. Importantly, our analysis took into account the variation in detection attributable to grid location through the inclusion of a random intercept in all of our models. This process revealed differences in the effect of this variable across species, which likely reflects variation in species-specific densities across the sampled grids (O. R. Wearn, unpubl. data). Although we would have liked to verify this statement using independently estimated densities for the four species, these were not available at the time.
Conclusions
Our study has shown that small-scale variation in tropical forest structure can have an important influence on the spatial patterns of Bornean terrestrial rodents. Each of the four species considered here showed unique preferences for forest attributes measured at the level of the individual trap. They also responded differently to the level of microhabitat disturbance found within logged forest, with native species appearing to be associated with less intensive disturbance whilst the invasive R. rattus, which is known to pose a threat to native species through competition for resources and direct interference, demonstrated a preference for more degraded sites. We suggest that reduced-impact logging policies that minimize forest disturbance, as well as the rehabilitation of highly degraded areas that have already been logged, will provide added benefits in terms of invasion resistance, in addition to well-documented direct benefits in terms of carbon and biodiversity (Putz et al. Reference PUTZ, ZUIDEMA, PINARD, BOOT, SAYER, SHEIL, SIST, ELIAS and VANCLAY2008b). Without these, we risk compromising the biodiversity value of logged secondary forests by facilitating the spread of invasive species.
ACKNOWLEDGEMENTS
We would like to thank the Economic Planning Unit and the Sabah Biodiversity Centre for providing research permits, Sime Darby Foundation, the Sabah Foundation and Benta Wawasan, the Sabah Forestry Department and the Royal Society South East Asia Rainforest Research Programme. We are also grateful to the SAFE project staff and scientists for invaluable help in the field. We also thank three anonymous reviewers for providing comments on earlier drafts of the manuscript. The SAFE Project is funded by the Sime Darby Foundation.
Appendix 1. Description of microhabitat disturbance score levels recorded within a 5-m radius of each trap site. The influence of each of these levels on the probability of detection of three native (Maxomys surifer, Maxomys whiteheadi and Leopoldamys sabanus) and one non-native (Rattus rattus) species of Bornean murid was tested using generalised linear mixed effects models with the variable grid as a random factor. We collected detection/non-detection data at 500 trap sites across logged and unlogged forests in northern Borneo between April and July 2011.

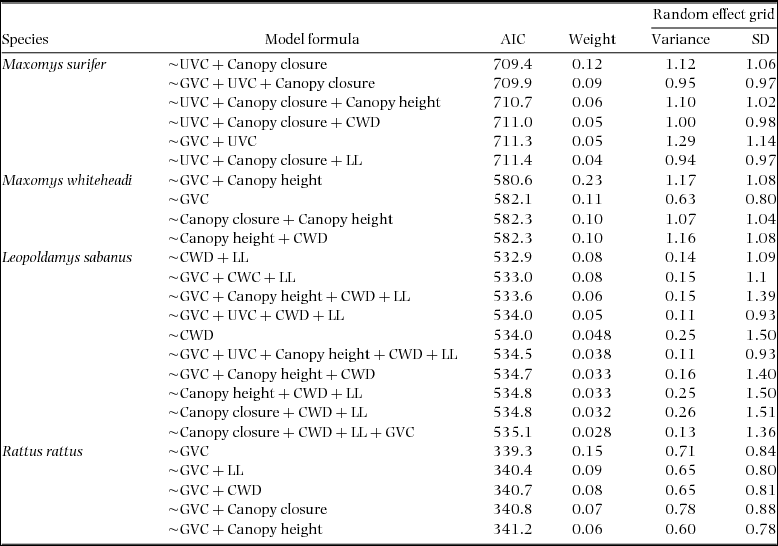
Appendix 2. Formula, AIC and weight values associated with generalised mixed-effects models within 2 ∆AIC of the top model for three native (Maxomys surifer, Maxomys whiteheadi and Leopoldamys sabanus) and one non-native (Rattus rattus) species of Bornean murid. The variance and standard deviance associated with the random intercept of the variable grid are also given for each model. Fixed effects included canopy closure (z standardized), canopy height (z standardized), ground vegetation cover (GVC – four-level factor), understorey vegetation cover (UVC – four-level factor), number of coarse woody debris with diameter >10 cm (CWD – z standardized) and leaf litter depth (LL – z standardized). These were collected across 500 trap sites located in logged and unlogged tropical forests of northern Borneo. In all cases, the response variable is binary and describes detection (1) and non-detection (0) of the corresponding species.