Introduction
Patients with amyotrophic lateral sclerosis (ALS) may present with disproportional wasting and weakness of the abductor pollicis brevis (APB) and first dorsal interosseous (FDI) muscles, with relative sparing of the abductor digiti minimi muscle (ADM), which is known as the split-hand syndrome. Reference Wilbourn1 Consequently, the split-hand index (SHI) ((FDI × APB)/ADM) has been suggested as a useful measure for ALS diagnosis, using electrophysiological indices such as the compound muscle action potential (CMAP) amplitudes, Reference Menon, Kiernan, Yiannikas, Stroud and Vucic2–Reference Wang, Liu, Ding, Hu and Cui4 F-waves, Reference Wang, Liu, Ding, Hu and Cui4,Reference Wang, Liu, Cai, Ding, Hu and Cui5 and motor unit number index (MUNIX), Reference Kim, Hong, Shin, Park, Sohn, Lee, Park and Sung3,Reference Günther, Neuwirth and Koch6 and recently also by using sonographic indices including muscle echointensity Reference Seok, Park, Kim, Oh, Kim and Kim7 and thickness. Reference Abraham, Fainmesser, Drory and Bril8 In addition, split hand was recently demonstrated also in patients with spinal muscular atrophy (SMA) using MUNIX Reference Günther, Neuwirth and Koch6 and muscle ultrasound (MUS). Reference Abraham, Fainmesser, Drory and Bril8 However, the sensitivity and specificity of split hand are somewhat limited, as split hand can be found in 20% of disease controls, Reference Menon, Kiernan, Yiannikas, Stroud and Vucic2,Reference Shibuya, Misawa and Uzawa9 and at a higher rates of 30% in patients with inflammatory polyneuropathies. Reference Shibuya, Misawa and Uzawa9
We have previously shown that SHI can be determined by quantitative sonographic assessment of hand muscle thickness, with excellent diagnostic accuracy for differentiating healthy controls from patients with ALS, and with good diagnostic accuracy for differentiating healthy controls from patients with SMA. Reference Abraham, Fainmesser, Drory and Bril8 In the present study, we aimed to explore the specificity of SHI derived by MUS for differentiating ALS and SMA from patients with other neuromuscular disorders.
Materials and Methods
Healthy controls were prospectively recruited at the Prosserman Family Neuromuscular clinic, Toronto General Hospital, University Health Network, from October to December 2018, as part of a study aimed to determine normative values for muscle thickness measured by US, including subjects from the clinic and hospital staff, and family members accompanying patients to the neuromuscular clinic. Reference Abraham, Drory, Fainmesser, Lovblom and Bril10 The Research Ethics Board of the University Health Network approved the study protocol and all subjects signed an informed consent. In addition, patients with ALS, SMA, polyneuropathy, and myopathy were prospectively recruited at the neuromuscular clinic at Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, from December 2018 to December 2020. The Institutional Review Board of Tel Aviv Sourasky Medical Center approved the study protocol and all subjects signed an informed consent. Clinical scales applied to assess disease severity included ALS Functional Rating Scale Revised (ALSFRS-R) Reference Cedarbaum, Stambler and Malta11 in patients with ALS and SMA, Hammersmith Functional Rating Scale Expanded (HFMSE) Reference O’Hagen, Glanzman and McDermott12 in patients with SMA, and the Overall Neuropathy Limitations Scale (ONLS) Reference Graham and Hughes13 in patients with polyneuropathy and myopathy. All healthy controls and patients underwent quantitative sonographic evaluation of muscle thickness, including the right APB, FDI, and ADM muscles, except for patients with significantly greater weakness in their left hand that underwent sonographic evaluation of their left hand. All subjects underwent MUS examination by a single examiner experienced with neuromuscular US (AA), in the supine position. MUS was performed in healthy controls using General Electric ultrasound device (LOGIQ S7 Expert, Toronto, Canada), and a transducer with a frequency of 15 Hz (ML6-15), Reference Abraham, Drory, Fainmesser, Lovblom and Bril10 while MUS in all patients was performed using Mindray M7 ultrasound device (Mindray, Shenzen, China), with a linear array transducer (L14-6Ns, Mindray, Shenzen, China). Muscle compression apart from the weight of the probe, was avoided, and the mean value of two to three consecutive thickness measurements was calculated to improve reliability. Reference Abraham, Drory, Fainmesser, Lovblom and Bril10 All muscles were imaged at their short axis, with electronic calipers placed online to measure muscle thickness at the location of maximum thickness. For FDI imaging, the probe was placed with a hand at semipronation perpendicular to the axis of the second metacarpal at the level of the first metacarpophalyngeal joint, Reference Simon, Ralph and Lomen-Hoerth14 for the APB with the hand at supination perpendicular to the axis of the first metacarpal slightly proximal to its center, and for the ADM with the hand at supination laterally and perpendicular to the axis of the fifth metacarpal slightly proximal to its center (Figure 1).
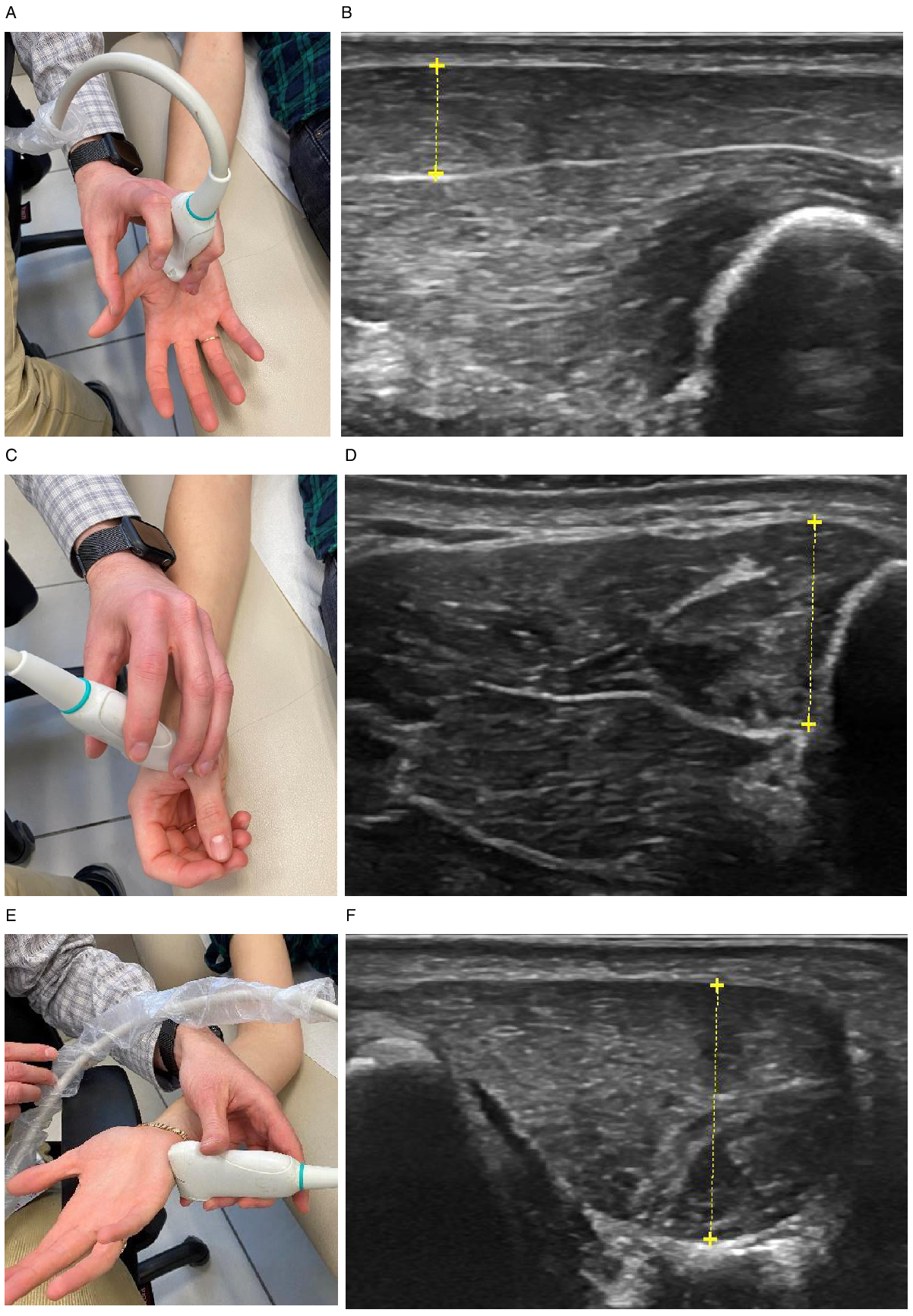
Figure 1: Ultrasound probe position and images of APB (A and B), FDI (C and D), and ADM (E and F) muscles in a healthy subject.
Statistical Analysis
Statistical analysis was performed using statistical package for social sciences (SPSS) software version 25 (IBM Corp., Armonk, NY, USA). Comparisons of demographics, clinical characteristics, between healthy controls and patients were performed using the Kruskal–Wallis one-way analysis of variance, Mann–Whitney U test, or the χ2-test, as appropriate. Comparisons of muscle thickness and SHI between healthy controls and each patient subgroup were performed using the Kruskal–Wallis one-way analysis of variance. Pairwise comparisons have been adjusted by the Bonferroni correction for multiple tests. Diagnostic accuracy for differentiating between healthy controls and each patient subgroup and between different patient subgroups was performed using the area under curve (AUC) calculated from the receiver operating characteristics curves formed using sensitivity and (1-specificity). Optimal cut-off point for SHI maximizing sensitivity and specificity was determined using Youden index. P values <0.05 were considered as significant.
Results
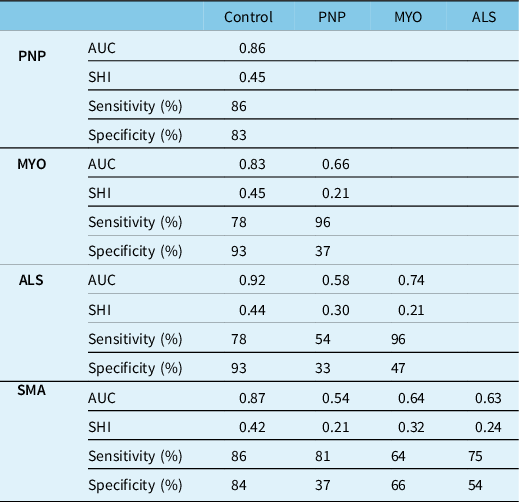
Sixty-five controls, 91 patients with ALS, 33 with SMA, 35 with polyneuropathy, and 22 patients with myopathy were included (Tables 1S and 2S). There were significant differences between subgroups. While SMA patients were the youngest with longest disease duration, ALS patients were the oldest with shortest disease duration. ALS patients had a more severe disease compared with SMA patients as captured by the ALSFRS-R, while there was no difference in disease severity between patients with polyneuropathy and myopathy as captured by the ONLS (Table 1). Compared with healthy controls, FDI muscle thickness was lower in all patients, APB in all patients except those with myopathy, and ADM only in patients with ALS and SMA. The SHI was lower in all patient subgroups compared with controls, most prominently in ALS (Figure 2). Although SHI showed excellent accuracy for differentiating ALS patients from controls (AUC – 0.92), and good accuracy for differentiating other patient subgroups from controls (AUC 0.83–0.87), poorer diagnostic accuracy was shown for differentiating between different patient subgroups (AUC 0.54–0.74), with the lowest diagnostic accuracy shown for differentiating SMA from polyneuropathy (AUC 0.54) (Table 2).
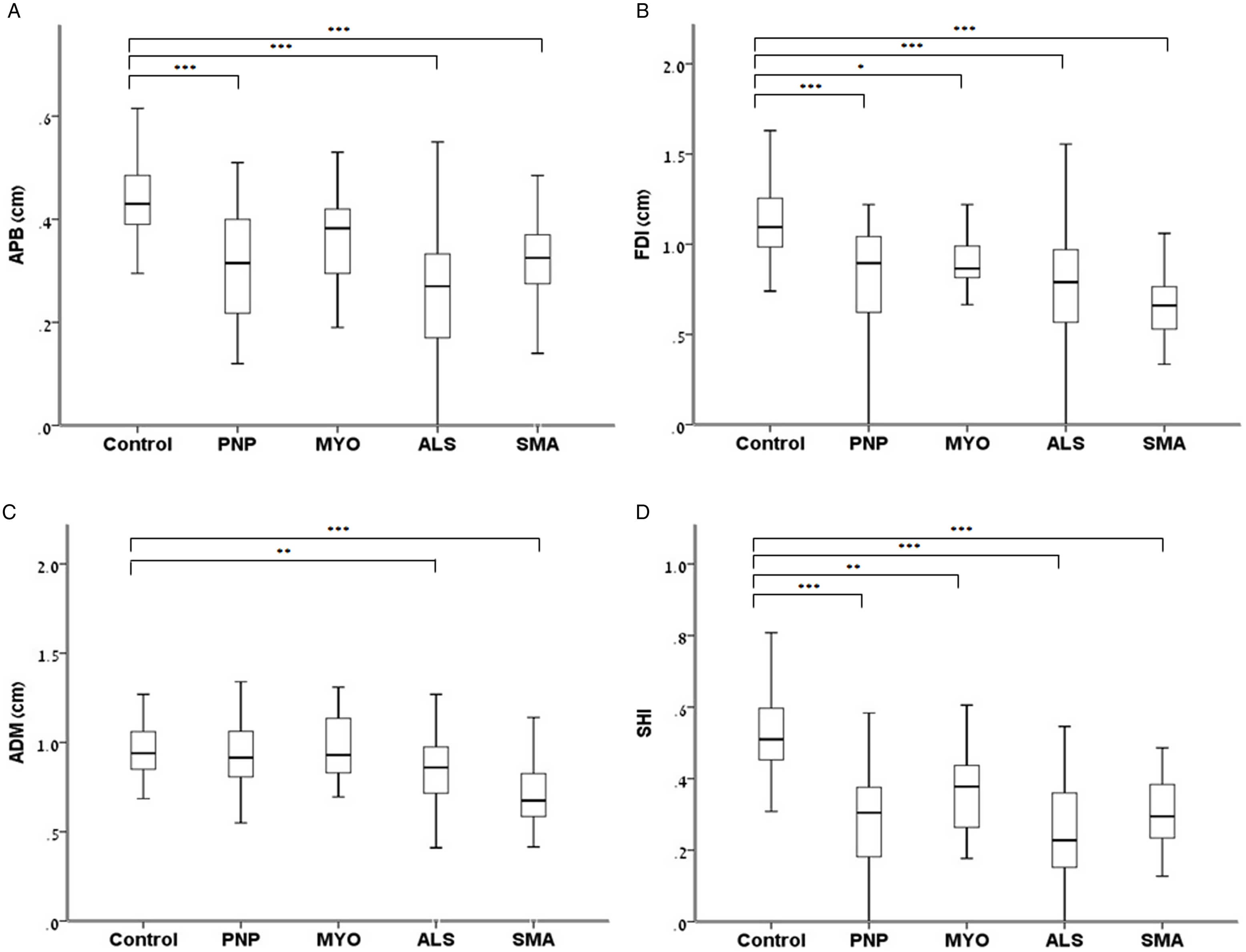
Figure 2: Comparisons of hand muscle thickness and SHI between healthy controls and patients with various neuromuscular disorders.
Table 1: Comparisons of demographics and clinical characteristics between 65 healthy controls and 181 patients with various neuromuscular disorders
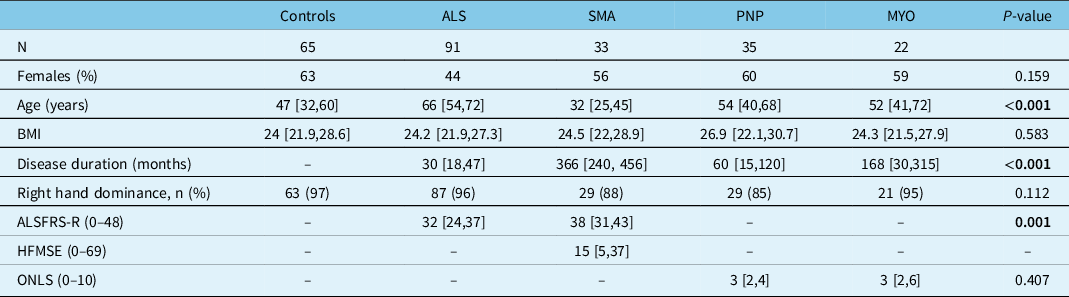
PNP: peripheral neuropathies; MYO: Myopathies; ALS: Amyotrophic Lateral Sclerosis; SMA: Spinal Muscular Atrophy; BMI: Body mass index; ALSFRS-R: ALS functional rating scale-revised; HFMSE: Hammersmith functional rating scale Expended. Data are presented as median [interquartile range] unless indicated otherwise. Significant p-values (<0.05) are bolded.
Table 2: Sensitivity, specificity, and area under the curve (AUC) calculated from the receiver operating characteristics curves for differentiating between different neurological conditions and healthy controls using the sonographic Split-Hand Index
Discussion
We have previously shown that the SHI determined by quantitative sonographic evaluation of hand muscle thickness has excellent diagnostic accuracy for differentiating patients with ALS from healthy controls (AUC – 0.92), and good diagnostic accuracy for differentiating patients with SMA from healthy controls (AUC – 0.87). Reference Abraham, Fainmesser, Drory and Bril8 The ability of the sonographic SHI to differentiate between patients with motor neuron disease and healthy controls is in line with previous studies using electrophysiological and sonographic indices. Reference Kim, Hong, Shin, Park, Sohn, Lee, Park and Sung3,Reference Wang, Liu, Ding, Hu and Cui4,Reference Günther, Neuwirth and Koch6,Reference Seok, Park, Kim, Oh, Kim and Kim7 However, our current study results suggest that sonographic SHI cannot differentiate between motor neuron diseases, and other neuromuscular disorders, including polyneuropathy or myopathy (AUC 0.54–0.74). The sonographic SHI showed good to excellent accuracy for differentiating all patient subgroups from controls (AUC – 0.83–0.92), especially in ALS (AUC – 0.92), but had inferior performance for differentiating between various neuromuscular disorders, including between motor neuron diseases and other neuromuscular disorders. These findings are in contrast to previous studies suggesting that the SHI is a specific measure, which can differentiate between ALS and other neuromuscular diseases. Reference Menon, Kiernan, Yiannikas, Stroud and Vucic2,Reference Wang, Liu, Cai, Ding, Hu and Cui5,Reference Seok, Park, Kim, Oh, Kim and Kim7 However, only one study showed sonographic SHI specificity for motor neuron disease using echointensity, and had a limited number of nine patients with other neuromuscular conditions. Reference Seok, Park, Kim, Oh, Kim and Kim7 The discrepancy of SHI performance regarding specificity between electrophysiological and sonographic findings is surprising in light of the correlation between CMAP amplitude and muscle thickness. Reference Abraham, Drory, Fainmesser, Algom, Lovblom and Bril15 However, Wang et al. found in patients with carpal tunnel syndrome and ulnar neuropathy association between neuropathy and sonographic hyper-echointensity in APB and FDI, but not in ADM, Reference Wang, Gutierrez and Martucci16 in line with our study finding, showing more prominent muscle thickness loss in the APB and FDI compared with ADM across all patient subgroups. Furthermore, we found that ADM muscle thickness was reduced only in patients with ALS and SMA. These finding suggest possible discrepancy regarding the association between electrophysiology and MUS for ADM, possibly explaining the lack of sonographic SHI specificity. An additional contribution for the lack of specificity, which was most prominent for differentiating between polyneuropathy and motor neuron diseases, might be related to our cohort composition. It has been previously shown that 30% of patients with inflammatory polyneuropathies such as chronic inflammatory demyelinating disease (CIDP) show split hand characteristics, and indeed, 16 out of 35 patients with polyneuropathy in our cohort had CIDP. Reference Shibuya, Misawa and Uzawa9
Our study has several limitations. We have not performed electrophysiological evaluations at the time of the MUS study, and therefore could not explore the specificity of SHI using electrophysiological indices, or assess for additional confounding factors such as median or ulnar entrapments which might affect muscle thickness. We recorded muscle thickness, but not cross sectional area (CSA), which might be a more robust measure. However, there is a very strong correlation between muscle thickness and CSA, Reference Duráo, Morosolli, Brown and Jacobs17 and in some muscles, such as the APB, CSA measurement might be more challenging compared with thickness measurement. In addition, the cross-sectional area have not recorded echointensity, as healthy controls and patients were studied using different ultrasound devices. However, while echointensity depends on device type and setting, and has to be analysed offline using an external computer and dedicated software, muscle thickness is expected to be similar across different devices and could be determined online or offline by the US device, and therefore might be a more practical parameter at clinic, and yielding more generalisable results. Although age was different between subgroups, we have not adjusted muscle thickness accordingly. However, our previous study showed that age had the most prominent effect on the quadriceps muscle, affecting much less small hand muscle thickness. Reference Abraham, Drory, Fainmesser, Lovblom and Bril10 Finally, we have not restricted ultrasound studies only to patients with specific neuromuscular disorders such as multifocal motor neuropathy and inclusion body myositis, which has the potential to mimic motor neuron disease, due to the relative rarity of these diseases.
In conclusion, sonographic SHI is useful for differentiating patients with various neuromuscular disorders from healthy controls, but might be not specific for motor neuron disease.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.20
Disclosures
Reuven Avidan, Alon Abraham, and Yaara Fainmesser have no conflicts of interest to declare.
Vera Bril is a consultant to CSL Behring, Grifols, Union Chimique Belge, ArgenX Alexion, Alnylam, Akcea, Takeda, Octapharma, and Immunovant. She serves on international scientific advisory boards for the Myasthenia Gravis Foundation of America and Guillain-Barré Syndrome/Chronic Inflammatory Demyelinating Polyneuropathy (CIDP/GBS) Foundation International, and has received research support from CSL Behring, Grifols, Union Chimique Belge, ArgenX, Immnovant, Momenta, Talecris, Takeda, Octapharma, and Akcea.
Vivian E Drory reports consultancy fees from Biogen, Neurosense Therapeutics, and Eyecontrol.
Funding
None.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Statement of Authorship
The authors RA, YF, VED, VB, and AA were all involved reviewing literature, writing parts of the manuscript, and editing the final version of the manuscript for submission.






