Introduction
Today, weaver ants (Formicidae, Oecophylla Smith, F.) are comprised of two Old World species that occupy humid tropical to subtropical forests: O. smaragdina (Fabricius) in southeast Asia from northern India through Queensland, Australia, and O. longinoda (Latreille) in the Afrotropics (Lokkers Reference Lokkers1986; Wetterer Reference Wetterer2017a, Reference Wetterer2017b).
Weaver ants are canopy dwellers, where they form multiple nests per colony across multiple trees, with a single queen in the main nest. Nests are made of leaves that workers join using the silk glands of larvae that they hold in their mandibles, hence their common name (Hölldobler and Wilson Reference Hölldobler and Wilson1990; Crozier et al. Reference Crozier, Newey, Schlüns and Robson2010). The ants have long legs suitable for rapid movement from leaf to leaf and a reduced petiole with an elongate, low node, which allows the gaster to be reflexed over the alitrunk, enabling greater agility in moving through treetops by shifting the centre of gravity forwards (Dlussky Reference Dlussky, Vishnjakova, Dlussky and Pritykina1981; Dlussky et al. Reference Dlussky, Wappler and Wedmann2008). Weaver ants feed upon insects and the honeydew produced by the sternorrhynchs and auchenorrhynchs (Hemiptera) and lycaenid caterpillars (Lepidoptera) that they tend.
Oecophylla has a rich fossil record beginning in the Eocene (Bolton Reference Bolton2003) of about 16 species, summarised and treated in detail by Dlussky et al. (Reference Dlussky, Wappler and Wedmann2008), Perfilieva (Reference Perfilieva2015, Reference Perfilieva2021), and Perfilieva et al. (Reference Perfilieva, Dubovikoff and Dlussky2017). In the Western Hemisphere, Oecophylla species are known from their oldest occurrences in the mid-Ypresian Okanagan Highlands series of fossil sites at Quilchena, south–central British Columbia, Canada and at Republic, north–central Washington, United States of America (below references) and from the middle Eocene of Mississippi, United States of America (Johnston Reference Johnston1993). Fossil weaver ants have been reported in Europe from the latest Ypresian (Messel, Germany; Dlussky et al. Reference Dlussky, Wappler and Wedmann2008) to the Miocene (Radoboj, Croatia; Heer Reference Heer1849), and from the Miocene of Africa (Mfangano Island, Kenya; Wilson and Taylor Reference Wilson and Taylor1964).
The Republic species is based on a queen that was first briefly mentioned and figured by Douglas and Stockey (Reference Douglas and Stockey1996, fig. 15), who treated it as Formicidae indet. Dlussky and Rasnitsyn (Reference Dlussky and Rasnitsyn1999) described this fossil as Camponotites kraussei Dlussky and Rasnitsyn, later distinguishing it from Oecophylla by its distinctively elongated head (Dlussky and Rasnitsyn Reference Dlussky and Rasnitsyn2003). Dlussky et al. (Reference Dlussky, Wappler and Wedmann2008), however, described Oecophylla longiceps Dlussky from Messel with such a head shape, recognising this as a species-level variation. Perfilieva et al. (Reference Perfilieva, Dubovikoff and Dlussky2017) and Perfilieva (Reference Perfilieva2021) subsequently suggested that C. kraussei belongs to Oecophylla due to its forewing venation and elongated petiole, although the authors did not mention its head nor formally transfer it by providing a new diagnosis to separate it from other species of the genus (International Commission on Zoological Nomenclature 1999, International Code of Zoological Nomenclature (ICZN) article 13.1.1 and recommendation 13A).
Douglas and Stockey (Reference Douglas and Stockey1996) listed but neither illustrated nor described ants from Quilchena. Archibald and Mathewes (Reference Archibald and Mathewes2000) later discussed Quilchena ants, grouping them as Formicidae types A, B, and C, noting the type A queens’ similarity to Camponotites kraussei. It is not clear if these are the same as Douglas and Stockey’s ants. Still undescribed, Perfilieva (Reference Perfilieva2021) informally considered a type A queen figured by Archibald et al. (Reference Archibald, Rasnitsyn, Brothers and Mathewes2018, fig. 10A and B) to be a species of Oecophylla without further comment.
Here, we describe and name the type A and B Quilchena species of Archibald and Mathewes (Reference Archibald and Mathewes2000) as a new genus and new species within the tribe Oecophyllini, treating type B ants as the males of type A queens. We formally place the Republic species in Oecophylla, providing a diagnosis that distinguishes the species within the genus. We also describe but do not name a new species of Oecophyllini from the Okanagan Highlands McAbee locality in British Columbia based on a worker fossil specimen. We briefly discuss Oecophyllini occurrences through the Cenozoic, including their climatic and biogeographic implications; these will be examined in detail in a future work.
Material and methods
We examined 30 fossil specimens – 28 from Quilchena, one from McAbee, and one from Republic.
Figures were made with Adobe Photoshop (Adobe, San Jose, California, United States of America) from photographs taken in the laboratory of Parks Canada (Vancouver, British Columbia) with a digital camera mounted on a Zeiss microscope (Zeiss, Oberkochen, Germany). Line drawings were made from these with Adobe Illustrator and are taken from both the part and counterpart, which may preserve different portions of the fossil.
Institution and collection abbreviations used in this paper are as follows: Beaty Biodiversity Museum, University of British Columbia, Vancouver, British Columbia, Canada (BBM); Quilchena collection of Simon Fraser University (SFU), Department of Biological Sciences, Burnaby, British Columbia, Canada (Q); Burke Museum, University of Washington, Seattle, Washington, United States of America (UWBM); the collection of the late Rene Savenye (RS); Archibald collection (SBA).
We use Republic locality codes B4876 and A0307 of the Burke Museum, also used by the Stonerose Interpretive Center in Republic.
We follow the wing venation terminology of Dlussky et al. (Reference Dlussky, Wappler and Wedmann2008, fig. 2), with slight modifications, and cf. the hind wing terminology of Cantone and Von Zuben (Reference Cantone and Von Zuben2019, fig. 1).
Measurements are presented in millimetres.
Abbreviations are defined as follows: head length without mandibles (HL); head width (HW); maximum eye diameter (ED); mandible length (MdL); total body length (BL); alitrunk length (AL); alitrunk width (AW); alitrunk height (AH); forewing length (FwL); forewing width (FwW); petiole length (PtL); petiole width (PtW); scape length (SL); gaster length (GL); gaster width (GW).
Contrary character states of compared taxa are provided in square brackets.
We use the mean annual temperature categories of Wolfe (Reference Wolfe1975): microthermal, ≤ 13 °C; mesothermal, > 13 °C, < 20 °C; megathermal, ≥ 20 °C.
Localities
Quilchena
Quilchena is an exposure of Kamloops Group lacustrine shales (Coldwater beds) near Nicola Lake, British Columbia, Canada (Mathewes et al. Reference Mathewes, Greenwood and Archibald2016). A tephra bed within the shales is 51.5 ± 0.4 Ma old by 40Ar–39Ar dating of sanidine crystals (Villeneuve and Mathewes Reference Villeneuve and Mathewes2005). It is estimated to have had a mesic-to-moist climate with a low- to mid-mesothermal mean annual temperature and a minimum coldest month mean temperature of at least 5 °C and perhaps as high as 8 °C by paleobotanical and insect analyses (Archibald et al. Reference Archibald, Morse, Greenwood and Mathewes2014; Mathewes et al. Reference Mathewes, Greenwood and Archibald2016).
McAbee
The ant described here is from the Hoodoo Face beds of the McAbee, an unnamed formation of Kamloops Group lacustrine shale about 8 km east of the village of Cache Creek, British Columbia, Canada. These beds have a U–Pb maximum likelihood age of 52.10 ± 0.26 Ma (Rubino et al. Reference Rubino, Leier, Cassell, Archibald, Foster-Baril and Barbeau2021). McAbee’s climate is estimated as moist and upper microthermal, with a coldest month mean temperature similar to that of Quilchena, as determined by similar analyses (Archibald et al. Reference Archibald, Morse, Greenwood and Mathewes2014; Gushulak et al. Reference Gushulak, West and Greenwood2016).
Republic
The fossil is from the Golden Promise Mine (B4876) exposure of the Tom Thumb Tuff Member of the Klondike Mountain Formation, near Republic, Washington, United States of America. B4876 does not have an estimated age, but the nearby exposure A0307 has a U–Pb maximum likelihood age from zircons of 51.45 ± 0.12 Ma and exposure B4131 of 51.18 ± 0.09 Ma (Rubino et al. Reference Rubino, Leier, Cassell, Archibald, Foster-Baril and Barbeau2021). Republic’s climate is estimated as mesic and upper microthermal with a coldest month mean temperature similar to that of Quilchena and McAbee (Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005; Archibald et al. Reference Archibald, Morse, Greenwood and Mathewes2014).
Systematic paleontology
Order Hymenoptera Linnaeus
Family Formicidae Latreille
Subfamily Formicinae Latreille
Tribe Oecophyllini Emery
Emended diagnosis. Further to the diagnosis of Bolton (Reference Bolton2003), except eyes may be positioned more forwards (cf. O. kraussei): forewings separated from those of other Formicinae by combination of 5Rs notably curved towards anterior margin [towards posterior margin]; cell 3r narrow, about as long as cell 1+2r [notably longer]; cell 1+2r bounded below by Rs+M notably curved towards posterior margin or bent where m-cu would be (where stub of m-cu is in Eoecophylla) (compare Dlussky et al. Reference Dlussky, Wappler and Wedmann2008, fig. 2, with Dlussky et al. Reference Dlussky, Karl, Brauckmann, Brauckmann, Gröning and Reich2011, fig. 1, and Perfilieva Reference Perfilieva2021, plate 9, fig. 2).
Composition. Oecophylla Smith, F., type genus, and Eoecophylla n. gen.
Genus Eoecophylla, Archibald, Mathewes, and Perfilieva n. gen.
ZooBank Registration number: urn:lsid:zoobank.org:act:0B7CBA6B-C6D3-4A6F-B511-1AE462986217

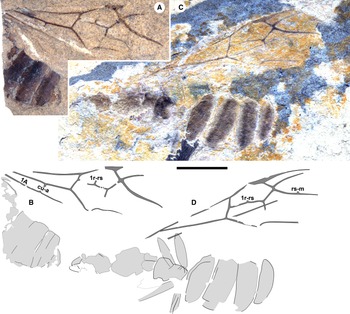
Figure 1. Eoecophylla quilchenensis n. sp. queens. Paratype BBM-PAL-P000017: A, photograph and B, drawing. Holotype BBM-PAL-P000016A: C, photograph; D, drawing; and E, labelled drawing of right wings. 1A–1D and 1E to scales, 5 mm.
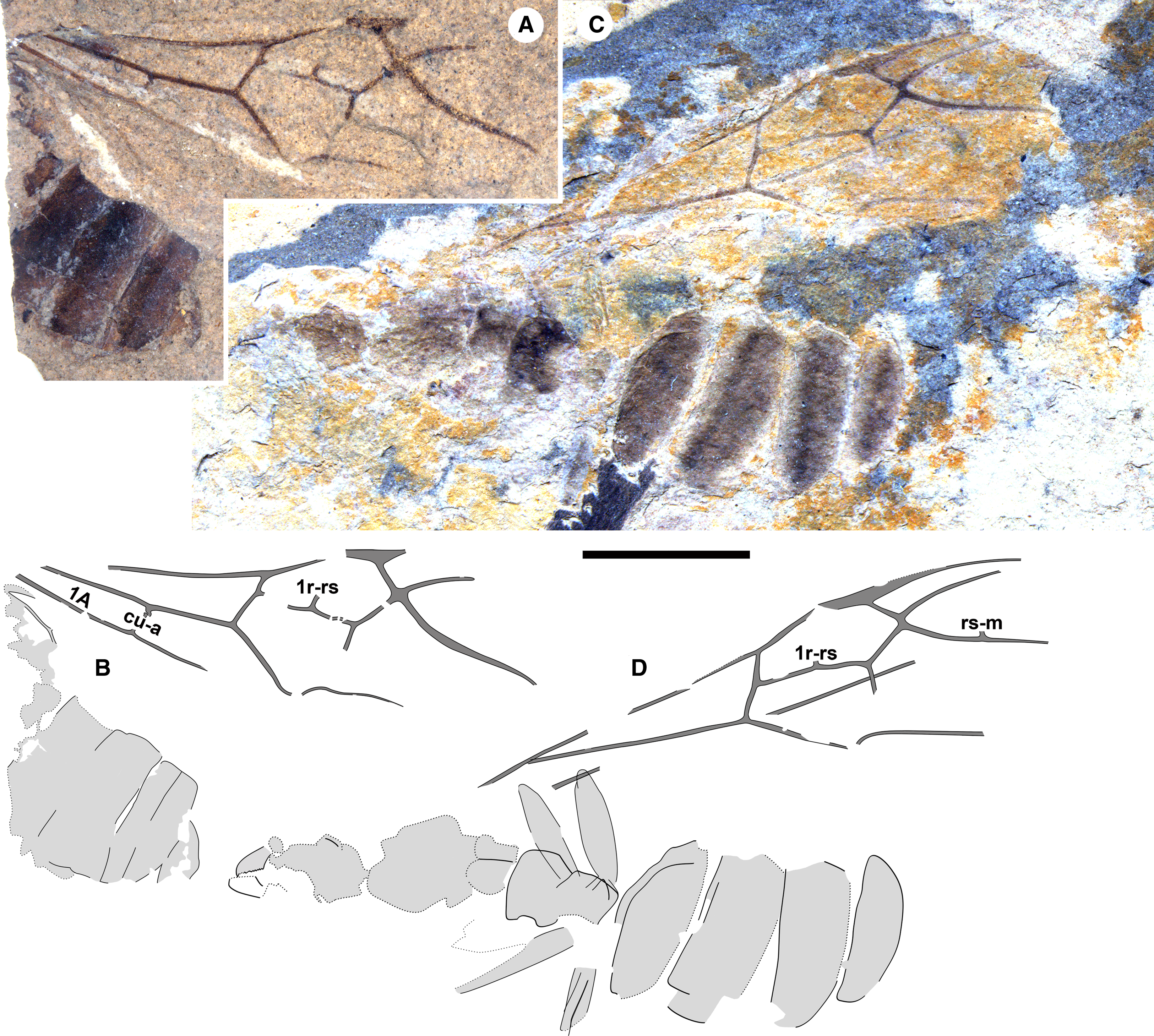
Figure 2. Eoecophylla quilchenensis n. sp. queens. BBM-PAL-P000018: A, photograph and B, drawing. Paratype BBM-PAL-P000019: C, photograph and D, drawing. All to scale, 5 mm.

Figure 3. Eoecophylla quilchenensis n. sp. queen. BBM-PAL-P000020: A, photograph and B, drawing. Both to scale, 5 mm.

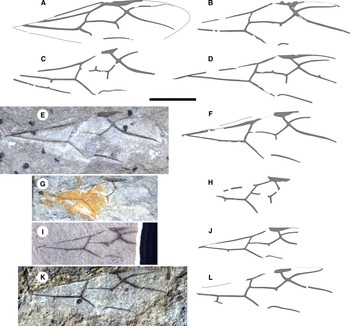
Figure 4. Eoecophylla quilchenensis n. sp. queen forewings. A, holotype BBM-PAL-P000016; B, BBM-PAL-P000020; C, BBM-PAL-P000018; D, paratype BBM-PAL-P000019. BBM-PAL-P000021: E, photograph and F, drawing. BBM-PAL-P000022: G, photograph and H, drawing. BBM-PAL-P000023: I, photograph and J, drawing. BBM-PAL-P000024: K, photograph and L, drawing. All to scale, 5 mm.

Figure 5. Eoecophylla quilchenensis n. sp. males. Paratype BBM-PAL-P000026: A, photograph and B, drawing. Paratype BBM-PAL-P000027: C, photograph and D, drawing. Allotype BBM-PAL-P000025: E, photograph and F, drawing. BBM-PAL-P000028: G, photograph. BBM-PAL-P000029: H, photograph. BBM-PAL-P000030: I, photograph. BBM-PAL-P000031: J, photograph. All to scale, 5 mm.
Formicidae Type A and Type B: Archibald and Mathewes Reference Archibald and Mathewes2000, pp. 1451, 1454, fig. 12A–B.
Formicidae sp. (all specimens listed below), Quilchena: Archibald et al. Reference Archibald, Rasnitsyn, Brothers and Mathewes2018, pp. 18–20, fig. 10A, B, and D.
Oecophylla sp., SFU Q-0409 (here BBM-PAL-P000016): Perfilieva Reference Perfilieva2021, pp. 79, 84, plate 9, fig. 1.
Diagnosis. Queen. Forewings distinct from those of Oecophylla by presence of m-cu stub (originating on Rs+M, not joining Cu) of varying lengths, from very small to less than half length to Cu [Oecophylla: absent], sometimes small stubs of 1r-rs, rs-m present [Oecophylla: absent]; pterostigma similar lengths basad, distad 2r-rs [Oecophylla: considerably longer distad]; by hind wings with well-developed 2M [Oecophylla: absent].
Type species. Eoecophylla quilchenensis n. sp. by monotypy.
Description. As for Eoecophylla quilchenensis, below.
Remarks. The stub of m-cu is longest in the holotype and varies in other specimens to very short; e.g., see BBM-PAL-P000023 (Fig. 4I, J) and Q-0492 (Fig. 4K). Specimens also vary in the presence of very short stubs of 1r-rs in BBM-PAL-P000019 (Fig. 4D) and rs-m in BBM-PAL-P000018 (Fig. 4C) and BBM-PAL-P000019 (Fig. 4D).
Hind wing venation is preserved only in the holotype (Fig. 1C–E). This conforms to the type I hind wing venation of Cantone and Von Zuben (Reference Cantone and Von Zuben2019) by its well-developed 2M and the 2M’s relationships with 1M and rs-m (Fig. 1E). This is unique within Formicinae (Perfilieva Reference Perfilieva2010), which we consider plesiomorphic, suggesting that Oecophyllini is sister to the rest of the subfamily. A jugal lobe may be present or absent in Cantone and Von Zuben’s (Reference Cantone and Von Zuben2019) type I hind wings but is always absent in their type II; this cannot be evaluated in E. quilchenensis due to poor preservation. It is very different from their type III, which has very reduced venation.
Specimens that we treat as males of Eoecophylla quilchenensis resemble those of Eoformica Cockerell in many ways, cf. the figures of Cockerell (Reference Cockerell1921, plate 8, fig. 11), Carpenter (Reference Carpenter1930, plate 2, fig. 6), Dlussky and Rasnitsyn (Reference Dlussky and Rasnitsyn2003, figs. 26–34), and Lapolla and Greenwalt (Reference Lapolla and Greenwalt2015, figs. 13–14). Eoformica was originally erected as a nominal genus by Cockerell (Reference Cockerell1921), although specimens assigned to it are so indistinctly preserved, have such a range of morphologies, and so are of such indeterminate affinities that it was later treated as a collective genus “which includes poorly preserved wingless imprints of ants in which the waist is one-segmented and narrowly attached to the gaster and the gaster lacks a constriction between the first and second segments” (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009, p. 14).
The Quilchena specimens, however, include many with wings, and the forewing of the allotype has some preserved venation, with Rs+M, 2r-rs, 5Rs, and 4M meeting to form an ×, 5Rs curved towards the anterior margin, and the size and shape of cell 3r all consistent with Oecophyllini. General character states of their bodies agree with males of Oecophylla. In addition, the association of these males at the small outcrop at Quilchena with queens bearing the same wing character states strengthens this determination.
We suspect that once their wings are known, some ants currently assigned to the grab bag Eoformica might be recognised as Oecophylla or Eoecophylla males.
Etymology. The genus name is derived from Oecophylla and the Eocene. Gender, feminine.
Eoecophylla quilchenensis, Archibald, Mathewes, and Perfilieva, n. sp.
ZooBank Registration number: urn:lsid:zoobank.org:pub:BDF6655F-884D-4452-A8D8-24A338A67151
Material. All Quilchena insect fossils were collected by RWM, J. Mathewes, G. Guthrie, and Simon Fraser University undergraduate students over many years and are now transferred from the Simon Fraser University collection to the Beaty Biodiversity Museum.
Queens (type A of Archibald and Mathewes Reference Archibald and Mathewes2000): all preserved in dorsal aspect. Holotype: BBM-PAL-P000016 (SFU number Q-0409AB; Figs. 1C–D, 4A): body with a forewing and hind wing. Paratypes: BBM-PAL-P000017 (Q-0011; Fig. 1A–B): well-preserved body, no wings; BBM-PAL-P000019 (Q-0517; Figs. 2C–D, 4D): much of the body, poorly preserved, well-preserved forewing with partial wing beneath it, other forewing disarticulated from the body. Other specimens: BBM-PAL-P000018 (Q-0006; Figs. 2A–B, 4C): part of gaster, much of forewing, perhaps part of hind wing; BBM-PAL-P000020 (Q-0271; Figs. 3, 4B): poorly preserved body, two forewings, one rather complete; BBM-PAL-P000021 (Q-0001; Fig. 4E–F): forewing, mostly complete; BBM-PAL-P000022 (Q-0412; Fig. 4G–H): part of forewing, missing distal portion; BBM-PAL-P000023 (Q-0457; Fig. 4I–J): much of forewing missing distal portion. BBM-PAL-P000024 (Q-0492; Fig. 4K–L): partial forewing.
Male (type B of Archibald and Mathewes Reference Archibald and Mathewes2000). Allotype: BBM-PAL-P000025 (Q-0007; Fig. 5E–F): almost complete, wings with some venation preserved. Paratypes: BBM-PAL-P000026 (Q-0008; Fig. 5A–B): almost complete, well preserved, but no wings; BBM-PAL-P000027 (Q-0009; Fig. 5C–D): rather well preserved, but no wings, almost no legs. Other specimens: BBM-PAL-P000028 (Q-0013; Fig. 5G): mostly poorly preserved, parts of four faint wings; BBM-PAL-P000029 (Q-0258; Fig. 5H): body, some leg parts, no wings; BBM-PAL-P000030 (Q-0456; Fig. 5I): body, most legs, no wings; BBM-PAL-P000031 (Q-0485; Fig. 5J): poorly preserved body, no wings. The following are not figured. BBM-PAL-P000032 (Q-0002): complete but with poorly preserved wings; BBM-PAL-P000033 (Q-0010): partially preserved, no wings, no head, some legs; BBM-PAL-P000034 (Q-0012): poorly preserved, faint wings; BBM-PAL-P000035 (Q-0014): poorly preserved body with parts of two legs, no wings; BBM-PAL-P000036 (Q-0019): poorly preserved body, no legs, faint wing(s?); BBM-PAL-P000037 (Q-0021): well-preserved body, no wings; BBM-PAL-P000038 (Q-0366): part of body, leg bits, no wings; BBM-PAL-P000039 (Q-0410): poorly preserved body, no wings; BBM-PAL-P000040 (Q-0453): body, some legs, no wings; BBM-PAL-P000041 (Q-0510): poorly preserved body, no legs, no wings; BBM-PAL-P000042 (RS-160): poorly preserved body, no wings; BBM-PAL-P000043 (RS-281): poorly preserved body, some leg parts, no wings.
Diagnosis. As for the genus, above.
Description. Holotype queen BBM-PAL-P000016: preserved in dorsal aspect (Figs. 1C–E, 4A). BL ca. 14.5. Head: HL ca. 1.7, HW ca. 2.0; mandibles large, MdL ca. 1.0, subtriangular, masticatory margin indistinctly preserved; eyes, antennae poorly preserved; occipital corners well developed, rounded. Alitrunk indistinctly preserved. Forewing: FwL ca. 20.0; FwW indistinct, ca. 5.5; 1M, 1Rs nearly aligned; pterostigma long, narrow, similar lengths basad, distad crossvein 2r-rs; 2r-rs angled, anterior basad posterior; 5Rs curved towards anterior margin; stub of m-cu present, extending almost halfway between Rs+M, Cu; Rs+M distinctly bent at stub; 1Rs+M, 2Rs+M distinctly angled; 2Rs+M, 2r-rs, 5Rs, 4M form a ×; fragment of cu-a preserved on left forewing, better-preserved on BBM-PAL-P000018 forewing, see Fig. 2B. Hind wing: 1M, 2M free, 2M well developed, rs-m curved, portions of 2M+Cu, R, 2Rs preserved, A not preserved, nor region where jugal lobe might be present. Waist of one segment; petiole poorly preserved. Legs: fragments, poorly preserved. Gaster: indistinct, without constriction between AIII, AIV (first two segments); GL ca. 5.4; GW ca. 5.0.
Paratype queen BBM-PAL-P000019: preserved in dorsal aspect (Figs. 2C–D, 4D). BL ca. 19.0. Head, alitrunk indistinctly preserved but with subtriangular mandibles bearing probably 9? or more teeth. Forewing: FwL ca. 19.0 preserved, missing apical portion; FwW ca. 5.5; 1M, 1Rs aligned; pterostigma long, narrow, apparently similar lengths basad, distad crossvein 2r-rs; 2r-rs angled, anterior basad posterior; 5Rs curved towards anterior margin; stub of m-cu present, extends less than halfway between Rs+M, Cu; 1Rs+M, 2Rs+M distinctly angled; 2Rs+M, 2r-rs, 5Rs, 4M meet to form an ×. Hind wings absent. Legs: Fragment present, mesofemur, metafemur ca. 3.5 mm long. Waist of one segment; petiole indistinct. Without constriction between AIII, AIV. Gaster.:GL ca. 8.0 as preserved; GW ca. 5.5.
Paratype queen BBM-PAL-P000017: preserved in dorsal aspect (Fig. 1A–B). BL 16.1. Head: with mandibles subtriangular, otherwise indistinct; HL+MdL 2.9, HW 2.6; occipital corners well developed, rounded. Alitrunk: AL 5.3. Wings: absent. Legs: fragments present. Petiole: PtL ca. 1.6. Gaster: GL 6.7, GW 5.8.
Allotype male BBM-PAL-P000025: preserved in dorsal aspect (Fig. 5E–F). BL 11.2. Head: square with rounded corners; HL 1.5; HW 1.7; mandibles poorly preserved; eyes indistinct. Alitrunk: AL 4.9, AW ca. 2.6. Forewing: mostly poorly preserved, 2Rs+M, 2r-rs, 5Rs, 4M meet to form ×; 5Rs curved towards anterior margin. Hind wing: not known. Legs: long. Waist: single segmented; petiole without scale. Gaster: GL ca. 4.1, GW 3.5, large, semi-spherical without constriction between first two segments.
Paratype male BBM-PAL-P000026: preserved in dorsal aspect (Fig. 5A–B). BL 10.4. Head: HL 1.4, HW 1.4; occipital corners well developed, rounded; MdL 0.6. Alitrunk: AL 4.3. Legs: long, metafemur ca. 3.3, metatibia ca. 3.0. Waist single-segmented, petiole without scale, PtL 1.1, PtW 0.8. Gaster: GL 4.0, GW 3.1.
Paratype male BBM-PAL-P000027: head in dorsolateral, alitrunk in lateral, petiole, gaster in dorsal aspect (Fig. 5C–D). Alitrunk: AL 4.0, AH 3.0. Waist: single-segmented; petiole without scale, PtL 1.1, PtW 0.8. Gaster: GL 4.0, GW 3.5.
Etymology. The specific epithet is a toponym referring to the species’ only known locality.
Genus Oecophylla Smith, F.
Oecophylla kraussei (Dlussky et Rasnitsyn) n. comb.
Fig. 6

Figure 6. Oecophylla kraussei (Dlussky and Rasnitsyn), holotype UWBM-78047 from Republic, WA: A, photograph; B, drawing, and C, drawing of left forewing. Scales 5 mm.
Formicidae: Douglas and Stockey Reference Douglas and Stockey1996, pp. 1145, 1149, fig. 15.
Camponotites kraussei: Dlussky and Rasnitsyn Reference Dlussky and Rasnitsyn1999, pp. 547, 548, fig. 2.
Camponotites krausei [sic]: Dlussky and Rasnitsyn Reference Dlussky and Rasnitsyn2003, pp. 418, 419, fig. 10.
Camponotites kraussei: Dlussky et al. Reference Dlussky, Karl, Brauckmann, Brauckmann, Gröning and Reich2011, pp. 452–455, fig. 4.
Camponotites kraussei: referred to as Oecophylla: Perfileva et al. 2017, p. 399.
Camponotites kraussei: Archibald et al. Reference Archibald, Rasnitsyn, Brothers and Mathewes2018, pp. 224, 225, fig. 12G.
Camponotites kraussei referred to as Oecophylla: Perfileva Reference Perfilieva2021, pp. 78, 83, 85.
Material. Holotype UWBM-78047 (Fig. 6): a queen preserved in dorsal aspect with an almost complete body, one rather complete forewing, and one forewing missing the apical portion, and lacking hind wings from the Klondike Mountain Formation near Republic, Washington, United States of America. Collected by Rob Krausse, 1994.
Emended diagnosis. Queen differs from those of all other species of Oecophylla except O. longiceps Dlussky by head elongate [both about 1.2× longer than wide; all others as wide or wider than long]. The species differs from O. longiceps by compound eyes larger, longer, positioned at anterior margin of head capsule [small, positioned mid-head]; by mandibles protruding ca. slightly > third head length [ca. slightly less than half].
Description. See Dlussky and Rasnitsyn (Reference Dlussky and Rasnitsyn1999).
Remarks. When Steinbach (Reference Steinbach1967) published the genus name Camponotites Steinbach, his description was cursory, he provided no illustration, and he did not provide a diagnosis as required by the IZCN for names of nominal taxa published after 1930 (International Commission on Zoological Nomenclature 1999, ICZN article 13.1.1). The name was then unavailable. He also did not explicitly designate a type species as required by article 13.3, although Camponotites silvestris Steinbach is implied by monotypy.
Unaware of this rather obscure paper, Dlussky (Reference Dlussky, Vishnjakova, Dlussky and Pritykina1981) separately erected Camponotites as a collective group, writing it in quotation marks throughout this work. He provided a diagnosis and description and designated a type species, although these cannot be done for a collective group by ICZN articles 13.3.2, 42.3.1, 66, and 67.14, where they are replaced by a definition. Although Dlussky’s diagnosis did not distinguish the genus from others as would be necessary for a nominal taxon (article 13.1.1), it did function as a definition, which can be understood as (paraphrased from the Russian): those fossil ant species that possess the combination of wing character states listed by Dlussky distinctive of the formicine tribes Plagiolepidini, Camponotini, and Oecophyllini, but that cannot be assigned to any of these by preservation. This intention was made explicit by Dlussky and Rasnitsyn (Reference Dlussky and Rasnitsyn1999, p. 548, and Reference Dlussky and Rasnitsyn2003, p. 413) and Dlussky et al. (Reference Dlussky, Karl, Brauckmann, Brauckmann, Gröning and Reich2011, p. 455).
When Dlussky et al. (Reference Dlussky, Karl, Brauckmann, Brauckmann, Gröning and Reich2011) discovered the previous use of the name Camponotites by Steinbach, they considered it both a homonym and a synonym, transferring C. silvestris Steinbach to their collective genus, which they treated as Camponotites Steinbach. However, as “Camponotites Steinbach” was unavailable, the collective group is Camponotites Dlussky, which does not compete for priority with Steinbach’s nominal genus name (ICZN article 23.7.2, and see article 67.14). Treatments of Camponotites by other authors following the above are discussed by Dlussky et al. (Reference Dlussky, Karl, Brauckmann, Brauckmann, Gröning and Reich2011).
With the transfer of “Camponotites” kraussei to Oecophylla, Camponotites consists of C. silvestris Steinbach, C. steinbachi Dlussky et al., and C. xiejiaheensis Hong. “Camponotites” macropterus Dlussky is now Oecophylla macroptera (Dlussky) (see Perfilieva Reference Perfilieva2015 and Perfilieva Reference Perfilieva2021).
Oecophyllini sp. A
Fig. 7

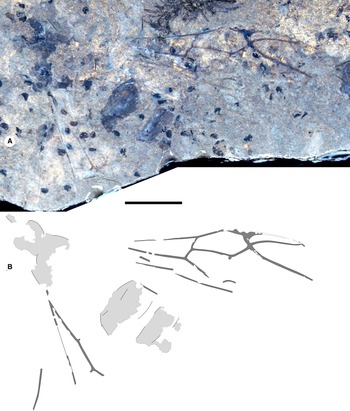
Figure 7. Oecophyllini sp. A, BBM-PAL-P000044, worker of unnamed species of Oecophyllini from McAbee, British Columbia, Canada: A, photograph and B, drawing. Both to scale, 5 mm.
Material. BBM-PAL-P000044A, B (collection number SBA-109A, B; Fig. 7): a worker, well preserved and rather complete, legs somewhat disarticulated. Collected by SBA at McAbee Hoodoo face beds 21 June 2000. Housed in the collection of the Beaty Biodiversity Museum.
Description. Worker BBM-PAL-P000044A, B: head rounded, HL 2.0, HW 2.2, MdL 1.0. Legs long. AL 4.0, AH 1.5. Waist of one segment; petiole elongate, node low, without scale, PtL 1.1, PtW 0.8. Gaster: incomplete, without constriction between first two segments.
Range and age. McAbee, British Columbia, Canada; mid-Ypresian.
Remarks. This ant agrees with Oecophylla workers, including their characteristic elongate, low node; however, we cannot rule out that this is an Eoecophylla worker, which are unknown; therefore, we treat it as Oecophyllini sp. A. Although the workers of arboreal species are common in amber, all worker ants are rare in lacustrine shales due to taphonomic barriers to the transport of wingless insects to the depositional environment.
Discussion
Oldest occurrences
The Okanagan Highlands specimens are the oldest known occurrences of Oecophyllini and Oecophylla by a few million years. Their queens show several conditions that we consider to be plesiomorphic. Dlussky et al. (Reference Dlussky, Wappler and Wedmann2008) noted a trend in head shape from longer than wide, without distinct occipital corners in the late Ypresian Oecophylla longiceps from Messel, Germany (age of Messel; Lenz et al. Reference Lenz, Wilde, Mertz and Riegel2015), to heads that are as wide or wider than long with well-developed occipital corners. Dlussky et al. (Reference Dlussky, Wappler and Wedmann2008) suggested that this may be due to a strengthening of the mandibles for an unknown function, with an associated change in interior space and attachment surface. Oecophylla kraussei, which is a few million years older than O. longiceps, also has this lengthened, oval shape. With intercontinental dispersal of plants and animals across far-northern land bridges at this time (Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011, Reference Archibald, Mathewes and Aase2023; Brikiatis Reference Brikiatis2014), it is possible that these species are closely related. We consider the stub of crossvein m-cu and occasional stubs of 1r-rs and rs-m in the E. quilchenensis forewing and the presence of a well-developed 2M in its hind wing to be plesiomorphic.
Abundance
Eoecophylla quilchenensis is the most common ant by far at Quilchena, comprising all but one specimen, and is more common there than any ant species at any other Okanagan Highlands site (although Okanagan Highlands amber has been only briefly examined to date; e.g., Archibald and Makarkin Reference Archibald and Makarkin2004). Oecophylla species are the most abundant arboreal ants at late Ypresian Messel and Lutetian Eckfeld in Germany (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009). Antropov et al. (Reference Antropov, Belokobylskij, Compton, Dlussky, Khalaim and Kolyada2014) called the two species of Oecophylla in the Priabonian Bembridge Marls (Isle of Wight, United Kingdom) hyperabundant, with 720 specimens out of 1136 ants and 1460 Hymenoptera. Dlussky et al. (Reference Dlussky, Wappler and Wedmann2008, Reference Dlussky, Wappler and Wedmann2009) examined the composition of arboreal ant communities through the Cenozoic, finding the proportion of Oecophylla increasing from latest Ypresian through the early Oligocene and then decreasing. The numerous specimens of Eoecophylla at Quilchena move the onset of Oecophyllini abundance back a few million years to the mid-Ypresian.
Community
To build and maintain their nests year-round, Oecophylla requires evergreen broadleaf dicot trees or lianas. Extant Oecophylla are associated with a wide range of these plants – the Asian O. smaragdina with 175 species in 46 families and the African O. longinoda with 28 species in seven families (Lim et al. Reference Lim, Kirton, Salom, Kok, Fell and Pfeiffer2008). These numbers may be biased by research emphasis on agriculturally important trees, or they might be explained by the much wider range of O. smaragdina, which includes a greater number of plant community types. These include both confirmed host and possible host plants, that is, with and without known nesting.
The Australian O. smaragdina shows a preference for larger trees with medium-sized leaves (ca. 5 × 8 to 20 × 20 cm) of suitable texture, excluding those with large, tough leaves, and smaller understorey trees less than 15 m tall (Blüthgen and Fiedler Reference Blüthgen and Fiedler2002).
The preferences of trophobionts that Oecophylla tend appear to in large part drive host plant selection. These include treehoppers (Hemiptera: Auchenorrhyncha: Membracidae), scale insects (Hemiptera: Sternorrhyncha: Coccomorpha), aphids (Hemiptera: Sternorrhyncha: Aphididae), leaf hoppers (Hemiptera: Auchenorrhyncha: Cicadellidae), and the caterpillars of gossamer-winged butterflies (Lepidoptera: Lycaenidae).
Potential host plants at Quilchena include species of Theaceae, Pieris, Ternstroemites, Gordonia, and Trochodendron, and those at Republic and McAbee include Ilex, Gordonia, and Trochodendron. Rhus (Anacardiaceae) is also known from McAbee and Republic, but modern species do not reach the minimum 15-m height that Australian O. smaragdina require (Blüthgen and Fiedler Reference Blüthgen and Fiedler2002).
Aphids are known at all three Okanagan Highlands sites, and Auchenorrhyncha are abundant and diverse throughout the Okanagan Highlands fossils. Lychaenids and coccomorphs have not been found at any Okanagan Highlands locality, but this could be for taphonomic reasons. Adult Lepidoptera are likely excluded by loss during extended floating time due to the high surface area:mass ratio of their large, scaled wings and relatively small, light bodies. Their caterpillars would be rarely transported out into the lake and even more rarely preserved as fossils if they were. The tiny, soft-bodied coccomorphs with weak-flying males and flightless females would also be rarely preserved in shale, although they are well known in amber. Analysis of leaf-feeding damage has not yet been done at these localities and may reveal a wider diversity of herbivorous insects than are readily preserved in the shale body fossil record.
Climate
The presence of this Paleotropical genus in the Okanagan Highlands, especially in the microthermal forests of Republic and McAbee, might seem contradictory (Wheeler Reference Wheeler1914; Archibald and Farrell Reference Archibald and Farrell2003). They may, however, be restricted to low latitudes today by intolerance to cold winters and not by a requirement of hot climates (Archibald et al. Reference Archibald, Mathewes and Aase2023). This Ypresian distribution might then be explained by the Eocene greenhouse world global climatic regime of mild winters into microthermal higher latitudes and elevations. This would have allowed them to thrive well outside of their modern regions of high mean annual temperature (Daley Reference Daley1972; Archibald and Farrell Reference Archibald and Farrell2003) into microthermal localities of the Okanagan Highlands with few or only mild frost days.
Acknowledgements
The authors thank the following: Caroline Strömberg, Curator of Paleobotany, and Paige Wilson Deibel, Paleobotany Collections Manager at the Burke Museum, for loan of the Oecophylla kraussei holotype UWBM-78047; James Wetterer, Florida Atlantic University, for a helpful discussion of weaver ants; Melanie DeVore, Georgia College and State University, for discussion of host plants at the Republic and McAbee sites; and Marlow Pellatt, Parks Canada, for access to his laboratory, microscope, and digital camera. We thank two anonymous reviewers for comments that greatly improved the manuscript.
Competing interests
The authors declare they have no competing interest.