Studies in Ghana( Reference Adu-Afarwuah, Lartey and Brown 1 , Reference Adu-Afarwuah, Lartey and Okronipa 2 ), Malawi( Reference Maleta, Phuka and Alho 3 , Reference Mangani, Maleta and Phuka 4 ), Haiti( Reference Iannotti, Dulience and Green 5 ) and Burkina Faso have tested the growth-promoting efficacy of small-quantity, lipid-based nutrient supplements (LNS) as a low-cost solution for tackling growth-faltering in young children. The studies have demonstrated modest( Reference Adu-Afarwuah, Lartey and Brown 1 , Reference Adu-Afarwuah, Lartey and Okronipa 2 , Reference Iannotti, Dulience and Green 5 ) or no effects( Reference Maleta, Phuka and Alho 3 , Reference Mangani, Maleta and Phuka 4 ) of LNS supplementation on child growth. At least three possible explanations for limited growth response to supplementation exist. First, supplementation starting at 6 months of age might come too late as children in resource-poor settings are commonly affected by intra-uterine growth restriction and thus are born stunted( Reference de Onis, Dewey and Borghi 6 ). Second, subclinical infection or inflammation may block the growth response to nutrient supplements, suggesting that interventions combining nutrition interventions with prevention and management of infections might increase the effect( Reference de Onis, Dewey and Borghi 6 – Reference Hess, Abbeddou and Jimenez 8 ). Third, energy from the supplementary food may be diverted to other needs of the children, such as physical activity( Reference Beckett, Durnin and Aitchison 9 , Reference Manary, Ndkeha and Ashorn 10 ).
Physical activity, which in young children is exhibited commonly in the form of play( Reference Cliff, Reilly and Okely 11 ), is an important determinant of young children’s cognitive and motor development( Reference Jahari, Saco-Pollitt and Husaini 12 , Reference Timmons, Naylor and Pfeiffer 13 ) and a contributor to their health in later life( Reference Timmons, Leblanc and Carson 14 , Reference Ekelund, Luan and Sherar 15 ). Some studies have found that undernourished children are less physically active than well-nourished children, but increase their activity level as their nutritional status improves( Reference Grantham-McGregor and Baker-Henningham 16 – Reference Worobey 18 ). This is plausible, given that the human body undergoes a series of physiological and behavioural changes, including reduction of physical activity, as a response to lowered energy intake( Reference Waterlow 19 , Reference Shetty 20 ). In addition to energy deficiency, micronutrient deficiencies, especially Fe deficiency, have been shown to result in reduced activity levels( Reference Aburto, Ramirez-Zea and Neufeld 21 – Reference Yaméogo, Cichon and Fabiansen 24 ). However, there is limited information on physical activity, measured by modern, objective methods( Reference Worobey 18 , Reference Yaméogo, Cichon and Fabiansen 24 – Reference Pulakka, Cheung and Ashorn 26 ), of young children in resource-poor settings, although some studies from high-income countries exist( Reference Hnatiuk, Ridgers and Salmon 27 – Reference Johansson, Hagstromer and Svensson 29 ).
Reduced physical activity of undernourished children is of concern, because it can be one of the mediators leading to poor child development. This theory is known as the functional isolation hypothesis: behaviour of undernourished children – that is, reduced activity and exploration and increased apathy – results in children having less interaction with their environment, and this in turn may restrict optimal development( Reference Lozoff, Klein and Nelson 23 , Reference Gardner and Grantham-McGregor 30 ). Moreover, in response to the undernourished children’s behaviour, caregivers may offer them less stimulation, which again hinders their development( Reference Grantham-McGregor and Baker-Henningham 16 , Reference Gardner and Grantham-McGregor 30 ).
Studies on the effect of nutrient supplementation on physical activity, using accelerometer-measured counts as an outcome, in areas with high prevalence of undernutrition are rare( Reference Faurholt-Jepsen, Hansen and Van Hees 17 , Reference Pulakka, Ashorn and Cheung 31 ). The International Lipid-Based Nutrient Supplements Study Group (iLiNS) has previously reported that small-quantity LNS did not increase the physical activity of the children in the Prevention of Linear Growth Failure in Infants and Young Children With Lipid-based Nutrient Supplements (iLiNS-DOSE) trial( Reference Pulakka, Ashorn and Cheung 31 ). However, the iLiNS-DOSE intervention was postnatal only, from 6 to 18 months of age, thus excluding the fetal period, which is crucial for brain growth and for later growth( Reference de Onis, Dewey and Borghi 6 ) and development( Reference Black, Walker and Fernald 32 ) of children. The currently reported study (Supplementing Maternal and Infant Diet With High-energy, Micronutrient Fortified Lipid-based Nutrient Supplements; iLiNS-DYAD-M NCT01239693, https://clinicaltrials.gov/ct2/show/NCT01239693) was designed to assess the health impacts of small-quantity LNS given to mothers during pregnancy and 6 months postpartum and to their infants from 6 to 18 months of age( Reference Ashorn, Alho and Ashorn 33 ). Our hypothesis was that children in the intervention group would be more physically active at the age of 18 months than children in the control group.
Methods
Setting and participants
The study was conducted in a semi-urban and rural area of Mangochi District, Southern Malawi. This area has high prevalence of chronic infant undernutrition( 34 ). The activity sub-study was a part of a larger trial, iLiNS-DYAD-M, details of which have been published earlier( Reference Ashorn, Alho and Ashorn 35 ). In brief, iLiNS-DYAD-M was a randomised, single-blind, parallel-group controlled trial testing the health effects of supplementing maternal diet during pregnancy and lactation and infant diet from 6 to 18 months of age with LNS. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the College of Medicine Research and Ethics Committee, University of Malawi, and by the Ethics Committee of Pirkanmaa Hospital District, Finland. We performed the trial according to Good Clinical Practice guidelines. Written or thumb-printed informed consent was obtained from all subjects.
The maternal enrolment took place through the antenatal clinics in Mangochi district hospital, Malindi hospital and Lungwena health centre. We included mothers whose ultrasound scan confirmed pregnancy of ≤20 completed gestation weeks. Detailed maternal inclusion and exclusion criteria were published previously( Reference Ashorn, Alho and Ashorn 35 ). For the physical activity sub-study, we recruited all participants who came to the last clinic visit of the main trial at the age of 18 months when they were still receiving the intervention. Those children who had moved out of the study area or whose guardian did not give consent for the sub-study were excluded. Data collection was conducted between January 2013 and March 2014.
Randomisation and blinding
The details of the randomisation procedure can be found in an earlier publication( Reference Ashorn, Alho and Ashorn 35 ). In brief, a researcher not involved in the data collection created randomisation slips in blocks of nine and sealed the slips in opaque, numbered envelopes. Eligible pregnant women were requested to choose one of the top six envelopes in a stack. The contents of the envelope indicated her participant number and group allocation.
We used single-masked procedures for the LNS intervention; that is, field workers who delivered the supplements knew which mothers were receiving LNS. The research assistants measuring activity and anthropometric outcomes were kept blinded to the group allocation until the end of data collection. The researchers doing the analyses were blinded until the data were cleaned and the statistical analysis plan published online (www.ilins.org and online Supplementary Material S1).
Interventions
The trial included three study groups( Reference Ashorn, Alho and Ashorn 35 ), which were collapsed into two groups for the purpose of this analysis. Children in the LNS group received 20 g of small-quantity LNS (20 g LNS) daily from 6 to 18 months of age. Their mothers had received 20 g of LNS during pregnancy and lactation (20 g LNS-P&L), daily during pregnancy and for 6 months thereafter. Both the 20 g milk containing LNS and 20 g LNS-P&L included twenty-two vitamins and minerals, 10 g fat (including linolenic acid and α-linolenic acid) and 2·6 g protein( Reference Ashorn, Alho and Ashorn 33 , Reference Arimond, Zeilani and Jungjohann 36 ). The main ingredients of the LNS were peanuts, vegetable oil, milk powder, and a vitamin and mineral mix. The 20 g daily ration of LNS would provide the RDA of most micronutrients for a healthy, breast-feeding infant( Reference Arimond, Zeilani and Jungjohann 36 ). LNS was produced and packed in individual 20 g foil sachets by Nutriset S.A.S.
Children in the control group did not receive supplementation, but their mothers had received either (a) one capsule daily of Fe-folic acid until delivery (60 mg Fe+400 µg folic acid) and one daily tablet of Ca (200 mg), akin to placebo, from delivery to 6 months postpartum (original Fe-folic acid (IFA) group) or (b) one tablet of multiple micronutrients (containing Fe-folic acid and sixteen additional micronutrients( Reference Arimond, Zeilani and Jungjohann 36 )) daily through pregnancy and 6 months postpartum (original micronutrients (MMN) group). Because of the absence of difference in child outcomes between the original IFA and MMN groups in our previous analyses( Reference Ashorn, Alho and Ashorn 33 , Reference Ashorn, Alho and Ashorn 35 ), and because children in these groups received no intervention from 6 to 18 months, we collapsed the IFA and MMN groups into a single control group.
Follow up
Data collectors made weekly home visits to collect information on supplement use and fortnightly visits to deliver the supplements. Guardians were advised to divide the daily LNS ration into two equal proportions, mixed with porridge. As a result of a new quality assurance procedure in the supplement production, there was a brief interruption in LNS delivery during the trial implementation. Because of this episode, 121 children missed receiving LNS for a period that ranged from 1 to 41 d between 1 August and 11 September 2012. Further details on this episode were published earlier( Reference Ashorn, Alho and Ashorn 35 ). Apart from malaria, which was treated with lumefantrine/artemether at the study clinic, the participants’ medical conditions were treated in Malawi’s national health system, with the study team reimbursing the participants for all medical costs.
Measurement of outcomes
Physical activity was measured over 1 week with the ActiGraph GT3X+ (ActiGraph LLC), a small accelerometer that records accelerations in three different axes: vertical, antero-posterior and medio-lateral( Reference Pulakka, Cheung and Ashorn 26 , Reference John and Freedson 37 ). While at the clinic, research assistants instructed the guardians to secure the accelerometer on the child’s right hip using an elastic belt and to allow the child to wear the device continuously throughout the day and night and to remove it only if the child showed signs of discomfort.
Measurement of covariates
At the maternal enrolment visit, trained anthropometrists measured the mothers’ weight with digital scales (SECA 874 flat scale; Seca GmbH & Co.) and height with stadiometers (Harpenden stadiometer; Holtain Limited). Research assistants obtained maternal age and years of schooling by interviewing mothers at the enrolment visit. Household food insecurity was assessed with the Household Food Insecurity Access Scale (HFIAS)( Reference Coates, Swindale and Bilinsky 38 ). On the basis of the length of follow up and the number of supplement doses delivered home and returned unused, the mean adherence (proportion of days when the children consumed LNS supplements) was 77 %( Reference Ashorn, Alho and Ashorn 33 ).
Children’s date of birth was verified by the research assistants who visited the child soon after birth( Reference Ashorn, Alho and Ashorn 35 ). At the clinic visits at 6 and 18 months of age, research anthropometrists measured participants’ weight in triplicate to the nearest 20 g using an electronic infant-weighing scale (SECA 381 baby scale; Seca GmbH & Co) and length to the nearest 1 mm using a high-quality length board (Harpenden Infantometer; Holtain Limited). We calculated length-for-age z-score (LAZ) and weight-for-length z-score (WLZ) using the WHO 2006 Child Growth Standards( 39 ). Research assistants observed the children to assess their ability to walk at 18 months of age. Guardians were interviewed on how much the children were being carried by others, and children who were reportedly being carried 1 h or more every day or almost every day were classified as carried.
Data processing and analyses
We analysed data using Stata/IC software, version 12.1 (StataCorp.). We set the level of statistical significance at 0·05 for all analyses. All the analyses were based on the principle of modified intention to treat; that is, we included all participants randomly assigned in the analyses, with the exception that two participants whose group allocations were incorrectly transcribed and assigned during enrolment were included in the group corresponding to the actual intervention they received throughout the trial. We considered physical activity data to be missing if the actual onset of measurement was over 30 d from the planned date. There were two reasons for this choice: children were no longer receiving intervention at this stage and improved motor skills at older age could result in accelerometer readings different from those of children at 18 months of age. The data reduction was done similarly to our earlier study( Reference Pulakka, Ashorn and Cheung 31 ): we excluded the first and the last days of measurement as incomplete days, night time between 20.00 and 05.00 hours, and strings of ≥20 min of zeroes( Reference Cliff, Reilly and Okely 11 ). Participants with ≥4 d( Reference Hnatiuk, Ridgers and Salmon 27 ) with ≥6 h( Reference Trost, Pate and Freedson 40 ) of accelerometer data were included in the analyses. We set epoch length at 15 s( Reference Cliff, Reilly and Okely 11 , Reference Oliver, Schofield and Kolt 41 , Reference Pate, O’Neill and Mitchell 42 ).
We used daily mean vector magnitude (VM) counts/15 s as the main outcome. The VM counts were calculated by taking the square root of the sum of squared activity counts of each of the three axes. Secondary outcomes included mean vertical axis counts/15 s, percentage of time in moderate-to-vigorous physical activity (MVPA), percentage of time being sedentary and percentage of active children. We calculated mean VM and vertical axis counts/15 s by averaging mean counts/15 s of each day over all valid days for each of the participants. The percentage of time spent in MVPA was defined using validated cutoff points of vertical axis activity counts ≥419 counts/15 s( Reference Trost, Fees and Haar 25 ) and percentage of time being sedentary as vertical axis activity counts ≤48 counts/15 s( Reference Trost, Fees and Haar 25 ). The proportion of active children was calculated according to the guidelines of the US National Association for Sports and Physical Education as those children whose mean time in MVPA over all valid days was ≥90 min/d( 43 ).
We used Fisher’s exact test to test for differences in the rate of loss to follow up between groups. We tested the hypothesis that physical activity of infants in the intervention group would be greater than that of infants in the control group for each activity outcome using Student’s t test, and the hypothesis that a greater proportion of children in the intervention group would reach 90 min of MVPA/d with a log-binomial regression model. We also drew kernel density plots for each of the outcomes. As a secondary analysis for assessing the mean VM counts in the intervention and control groups, we built a regression model adjusting for eight pre-specified variables (LAZ at 6 months, WLZ at 6 months, sex, season of activity measurement, birth order, maternal education, maternal age and HFIAS score) and for child carrying (carried v. not). We also performed a sensitivity analysis by testing the differences in mean VM counts between the three original groups, LNS, IFA and MMN, with ANOVA.
The sample size was originally calculated in accordance with the main objective of the trial: 288/group to detect an effect size of 0·3 of LNS on child length. The sample size of about 190 LNS and 380 control participants for this sub-study offered about 80 % power to detect an effect size of 0·25 sd in continuous outcomes at 5 % two-sided type I error rate.
Results
Fig. 1 presents the flow of the study participants and their mothers. Originally, 869 pregnant mothers were enrolled into this study. The 786 mothers who were still in the study at the time of delivery gave birth to 790 infants. Of the 661 children who were measured for activity at 18 months of age, 570 had sufficient data and were included in the activity analysis (78 % of the 728 children who were in follow up at the beginning of child intervention at 6 months of age).

Fig. 1 Participant flow. LNS, lipid-based nutrient supplements.
Background characteristics of the children were similar in the intervention and control groups at 6 months of age (Table 1). Of the children who were in follow up at 6 months of age, 21 % in the LNS group and 22 % in the control group (P=0·85) were either lost to follow up or had insufficient accelerometer data. Children who were included in the analysis were generally similar in their mean background characteristics compared with the 158 children who were available at 6 months of age but were not included in the analysis, except that their mothers had fewer years of schooling. The included participants had a mean of 5·9 (sd 1·5, range 4–10) d of activity measurement with a median measurement time of 13·5 h/d (interquartile range 12·1–14·3 h).
Table 1 Background characteristics of participants and their mothers (Mean values and standard deviations; numbers and percentages)

LNS, lipid-based nutrient supplements.
* Carried 1 h or more every day or almost every day by maternal report.
The main outcome – mean VM accelerometer counts/15s – was 302 (SD 57) for all the included participants. The mean VM counts/15 s was 303 (sd 59) for children in the LNS group and 301 (sd 56) for children in the control group (P=0·65) (Fig. 2 and Table 2). There was no statistically significant difference in the secondary outcomes between the groups (Table 2 and online Supplementary Fig. S1). In the LNS group, 38·4 % of the children reached the recommendation of 90 min of MVPA/d, whereas the corresponding figure for the control group was 35·8 (risk ratio 0·93; 95 % CI 0·74, 1·17) %. The results from the regression analysis also showed no association between intervention and physical activity (β-coefficient 4·3; 95 % CI 15, 6; P=0·41). In the sensitivity analysis, comparing the three original groups, the mean VM counts/15 s was 303 (sd 59) in the LNS group, 306 (sd 62) in the MMN group and 297 (sd 49) in the IFA group (P=0·28).

Fig. 2 Kernel density plots of mean vector magnitude counts/15 s by groups. ![]() , Lipid-based nutrient supplements;
, Lipid-based nutrient supplements; ![]() , control.
, control.
Table 2 Physical activity at 18 months of age (Mean values and standard deviations; difference in mean values and 95 % confidence intervals)
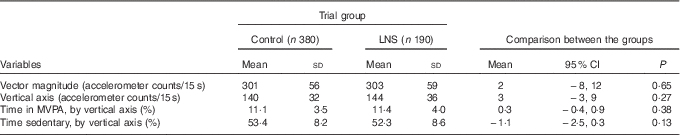
LNS, lipid-based nutrient supplements; MVPA, moderate-to-vigorous physical activity.
Discussion
The purpose of this sub-study of the randomised, controlled iLiNS-DYAD trial was to measure the effect of dietary supplementation with LNS, given to mothers during pregnancy and 6 months postpartum and to their infants from 6 to 18 months of age, on children’s physical activity in semi-rural Malawi. The mean accelerometer counts were slightly higher among the supplemented than among the non-supplemented participants in our sample, but the differences were small and statistically insignificant.
The results of this study are very similar to our previous findings where LNS did not increase the physical activity of Malawian children when given in 10–40 g quantities/d from 6 to 18 months of age( Reference Pulakka, Ashorn and Cheung 31 ). In the current study, giving LNS to mothers during pregnancy and lactation did not seem to offer additional benefits in terms of physical activity of the children. We are not aware of any other studies measuring the effects of LNS supplementation on physical activity; however, there are previous studies examining physical activity after supplementation with multiple micronutrients( Reference Jahari, Saco-Pollitt and Husaini 12 , Reference Gardner, Grantham-McGregor and Chang 44 – Reference Aburto, Ramirez-Zea and Neufeld 46 ). These studies have demonstrated varying results. Several of them reported positive effects on physical activity from supplementation with either Zn( Reference Sazawal, Bentley and Black 47 , Reference Bentley, Caulfield and Ram 48 ), multiple micronutrients( Reference Aburto, Ramirez-Zea and Neufeld 46 ) or milk and multiple micronutrients in children with Fe-deficiency anaemia( Reference Harahap, Jahari and Husaini 45 ). One of the studies found no effect( Reference Gardner, Grantham-McGregor and Chang 44 ) and one reported mixed results( Reference Jahari, Saco-Pollitt and Husaini 12 ) of a milk-based intervention. All of the studies used observation as the method for measuring activity.
There are several theoretically plausible explanations for why the LNS intervention had no significant effect on children’s physical activity. One limiting factor could be relatively low (77 %) adherence to consuming LNS. The energy provided by LNS might replace rather than be additive to energy from breast milk or complementary foods. However, our previous studies suggest that provision of LNS does not reduce breast milk or complementary food intake at the age of 9–10 months( Reference Kumwenda, Dewey and Hemsworth 49 , Reference Hemsworth, Kumwenda and Arimond 50 ). According to the accelerometer measurement, children (even in the control group) were at least as active as has been reported for toddlers in high-income countries( Reference Wijtzes, Kooijman and Kiefte-de Jong 28 , Reference Van Cauwenberghe, Gubbels and De Bourdeaudhuij 51 ), suggesting that they were already as active as might be expected at this age despite residing in a food-insecure area. There is considerable heterogeneity in the results of studies testing LNS for improving growth or treating moderate malnutrition( Reference Lenters, Wazny and Webb 52 ), implying that there could be underlying, context-specific mechanisms affecting children’s health and well-being and limiting response to nutrition interventions. Therefore, it is possible that nutrition interventions alone are not sufficient, but interventions that aim to improve children’s well-being also need to target other risk factors, such as infections( Reference Dewey and Mayers 7 , Reference Humphrey 53 , Reference Gough, Moodie and Prendergast 54 ).
The main strengths of this study include the following: randomisation, which controls for confounders and decreases selection bias, objective measurement of physical activity, which eliminates reporting bias inherent to subjective physical activity measurement methods; and broad inclusion criteria, which increase the generalisability of results. The sample size was large enough to detect an effect size of 0·25 sd with about 80 % power.
The main weakness of the study was that the guardians could not be blinded to the treatment allocation of their children. However, because we used objective measurement of the outcome, this is unlikely to have affected the physical activity measurement. The weaknesses of accelerometers in measuring physical activity include the fact that they do not provide information on activity type or context, nor do they distinguish between the causes of movement, which may lead to overestimation of activity in infants who are frequently being carried by their caregivers( Reference Cliff, Reilly and Okely 11 , Reference Worobey 18 , Reference Tsai, Burr and Thomas 55 ). However, overestimation of activity while the child is being carried appears to be more common among young infants who cannot walk( Reference Worobey 18 ), whereas only 17 % of the 18-month-old children in both the control and intervention groups in our study were reportedly being carried ≥1 h daily or almost daily. Furthermore, we did not perform per-protocol analysis – that is, including only the most adherent children – because we did not have a placebo control and were thus unable to identify and exclude non-adherent children in the control group. Although loss to follow up was about 22 %, differences in background characteristics between participants who were enrolled or not enrolled into this sub-study were small, suggesting that attrition bias was not likely. Taking into account these shortcomings, we believe that the main results are valid and sufficiently representative of the target population, suggesting that LNS supplementation does not markedly increase the mean physical activity of young children in the defined target group.
In conclusion, our results do not support the hypothesis that LNS, given to mothers during pregnancy and 6 months postpartum and to their infants from 6 to 18 months of age, increases physical activity among 18-month-old children in semi-rural Malawi. Further research is needed to identify interventions that could improve the health and well-being of children who are living in food-insecure areas. Given the bidirectional relationship between nutrition and infections, whereby poor nutrition increases the risk for infections and infections can worsen nutritional status, these interventions might need to target both nutrition and infections. Furthermore, current recommendations for children’s physical activity are mainly based on parental-reported physical activity, a measure that is known to be biased. It would thus be important to establish recommendations for sufficient physical activity to support the health and development of children based on research using objective physical activity measures, such as accelerometers.
Acknowledgements
The authors thank all the participating families and the data collection teams for their participation and contribution to the study. The accelerometer data were collected by teams lead by Martin Ndelemani.
This publication is funded in part by a grant to the University of California, Davis, from the Bill & Melinda Gates Foundation, with additional funding from the Office of Health, Infectious Diseases, and Nutrition, Bureau for Global Health, US Agency for International Development (USAID) under terms of cooperative agreement AID-OAA-A-12-00005, through the Food and Nutrition Technical Assistance III Project, managed by FHI 360. For data management and statistical analysis, the team received additional support from the Academy of Finland through grant no. 252075 and from the Medical Research Fund of Tampere University Hospital through grant no. 9M004. Y. B. C. was supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist Award. Lotta Hallamaa provided statistical support.
The findings and conclusions contained within the article are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation, USAID, the US government, or the other funders. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors’ contributions were as follows: A. P., Y. B. C., K. M., K. G. D. and P. A. were responsible for the study concept and design. A. P., C. K., J. B., U. A and P. A. conducted the data collection. A. P., Y. B. C. and P. A. designed the analysis. A. P. conducted the data analysis, wrote the first draft of the manuscript and had primary responsibility for the final content. All the authors critically reviewed and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/doi:10.1017/S0007114517000290






