Introduction
Corvids are highly adaptive birds that respond well to anthropogenic changes in their environment. Some central European corvids are sedentary and do not migrate extensively (Corvus corax, Pica pica, Garrulus glandarius), while others, such as Corvus frugilegus, make long migratory movements, and nesting populations are replaced in summer with those nesting in West Russia, Ukraine, and Belarus (Cepák et al. Reference Cepák, Formánek, Horák, Jelínek, Klvaňa, Schröpfer, Škopek and Zárybnický2008). Local populations of other corvid species may also be supplemented in winter by varying numbers of migratory individuals from the northeast. Many corvids are highly opportunistic regarding their diets and consume many anthropogenic food items when such sources are available. Other food items consist of plants, including seeds, and various invertebrates; some predate other birds and mammals and feed on carrion after other predators have exposed the insides or when they have become opened due to roadkill. Although all the species studied herein are omnivorous, they differ in dietary composition, which is directly related to the spectra of immature trematodes that the respective birds can ingest. The diet of C. frugilegus is composed mainly of earthworms, insects, snails, small mammals, crustaceans, seeds, fruits, and anthropogenic waste (particularly in winter) (Gromadzka Reference Gromadzka1980; Orlowski et al. Reference Orlowski, Kasprzykowski, Zawada and Kopij2009; Maciorowski et al. Reference Maciorowski, Bural, Gierszal and Urbanska2014; Kitowski et al. Reference Kitowski, Sándor, Czarnecka and Grzywaczewski2017). The diet of Corvus cornix is composed of mollusks, earthworms, insects, and other arthropods, frogs, fish, small birds, and mammals, particularly immature birds, bird eggs, and carrion. The plant part of the diet consists of grain, potatoes, and various fruits and seeds; when occurring close to human settlements, a large part of the diet consists of anthropogenic waste (Berrow et al. Reference Berrow, Kelly and Myers1992; Zduniak et al. Reference Zduniak, Kosicki and Goudyn2008; Annala et al. Reference Annala, Tillman, Backus, Keacher and Avery2012; Goldyn et al. Reference Goldyn, Książkiewicz-Parulska and Zduniak2016). The diet of C. corax is composed mainly of vertebrates of small or intermediate size, bird eggs, seeds, carrion, and anthropogenic waste; in some regions, insects are seasonally present in the C. corax diet as well (Nogales Reference Nogales1997). The diet of Coloeus monedula consists primarily of plant materials (60–84% except in May; can exceed 97% in winter months), mainly grain and other seeds, and less commonly various berries; the diet is also supplemented with insects, particularly beetles, other invertebrates, small mammals, bird eggs, and immature birds (Lockie Reference Lockie1955; Hell and Soviš Reference Hell and Soviš1958). As with most other bird species, nestlings consume a higher share of food of animal origin, consisting primarily of adult, larval, and pupal beetles and larval butterflies (Högstedt Reference Högstedt1980). The diet of P. pica contains mostly insects, mollusks, small mammals, reptiles, immature birds, bird eggs, carrion, and, to some extent, seeds and various fruits (Högstedt Reference Högstedt1980; Kryštofková et al. Reference Kryštofková, Fousová and Exnerová2011). When available, a large portion of the diet is composed of anthropogenic waste. The diet of G. glandarius is mainly composed of buckeyes and acorns and also contains hazelnuts, grains, berries and seeds, insects, snails and slugs, immature birds and bird eggs, small mammals, reptiles, and carrion; nestlings are fed primarily insects and spiders (Eigelis Reference Eigelis1965).
Although corvid populations are being subject to large-scale changes due to synanthropization, and population increases have been recently recorded in most corvid species associated with anthropogenic environments (Keller et al. Reference Keller, Herrando, Voříšek, Franch, Kipson, Milanesi, Martí, Anton, Klvaňová, Kalyakin, Bauer and Foppen2020), corvid populations have rarely been studied from the helminthological viewpoint. A series of helminthological studies were conducted in the Volga River delta, Russia, in the 1960s and early 1970s (Lugovoi and Kurochkin Reference Lugovoi and Kurochkin1962; Chernobai Reference Chernobai and Kobyshev1966, Reference Chernobai and Markov1969; Budkin Reference Budkin1974). This region has specific properties because the Volga River has strong seasonal dynamics (Gorelits et al. Reference Gorelits, Ermakova and Terskii2018); therefore, the study birds had a unique dietary composition following regular flood events. Several large-scale studies were conducted in the 1950s and 1960s in other Eastern and central European countries (Macko Reference Macko1957; Luft Reference Luft1960; Stoimenov Reference Stoimenov1962; Rutkowska Reference Rutkowska1963; Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972; Koubek and Vojtek Reference Koubek and Vojtek1973). Studies conducted later or in other regions are lacking or include only a few host individuals except for P. pica (Davies Reference Davies1958; Todd Reference Todd1964; Todd and Worley Reference Todd and Worley1967; Ryšavý et al. Reference Ryšavý, Baruš and Groschaft1970; Mizuno Reference Mizuno1984; Borgsteede et al. Reference Borgsteede, Okulewicz and Okulewicz2000; Halajian et al. Reference Halajian, Eslami, Mobedi, Amin, Mariaux, Mansoori and Tavakol2011; Dipineto et al. Reference Dipineto, Borrelli, Pepe, Fioretti, Caputo, Cringoli and Rinaldi2013; Girisgin et al. Reference Girisgin, Alasonyalilar Demirer, Büyükcangaz, Khider, Birlik and Ipek2019; Yilmaz et al. Reference Yilmaz, Azizoglu and Adizel2020; Sitko and Heneberg Reference Sitko and Heneberg2021) and a recent study on C. frugilegus from two cities in Ukraine (Greben et al. Reference Greben, Dupak, Lisitsyna and Kuzmin2023). A large study of helminths of Corvus corone orientalis in Japan lacked data on prevalence and intensity and reported the recorded helminths together with those from Corvus macrorhynchos japonensis (Mizuno Reference Mizuno1984). An overview of currently available information on the prevalence and infection intensity of trematodes in corvids is provided in Table 1.
Table 1. Overview of previously published records of trematode prevalence and intensity of infection in corvids

Prior studies of corvid trematodes have provided highly heterogeneous data, reporting the component communities of P. pica (21 trematode species), C. cornix, and C. frugilegus (16 trematode species each) to be the most diverse. In contrast, those of C. monedula, G. glandarius, and Nucifraga caryocatactes contained only three to six species. Those of C. corone contained a single species, and there were no trematodes reported for C. corax (see Table 1 for more details). Some additional host-species records were reported in national checklists without any detail regarding the respective findings (Bykhovskaya-Pavlovskaya Reference Bykhovskaya-Pavlovskaya1962; Sulgostowska and Czaplinska Reference Sulgostowska and Czaplinska1987; Iskova et al. Reference Iskova, Sharpilo, Sharpilo and Tkach1995; Sitko et al. Reference Sitko, Faltýnková and Scholz2006). In the present study, we aimed to analyze the species composition and community structure of trematodes parasitizing central European corvids. We examined a large series of host individuals of six corvid species (C. frugilegus, C. cornix, C. corax, C. monedula, P. pica, and G. glandarius), focusing on those living in rural environments outside large cities and large municipal waste dumps, thereby avoiding those who feed mainly on anthropogenic waste. As the dietary composition of adults and nestlings differ, we analyzed the component communities of first-year birds and those of adult females or males separately.
Materials and Methods
Using data we obtained from 1963 to 2023, we examined 206 specimens of C. frugilegus, consisting of 105 adult males, 85 adult females, and 16 birds in the first calendar year (1Y). We further examined 39 specimens of C. cornix. The dataset included 20 adult males, 13 adult females, and six 1Y birds. We examined 17 specimens of C. corax, specifically, 3 adult males, 7 adult females, and seven 1Y birds. We examined 44 specimens of C. monedula, including 22 adult males, 15 adult females, and seven 1Y birds. We examined 169 specimens of P. pica, specifically, 98 adult males, 59 adult females, and 12 1Y birds. We also examined 120 specimens of G. glandarius, including 59 adult males, 46 adult females, and 15 1Y birds. Note that the same dataset of P. pica was analyzed by Sitko and Heneberg in their study of longitudinal trends among farmland birds (Sitko and Heneberg Reference Sitko and Heneberg2021).
We assigned the examined birds to three age and sex categories: 1Y birds (born in the calendar year when they were examined), adult females, and adult males (we considered birds in their second calendar year of life or older as adults). All the examined birds originated from the eastern and central Czech Republic (48.7°N–49.80°N, 13.3°E–18°30′E). We obtained the dead birds when they were provided to the Comenius Museum collection (Přerov, Czech Republic). The birds were wounded and injured individuals sacrificed in rescue stations due to untreatable wounds, and as some corvid species are legally hunted in Czechia, some of the examined birds were also provided by licensed hunters. Birds provided by the rescue stations included only those untreated with antihelminthic agents before being sacrificed. Governmental and local authorities authorized our long-term research; the Ministry of the Environment of the Czech Republic issued our most recent permit on August 3, 2009 (No. 11171/ENV/09-747/620/09-ZS 25).
We performed full-body necropsies, fixed the trematodes in 70% ethanol, stained them with borax carmine, transferred them through an alcohol series to xylene, and mounted them in Canada balsam, as described by Sitko and Heneberg (Reference Sitko and Heneberg2015). We recorded the infection intensities and species richness of trematodes in each examined host individual. We stored representative specimens in the Comenius Museum collections (Přerov, Czechia). We published some of the host–parasite records from the examined datasets in revisions of specific trematode taxa and previously used some of the analyzed helminths for molecular analyses (Heneberg et al. Reference Heneberg, Sitko and Bizos2015, Reference Heneberg, Sitko and Bizos2016, Reference Heneberg, Sitko, Těšínský, Rzad and Bizos2018); data obtained until 2006 were also used in the checklist of Czech trematodes (Sitko et al. Reference Sitko, Faltýnková and Scholz2006). The nomenclature used follows the Fauna Europaea database (de Jong et al. Reference de Jong, Verbeek, Michelsen, de Place, Los, Steeman, Bailly, Basire, Chylarecki, Stloukal, Hagedorn, Wetzel, Glöckler, Kroupa, Korb, Hoffmann, Häuser, Kohlbecker, Müller, Güntsch, Stoev and Penev2014) and recently published reclassifications (Heneberg and Sitko Reference Heneberg and Sitko2023). For details concerning the life cycles of the examined helminths, refer to Sitko et al. (Reference Sitko, Faltýnková and Scholz2006).
We calculated the mean frequency of infection and trematode load, trematode species-specific mean relative prevalence (the proportion of host individuals infected by the respective trematode species), and mean intensity of infection (the number of trematode specimens per host calculated over all hosts that were positive for the respective trematode). We computed rarefaction curves to interpolate the trematode species richness (Willis Reference Willis2019). We calculated the Chao-1 estimator corrected for unseen helminth species to estimate the true trematode species richness. Additionally, we compared helminth species richness using the presence/absence-based Sørensen similarity index and the abundance-based Bray-Curtis similarity index. We tested for differences in helminth diversity among the analyzed component communities using the Shannon diversity t-test (comparing values of the Shannon H index with a Poole’s bias correction term of two abundance datasets while assuming equal sampling conditions). Further, we calculated Gini-Simpson’s dominance, evenness, equitability, Fisher’s alpha, and the Berger-Parker dominance index to describe the species richness and diversity of the analyzed datasets. In the Gini-Simpson index, a value of one indicates complete domination of a single taxon, and zero indicates equal representation of all taxa. We used two evenness measures: equitability, in which the Shannon index is divided by a logarithm of the number of taxa, and Buzas and Gibson’s evenness, in which the Shannon index is divided by the species number. Fisher’s alpha is a parametric diversity measure assuming that the abundance of a particular taxon follows the log series distribution. The Berger-Parker index is calculated as the number of cases in the dominant taxon relative to the total number of cases (Harper Reference Harper1999). To avoid bias caused by differences in prevalence and infection intensities in juveniles and adults and to avoid possible sex-related bias, we evaluated the adult females, adult males, and 1Y birds for each species separately. We employed one-way PERMANOVA to identify differences among adult male, female, and 1Y bird hosts. All C. corax host individuals were treated as a single group due to the lack of trematodes in 1Y C. corax and low diversity of trematodes in this host species. We further used nonmetric multidimensional scaling (NMDS) to analyze the effects of explanatory variables (age, sex, and host species). We performed all calculations in SigmaPlot 12.0 (Systat Software, San Jose, California), EstimateS 9.1.0 (https://www.robertkcolwell.org/pages/1407-estimates), and PAST 2.14 (https://www.nhm.uio.no/english/research/resources/past/). Data are shown as the mean±SE unless stated otherwise.
Results
Helminth component communities of central European corvids
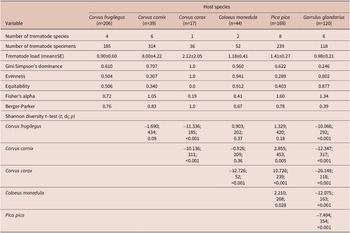
We collected a total of 944 specimens belonging to 16 species of trematodes. The trematode load differed among the host species by nearly one order of magnitude (Table 2). We analyzed host species-specific component communities in C. frugilegus (185 specimens, 4 trematode species), C. cornix (314 specimens, 6 trematode species), C. corax (36 specimens, 1 trematode species), C. monedula (52 specimens, 2 trematode species), P. pica (239 specimens, 8 trematode species), and G. glandarius (118 specimens, 6 trematode species) (Table 3; Figure 1). Although P. pica was represented by the second highest number of examined hosts, the dataset of obtained trematodes contained several singletons and doubletons (Table 3). The Chao-1 estimates of trematode species richness in the analyzed component communities were 4.0 (95% CI [4.0, 5.4]) species in C. frugilegus, 5.0 (95% CI [5.0, 6.0]) species in C. cornix, 1.0 (95% CI [1.0, 1.0]) species in C. corax, 2.0 (95% CI [2.0, 2.0]) species in C. monedula, 7.0 (95% CI [7.4, 8.1]) species in P. pica, and 5.0 (95% CI [5.0, 5.2]) species in G. glandarius.
Table 2. Diversity indices and outcomes of the Shannon diversity t-test

Table 3. Host-specific component communities of trematodes found in the present study


Figure 1. Rarefaction curves of the component communities of C. frugilegus, C. cornix, C. corax, C. monedula, P. pica, and G. glandarius sampled in the present study.
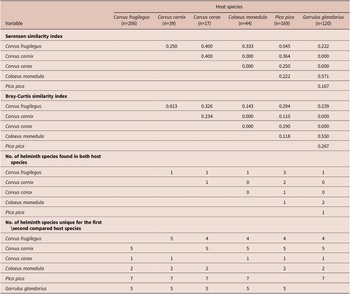
Species composition and diversity differed among the analyzed species. The Shannon diversity t-test revealed significant differences in all pairwise comparisons that involved G. glandarius or C. corax. The trematodes of P. pica differed from all other component communities except for that of C. frugilegus. The component communities of other host species were more similar, and there were no significant differences when comparing those of C. frugilegus with C. cornix or C. monedula or those of C. cornix with C. monedula (Table 2). The species of the component communities of C. frugilegus and P. pica (Sørensen similarity index 0.545; Table 4) and C. monedula and G. glandarius (Sørensen similarity index=0.571) overlapped most in terms of the number of species, while the similarity among all other species combinations was much lower. Abundance data also suggested the highest similarity between component communities of C. monedula and G. glandarius (Bray-Curtis similarity index=0.550; Table 4). In addition, the Bray-Curtis index identified high similarity between component communities of C. frugilegus and C. cornix (Bray-Curtis similarity index=0.613). All other abundance-based comparisons showed lower values. Despite sound Sørensen similarity indices, the Bray-Curtis similarity indices were low for comparisons involving P. pica, suggesting that although there was species overlap between the component communities of P. pica and other analyzed host species, the abundance of shared species was relatively low (Table 4). In absolute numbers, most component communities shared only one trematode species; the exceptions were component communities of C. frugilegus and P. pica (three species), C. cornix and P. pica (two species), and C. monedula and G. glandarius (two species) (Table 4).
Table 4. Comparison of the diversities of the analysed component communities

Alpha diversity, quantified using Fisher`s alpha, was low in all analysed host species. The highest alpha diversity was associated with P. pica (1.60) and G. glandarius (1.34), while the lowest alpha diversity was found for C. corax (0.19) and C. monedula (0.41). Gini-Simpson’s dominance values were generally high; the lowest Gini-Simpson’s dominance value was found for G. glandarius (0.246), and it exceeded 0.5 for all five other host species. Evenness differed among the analysed species, with a low value obtained for P. pica (0.29). In contrast, the C. corax, C. monedula, and G. glandarius component communities were associated with high evenness (1.00, 0.94, and 0.80, respectively). Equitability ranged between 0.00 (C. corax) and 0.912 (C. monedula). The Berger-Parker index ranged between 0.39 (G. glandarius) and 1.00 (C. corax) (Table 2).
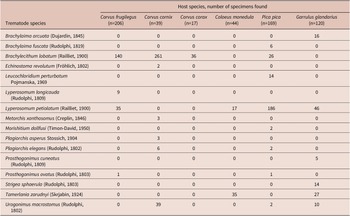
From the species-specific point of view, in all three Corvus spp., the most abundant trematode species was Brachylecithum lobatum. This species was also present in P. pica but was only the second most abundant trematode in P. pica after Lyperosomum petiolatum. Brachylecithum lobatum was lacking in representatives of the other corvid genera, Coloeus and Garrulus. In P. pica and G. glandarius, the most abundant species was L. petiolatum. It was also present in C. monedula and C. frugilegus (in both host species as the second most abundant trematode). The most abundant trematode in C. monedula was Tamerlania zarudnyi, which was also present in G. glandarius (Table 3). Other trematode species were represented chiefly by rare or incidental findings, except Urogonimus macrostomus (present abundantly in C. cornix and G. glandarius, with rare records in P. pica and absent in C. frugilegus, C. corax, and C. monedula). Findings of another species that was previously thought to be characteristic of corvids, Lyperosomum longicauda, were recently subjected to integrative morphological and molecular analysis, concluding that most putative L. longicauda represent L. petiolatum (particularly the immature specimens), and L. longicauda is present in C. frugilegus but is much less common than previously thought (Heneberg and Sitko Reference Heneberg and Sitko2023).
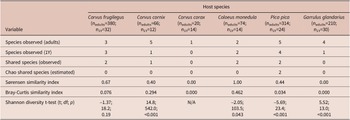
Trematode component communities in 1Y birds were mainly simplified compared to adult bird hosts. The trematodes were completely lacking in 1Y C. corax, and their diversity was significantly lower in 1Y C. cornix, C. monedula, P. pica, and G. glandarius. Only adult and 1Y birds of C. frugilegus hosted the same number of species. The number of trematode species shared between 1Y and adult birds of the respective host species ranged between zero (C. corax, G. glandarius) and two (C. frugilegus, C. monedula, and P. pica). The Bray-Curtis similarity index ranged from 0.0 (C. corax and G. glandarius), to low values (0.03–0.08 in P. pica and C. frugilegus), to 0.29 in C. cornix, and 0.46 in C. monedula (Table 5).
Table 5. Comparison of component communities in adult and 1Y host individuals

The combined data suggest that the trematodes of corvids have low prevalence and limited preferences for specific corvid host species, age, and sex. Their limited host preferences are supported by the NMDS analysis of all analysed host cases, using host species, age, and sex as explanatory variables, which did not identify any specific drivers of the analysed communities (Figure 2). Differences among component communities of adult males, adult females, and 1Y corvids were significant (one-way PERMANOVA (Bray-Curtis distance measure): permutation N=9999, total sum of squares=210.3, within-group sum of squares=193.7, F=6.735, p=0.0001). Subsequent pairwise comparisons revealed that all the variability was attributed to species-specific differences and that species-specific component communities of 1Y birds did not differ significantly from those in adult males or females of the same host species (Bonferroni-corrected p>0.05 each). Interestingly, all the differences between component communities of the host species were attributable to differences between component communities of adult host individuals. In contrast, there were no significant differences between species-specific component communities of 1Y birds of multiple corvid host species (Bonferroni-corrected p>0.05 each). Some of the singletons were found in 1Y birds. These included, for example, Prosthogonimus cuneatus (e.g., G. glandarius), Prosthogonimus ovatus (e.g., P. pica and C. frugilegus), Plagiorchis asperus (e.g., C. cornix), and Morishitium dollfusi (e.g., P. pica) (Figure 3). These records could be attributable to an increased share of insects (intermediate hosts of Prosthogonimus and Plagiorchis) and snails (intermediate hosts of Morishitium) in the diet of juveniles.

Figure 2. Nonmetric multidimensional scaling (Bray-Curtis distance measure) plots of the effects of explanatory variables (species, sex (adult M=1, adult F=0), and age (1Y=1, adult=0)) on the analysed trematode component communities in corvid birds. Colors indicate individual species, but there was complete overlap of the trematode communities from all six analysed host species. Points show host cases; convex hulls indicate host cases of the same type.

Figure 3. Heatmap comparing the prevalence [%] of the trematode species in adult and 1Y host birds. Trematode species are indicated on the left; host species and age are on the bottom. Color intensity corresponds to the prevalence in hosts of the respective age.
Discussion
We identified corvids as hosts of mutually overlapping component communities of only a few species of trematodes (B. lobatum, L. petiolatum, L. longicauda, T. zarudnyi, U. macrostomus), with the presence of many rare and incidental findings of other trematode species. The obtained data only partly overlapped with results of previous studies. Some of them were of limited size and thus may not be considered representative. Some originated from specific environments, like the river Volga delta, where the birds have different food opportunities compared to the examined habitats in the present study. Spatiotemporal factors may also play important roles, as different regions are associated with different intermediate hosts, and as most of the bird trematode communities have experienced simplification in recent decades dues to changes in food composition of the host birds and due to adverse effects of various chemicals, such as benzimidazoles that are massively used due to their fungicidal effects in agriculture.
Previous reports identified B. lobatum in only two of the eight studies on C. frugilegus listed in Table 1. This trematode was identified at 0.9% prevalence in Czechia and Slovakia (Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972) and at 1.7% prevalence in the Volga River region in Russia (Chernobai Reference Chernobai and Markov1969). However, other studies of C. frugilegus from the same regions failed to identify B. lobatum. In the present study, we found B. lobatum at a 1.9% prevalence in C. frugilegus. Previous studies did not report B. lobatum from C. cornix, C. corone, or C. monedula; we reported it previously from P. pica (Sitko and Heneberg Reference Sitko and Heneberg2021), and Macko (Reference Macko1957) found it in G. glandarius with a 4% prevalence (Table 1). In the present study, we report it from C. cornix at 15% prevalence, from C. corax (in 1 out of 17 examined birds), and P. pica at 2.4% prevalence. All examined C. monedula and G. glandarius were free of B. lobatum (Table 3). In line with previous findings (Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972), we identified B. lobatum in adult P. pica, C. corax, and C. frugilegus but not in 1Y P. pica, C. corax, or C. frugilegus. However, we found it equally in adult and 1Y C. cornix (Figure 3).
Previous studies of C. frugilegus trematodes identified L. petiolatum in only one host case (Table 1; Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972). It was absent from C. cornix, C. corone, and C. monedula but was present in two of 13 studies of P. pica trematodes (Table 1; Borgsteede et al. Reference Borgsteede, Okulewicz and Okulewicz2000; Sitko and Heneberg Reference Sitko and Heneberg2021), and Macko (Reference Macko1957) found it in 10% of examined G. glandarius. In the present study, we found L. petiolatum in 28% of P. pica, 8% of G. glandarius, 7% of C. monedula, and 6% of C. frugilegus. We did not find L. petiolatum in C. corax or C. cornix (Table 3). In contrast to previous findings (Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972), we found that the prevalence of L. petiolatum was higher or equal in 1Y C. frugilegus and C. monedula compared to adult individuals of the same host species, and the intensity of infection was also higher in 1Y than in adult individuals of these two host species. However, L. petiolatum was absent from 1Y P. pica and G. glandarius despite being present in adult individuals of the same species (Figure 3).
Previous studies identified L. longicauda in three of the eight studies on C. frugilegus listed in Table 1. This trematode was identified at 3.4% prevalence in Czechia and Slovakia (Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972), at 2.7% prevalence in the Volga River region in Russia (Chernobai Reference Chernobai and Markov1969), and at 2.2% prevalence in a recent study from Ukraine (Greben et al. Reference Greben, Dupak, Lisitsyna and Kuzmin2023). Other studies of C. frugilegus from the same regions failed to identify L. longicauda and also did not report other Lyperosomum spp. In previous studies, L. longicauda was absent from C. corone. Nevertheless, it was identified in a large study on C. cornix (0.6% prevalence) in Russia (Chernobai Reference Chernobai and Markov1969), one of six studies on C. monedula (3% prevalence, Great Britain (Davies Reference Davies1958)), one of 13 studies on P. pica (4.2% prevalence, Bulgaria (Stoimenov Reference Stoimenov1962)), and one of four studies on G. glandarius (2% prevalence, Poland (Luft Reference Luft1960)). In the present study, we found L. longicauda in 4% of C. frugilegus, but it was absent from all other corvids (Table 3). Recently published molecular analyses have shown that it is possible to identify only well-developed adult L. longicauda based on morphological traits, and it is challenging to distinguish the juvenile L. longicauda from L. petiolatum; therefore, previously published data on these two species must be interpreted with caution as misidentifications are likely common (Heneberg et al. Reference Heneberg, Sitko, Casero and Rząd2023).
Previous studies identified T. zarudnyi in two of the eight studies on C. frugilegus listed in Table 1. This trematode was identified at 0.3% prevalence in Czechia and Slovakia (Baruš et al. Reference Baruš, Ryšavý, Groschaft and Folk1972) and at 1.0% prevalence in the Volga River region in Russia (Chernobai Reference Chernobai and Markov1969). This trematode species was absent from studies on C. corone and G. glandarius. Nevertheless, it was identified in the course of the large study on C. cornix (0.6% prevalence) in Russia (Chernobai Reference Chernobai and Markov1969), in two of six studies on C. monedula (prevalence of 3% in Great Britain (Davies Reference Davies1958), and of 3% also in Russia (Chernobai Reference Chernobai and Markov1969)), and in two of 13 studies on P. pica (prevalence of 1.4% in Bulgaria (Stoimenov Reference Stoimenov1962) and of 0.3% in Russia (Chernobai Reference Chernobai and Markov1969)) (Table 1). In the present study, T. zarudnyi was present in both adult and 1Y C. monedula and adult G. glandarius (Figure 3).
The fifth trematode species, U. macrostomus, was previously reported as absent from all corvid species, and the only report consisted of our previous study on P. pica (Sitko and Heneberg Reference Sitko and Heneberg2021) (Table 1). In the present study, we confirmed it from adult C. cornix, 1Y P. pica, and both adult and 1Y G. glandarius (Figure 3).
Further research is needed to validate the differences between 1Y and adult birds and address the presence of the respective trematodes in nestlings. The nestlings are fed diets of different compositions, with a high share of insects and other soft-bodied animals. They are, therefore, more exposed to infections by trematodes hosted by insects (as shown for Prosthogonimus spp. and P. asperus). The component communities of trematodes in corvids are species-poor, but corvids serve as core hosts of several trematode species, including L. longicauda and B. lobatum). The limited prevalence of trematodes in C. corax in contrast to other corvid species is interesting and may be related to its specialization in carrion and vertebrate prey. Therefore, it would be interesting to compare the trematode data obtained for C. corax with that from regions where its diet includes a larger share of insects (Nogales Reference Nogales1997). However, it is also possible that the limited prevalence of trematodes in C. corax may be an artefact of relatively low sample size as we examined only 17 C. corax individuals.
Limitations
The present study relied on an opportunistic sampling design since we depended on the provision of carcasses of the examined hosts by rescue stations and hunters. A high proportion of the examined birds consisted of wounded or injured individuals. As such, the sample may not have necessarily contained representative trematodes that would be present in birds of good health. It is also highly likely that species with low prevalence escaped detection due to the limited number of examined host cases. However, none of these factors affected the ability of the present study to explore the composition of dominant species within the analysed component communities or to identify host species- and host age-specific differences in the analysed component communities.
Conclusions
We performed a large-scale analysis of the species composition and community structure of trematodes parasitizing central European corvids (C. frugilegus, C. cornix, C. corax, C. monedula, P. pica, and G. glandarius). The analysed carcasses represent one of the largest datasets analyzed thus far, as data for some corvid species (C. corax) are poorly available or include mostly host individuals analyzed in the 1950s through 1970s (C. frugilegus and G. glandarius). The examined samples originated from rural environments and, therefore, can serve as good comparative material for subsequent studies addressing issues associated with the synanthropization of most of the examined corvids and their increasing dependence on anthropogenic food sources or on feeding in urban habitats. Only a few trematode species use corvids as their core hosts (L. longicauda and B. lobatum), but corvids serve as permissive hosts for a broad range of trematodes as long as their intermediate hosts are included in the corvid diet.
Data availability
Representative specimens of the helminths analysed in this study are available in the collections of the Comenius Museum in Přerov. All data are available in the main text or the supplementary materials.
Acknowledgments
We thank the Bartošovice rescue station, landlords, and gamekeepers for providing us with carcasses of untreatable birds and for excellent long-term cooperation. We thank the governmental and local authorities for providing the necessary permissions to conduct this long-term research.
Authors’ contribution
JS and PH conceived the study, JS collected the data, PH analyzed the data and wrote the manuscript, and both authors revised the manuscript and agreed on its final version.
Financial support
The study was supported by the Ministry of Culture of the Czech Republic, project DE07P04OMG007.
Competing interest
On behalf of both authors, the corresponding author states that there is no conflict of interest.
Ethical standard
Not applicable.