Introduction
Montmorillonite (Mnt) is a 2:1 layered aluminosilicate mineral (Brigatti et al. Reference Brigatti, Galan, Theng, Bergaya and Lagaly2006). Each layer of Mnt consists of two tetrahedral silica sheets bonded to both sides of an octahedral alumina sheet (Fig. 1a,b). Some of the Al3+ in the octahedral sheet can be substituted by divalent metal cations (Mg2+, Fe3+, Zn2+, Ni2+) and some of the Si4+ in the tetrahedral sheet can be substituted by trivalent metal cations (predominantly Al3+). The isomorphous substitutions result in a net negative surface charge and, to maintain neutrality, the interlayer spaces of Mnt adsorb exchangeable hydrated metal cations.

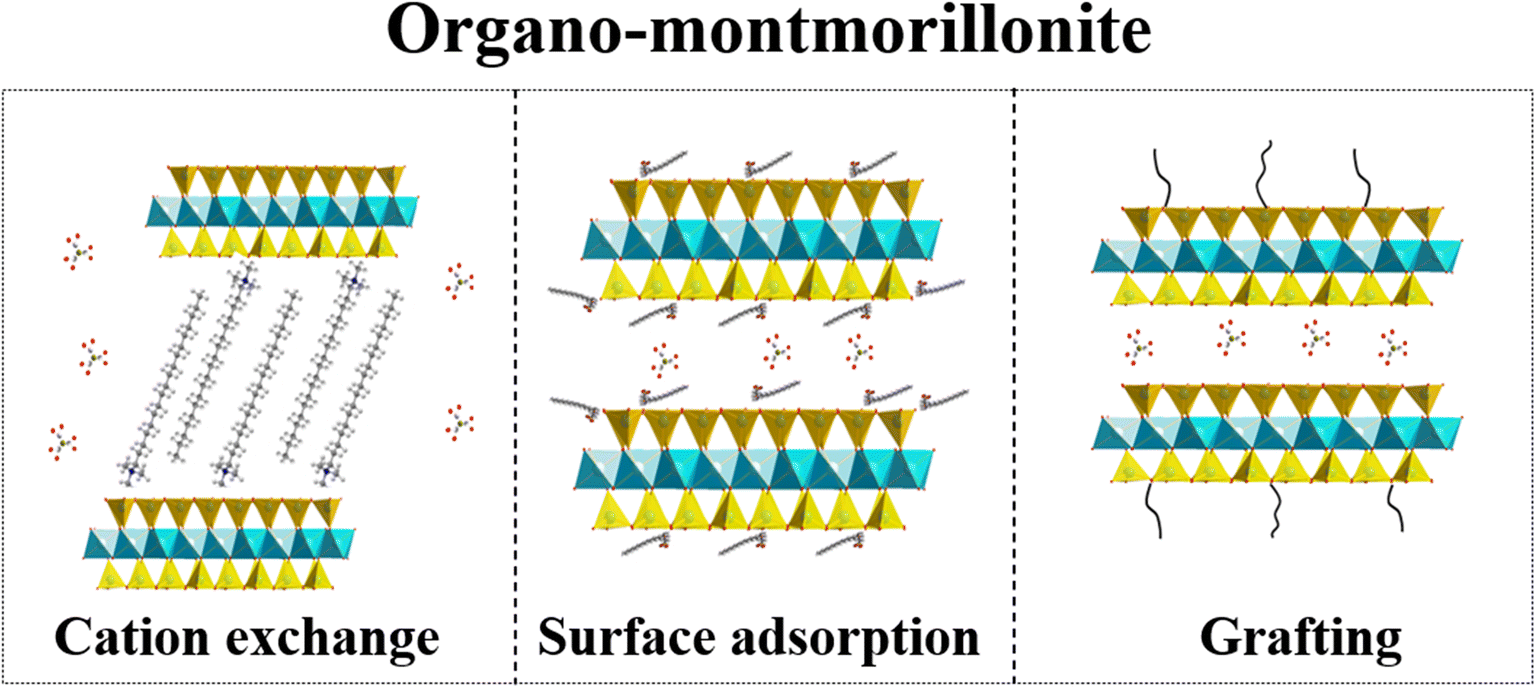
Fig. 1. Crystal structure of Mnt and schematic illustration of organo-modification of Mnt. a main view; b dioctahedron; c organic cation exchange; d surface adsorption; and e organo-grafting
The generally high hydrophilicity of natural Mnt limits its application in some organophilic polymerics (Jovic-Jovicic et al. Reference Jovic-Jovicic, Milutinovic-Nikolic, Bankovic, Mojovic, Zunic, Grzetic and Jovanovic2010; Xi et al. Reference Xi, Mallavarapu and Naidu2010). Organic modification is an effective method to solve this problem, most importantly in meeting the practical requirements of rheological control agents of, for example, drilling fluids (Silva et al. Reference Silva, Sousa, Menezes, Neves, Santana and Ferreira2014; Zhou et al. Reference Zhou, Zhang, Tang, Wang and Liao2016a), organic adhesives (Ye et al. Reference Ye, Zhong, Chen and Yang2005; Brantseva et al. Reference Brantseva, Antonov, Kostyuk, Ignatenko, Smirnova, Korolev, Tereshin and Ilyin2016), organic supplementary cementitious material (Taylor-Lange et al. Reference Taylor-Lange, Rajabali, Holsomback, Riding and Juenger2014; Ye et al. Reference Ye, Zhong, Chen and Yang2014), and organo-montmorillonite (OMnt)/polymer nanocomposites (Qin et al. Reference Qin, Hu, Luo, Yu and Xue2011; Al-Mulla et al. Reference Al-Mulla, Al-Mosawy, Abd-Almutalib and Mohamad2017; Bee et al. Reference Bee, Abdullah, Bee, Sin and Rahmat2018; Zhu et al. Reference Zhu, Zhou, Kabwe, Wu, Li and Zhang2019). OMnt is obtained through cation exchange, surface adsorption, or grafting (Fig. 1c–e). Until 10 years ago organic cations were the most commonly used modifiers for the preparation of OMnt. During the past 10 years, anions (Zhang et al. Reference Zhang, Liao and Xia2010), zwitterions (Lazorenko et al. Reference Lazorenko, Kasprzhitskii and Yavna2018), and other molecules (Borrego-Sánchez et al. Reference Borrego-Sánchez, Gómez-Pantoja, Morillo, Undabeytia and Sainz-Díaz2018) have been used increasingly as modifiers. OMnt is generally prepared in solution (Hu et al. Reference Hu, Tian, Zhan and Zhu2017; Martinez-Costa and Leyva-Ramos, Reference Martinez-Costa and Leyva-Ramos2017; Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a), but semi-solid-state (Zhou et al. Reference Zhou, Zhang, Tang, Wu and Zhao2016c) or solid-state reactions (Zhou et al. Reference Zhou, Zhang, Tang, Wang and Liao2016a, Reference Zhou, Zhang, Tang, Wu and Zhao2016c) are also common. Recently, by not being limited to heating, ultrasound (Zhang et al. Reference Zhang, Liao and Xia2010) and microwave (Cao et al. Reference Cao, Wang and Li2010) have also been shown to assist in the processing of OMnt.
Organo-modification of Mnt by introduced modifiers often increases the interlayer space (Gu et al. Reference Gu, Gao, Luo, Lu, Ye and Liu2014; Ezquerro et al. Reference Ezquerro, Ric, Miñana and Bermejo2015). Correspondingly, improving the adsorption capacity to organics (Zhou et al. Reference Zhou, Zhang, Tong, Wu, Yu and Ismadji2012) enhances compatibility in the polymer matrix (Theng Reference Theng2012; Scarfato et al. Reference Scarfato, Incarnato, Di Maio, Dittrich and Schartel2016) and improves the swellability in the organic solvent (Yu et al. Reference Yu, Zhu, Tong, Wang, Wu and Zhou2017b; Zhou et al. Reference Zhou, Li, Gates, Zhu and Yu2019). OMnt has been used widely as an additive in rheological control agents of paints, inks, adhesives, greases, varnishes (He et al. Reference He, Ma, Zhu, Yuan and Qing2010), adsorptive materials (Bajda and Klapyta, Reference Bajda and Klapyta2013; Yang et al. Reference Yang, Gao and Luo2014), drilling fluids (Zhou et al. Reference Zhou, Zhang, Tang, Wang and Liao2016a), and organic supplementary cementitious material in hydrating cement binders (Papatzani and Paine Reference Papatzani and Paine2017). OMnt can also be used as an adsorptive material for viruses (Liang et al. Reference Liang, Wei, Lee, Hsu, Lin and Lin2014), organic contaminants (Wang et al. Reference Wang, Ma, Li, He and Na2014; Hassani et al. Reference Hassani, Khataee, Karaca and Shirzad-Siboni2015), heavy metals (El Adraa et al. Reference El Adraa, Georgelin, Lambert, Jaber, Tielens and Jaber2017; Yu et al. Reference Yu, Xu, Jiang, Liu, McCall and Lu2017a), and dyes (Kohno et al. Reference Kohno, Inagawa, Ikoma, Shibata, Matsushima, Fukuhara, Tomita, Maeda and Kobayashi2011; Zhou et al. Reference Zhou, Zhang, Tong, Wu, Yu and Ismadji2012). Organic modification of Mnt can also strengthen the interfacial interaction between Mnt and polymers, significantly improving the mechanical strength and thermal stability and properties of the Mnt/polymer nanocomposites (Bujdak, Reference Bujdak2015; Gardi and Mishael Reference Gardi and Mishael2018; Zhu et al. Reference Zhu, Zhou, Kabwe, Wu, Li and Zhang2019). In the last 10 years, with the development of advanced technology, OMnt application has been transformed to high-tech fields such as gene engineering (Yu et al. Reference Yu, Li, Tong, Zhou, Lin and Xu2013; Hou et al. Reference Hou, Wu, Huang, Ruan, Liu and Zhu2014), bone-tissue engineering (Mauro et al. Reference Mauro, Chiellini, Bartoli, Gazzarri, Laus, Antonioli, Griffiths, Manfredi, Ranucci and Ferruti2017), and biosensors (Seleci et al. Reference Seleci, Ag, Yalcinkaya, Demirkol, Guler and Timur2012; Unal et al. Reference Unal, Yalcinkaya, Demirkol and Timur2018; Yilmaz et al. Reference Yιlmaz, Yalçιnkaya, Demirkol and Timur2020).
The past 10 years have increasingly witnessed new preparation technologies and new modification mechanisms for OMnt. This review summarizes the technological progress in organo-modified Mnt and highlights scientific insights into the modification mechanisms of Mnt. An exhaustive understanding of mechanisms of modification is important for the industrial design of OMnt-based nanomaterials. Finally, remaining problems and future work are noted.
ORGANIC CATION-MODIFIED MONTMORILLONITE
Cation-modified Mnt (OMntc) can be obtained using organic cations to modify Mnt through cation exchange (Fig. 2). The electrostatic interactions between organic cations and the negative charge of a Mnt surface (Lagaly et al. Reference Lagaly, Ogawa, Dékány, Bergaya and Lagaly2013), inherent hydrophobicity of alkyl chains, and hydrogen bonds are the main driving forces for surface modification (Bate and Burns Reference Bate and Burns2010; Michot et al. Reference Michot, Bihannic, Thomas, Lartiges, Waldvogel, Thieme, Funari and Levitz2013; Yu et al. Reference Yu, Ren, Tong, Zhou and Wang2014).

Fig. 2. Schematic illustrations of the cation modification mechanism of Mnt

Fig. 3. Intercalation of DS– in a Ca2+-Mnt, b Na+-Mnt, c Mnt-DS– (monomolecular of flat-lying), d Mnt-DS– (bimolecular of flat-lying ), e Mnt-DS– (monolayer of tilted chains), and f Mnt-DS– (pseudo tri-layer of tilted chains) (reproduced from Zhang et al. (Reference Zhang, Liao and Xia2010), copyright 2010, with permission from Elsevier). DS–: dodecylsulfate
OMntc can be prepared from suspension, a semi-solid-state, or a solid-state reaction (Zhou et al. Reference Zhou, Zhang, Tang, Wu and Zhao2016c; Lazorenko et al. Reference Lazorenko, Kasprzhitskii and Yavna2018). The preparation of OMntc from suspension, particularly aqueous suspension, is the preferred method at present (Wang et al. Reference Wang, Zhang, Hua, Su, Ma, Wang, Tao, Wang and Komarneni2017b; Ahmed et al. Reference Ahmed, Chaker, Belarbi, Abbas, Chotard, Abassi, Van Nhien, El Hadri and Bresson2018). In aqueous dispersion, the introduction of organic cations to the interlayer of Mnt occurs quickly even at room temperature with the assistance of stirring or sonification. Typically, however, surface coverage is more complete at slightly elevated temperatures (50–80°C) (Zhang et al. Reference Zhang, Mei, Chen, Chen and Zu2016; Lazorenko et al. Reference Lazorenko, Kasprzhitskii and Yavna2018), and microwave heating has been useful for cationic modification of Mnt (Peng et al. Reference Peng, Mao, Zheng, Wu, Wei, Zeng, Xiao and Sun2019). For example, Peng et al. (Reference Peng, Mao, Zheng, Wu, Wei, Zeng, Xiao and Sun2019) created OMntc by reacting the mixing suspension of dioctadecyltetrahydroxyethyldibromopropanediammonium and Mnt in a microwave synthesis system. Microwave heating can also be combined with ultrasonic dispersion to prepare OMntc. The combined action of microwave and ultrasound treatment was used by Luo et al. (Reference Luo, Ouyang, Antwi, Wu, Huang and Qin2019) to modify Mnt with butane-1,4-bis(dodecyldimethylammonium bromide) (gBDDA). The gBDDA-modified Mnt was better able to sequester phenol and chromate dyes.
OMntc created by solid-state or semi-solid-state reactions, such as ball milling, has advantages in water conservation and environmental protection by minimizing the amount of water used and wastes created (Zhou et al. Reference Zhou, Zhang, Tang, Wu and Zhao2016c; Wei et al. Reference Wei, Li, Zhang, Cai, Zhu, Li and Mo2018). OMntc is created through the solid-state reaction by adding Mnt and a cationic modifier into the ball mill and milling (Yan et al. Reference Yan, Chen, Feng, Xiang, Li, Shi, Wang and Lin2016a; Wei et al. Reference Wei, Li, Zhang, Cai, Zhu, Li and Mo2018). An alternative method is through semi-solid-state reaction by adding Mnt, cationic modifier, and a little water into the ball mill (Yan et al. Reference Yan, Chen, Feng, Xiang, Li, Wang and Lin2016b). The performances of OMntc prepared by these two methods, plus the aqueous dispersion method, were compared by Zhou et al. (Reference Zhou, Zhang, Tang, Wu and Zhao2016c). The properties tested included thermal stability, hydrophobicity, dispersibility, and thixotropy of the suspensions. At lower concentrations of hexadecyltrimethylammonium bromide (HDTMAB, also known as cetyltrimethylammonium bromide, CTAB), OMntc prepared in the semi-solid-state reaction was found to have similar properties to that prepared from aqueous dispersion. At high concentrations, the OMntc prepared from suspension had better property characteristics. Of the three preparation methods, OMntc prepared in the solid-state reaction had inferior properties in all HDTMAB/CTAB loadings tests. Modifiers in a liquid phase can cover the surface of Mnt evenly, thus leading to improved performance. For the preparation of OMntc by semi-solid-state or solid-state reaction, modifiers would be expected to be unevenly distributed on the surface of OMntc.
Cationic modification affects many properties of Mnt, including the basal spacing (Wu et al. Reference Wu, Yang, Mei, Qin, Liao and Lv2014a; Martinez-Costa and Leyva-Ramos Reference Martinez-Costa and Leyva-Ramos2017), surface hydrophobicity (Gu et al. Reference Gu, Gao, Luo, Lu, Ye and Liu2014; Yang et al. Reference Yang, Gao, Luo and Yang2016), catalytic properties (Wallis et al. Reference Wallis, Chaffee, Gates, Patti and Scott2010; Wang et al. Reference Wang, Song, Chen and Peng2010; Qin et al. Reference Qin, Troya, Shang, Hildreth, Helm and Xia2014), and the rheological behavior of suspensions (Tunç et al. Reference Tunç, Duman and Kancι2012; Yu et al. Reference Yu, Zhu, Tong, Wang, Wu and Zhou2017b). The extent of modification of the properties of OMntc depends on alkyl chain length (Lagaly et al. Reference Lagaly, Ogawa, Dékány, Bergaya and Lagaly2013; Wu et al. Reference Wu, Yang, Mei, Qin, Liao and Lv2014a; Zawrah et al. Reference Zawrah, Khattab, Saad and Gado2014; Acisli et al. Reference Acisli, Karaca and Gurses2017), chain number (He et al. Reference He, Ma, Zhu, Yuan and Qing2010; Wang et al. Reference Wang, Zhang, Hua, Su, Ma, Wang, Tao, Wang and Komarneni2017b), and modifier loading (Zhou et al. Reference Zhou, Zhu, Parker, Zhu, He and Molinari2015; Martinez-Costa and Leyva-Ramos Reference Martinez-Costa and Leyva-Ramos2017; Zhang et al. Reference Zhang, Zhang, Gao, Wang, Dong, Hou and Liu2017) (Table 1).
Table 1. Typical, recent methods and results of organo-cation modification of Mnt

*APTES: 3-triethoxysilylpropylamine; BTMA: benzyltrimethylammonium bromide; CTAB: cetyltrimethylammonium bromide; HDTMAB: hexadecyltrimethylammonium bromide; CTAC: hexadecyltrimethylammonium chloride; HDTMAC: hexadecyltrimethylammonium chloride; DC18: dimethyldioctadecylammonium chloride; LTAB: lauryltrimethylammonium bromide; DDTMAB: dodecyltrimethylammonium chloride; LTAC: lauryltrimethylammonium chloride; DDTMAC: dodecyltrimethylammonium chloride; DTDD: dioctadecyltetrahydroxyethyldibromopropanediammonium; HDTBPh: hexadecyltributylphosponium bromide; HDTMA: hexadecyltrimethylammonium (cation); HEMBP: hexamethylenebispyridiniumdibromide; STAC: octadecyltrimethylammonium chloride; ODTMAC: octadecyltrimethylammonium chloride; OMDAB: octadecylmethyldihydroxyethylammonium bromide; TMA: tetramethylammonium; TTAC: trimethyltetradecylammonium chloride.
After modification of Mnt by a long alkyl-chain organic cation, the basal spacing of OMntc increased with increases in chain length, chain number, and loading of the cationic modifier (He et al. Reference He, Ma, Zhu, Yuan and Qing2010; Sun et al. Reference Sun, Park, Zheng, Ayoko and Frost2013; Flores et al. Reference Flores, Undabeytia, Morillo and Torres Sanchez2017; Wang et al. Reference Wang, Zhang, Hua, Su, Ma, Wang, Tao, Wang and Komarneni2017b; Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a). For instance, the OMntc intercalated with hexadecyl trimethylammonium bromide and octyl trimethylammonium chloride were in a paraffin bilayer arrangement with basal spacing of 3.9 and 4.1 nm at 4.0 CEC (He et al. Reference He, Ma, Zhu, Yuan and Qing2010). When the added modifier was 4.0 CEC, the basal spacings of OMntc modified by a cationic modifier with a different alkyl chain number (octadecyl-trimethylammonium bromide and dioctadecyl-dimethylammonium bromide) were 4.1 nm and 5.5 nm, respectively. For a small loading of organo-cationic modifier, the alkyl chains occur in a parallel arrangement on the Mnt layer (Sun et al. Reference Sun, Park, Zheng, Ayoko and Frost2013; Naranjo et al. Reference Naranjo, Sham and Torres2017). For a large loading of organo-cationic modifier, the alkyl chains were arranged in a disordered bilayer or paraffin-type arrangement (Bagherifam et al. Reference Bagherifam, Komarneni, Lakzian, Fotovat, Khorasani, Huang, Ma, Hong, Cannon and Wang2014; Bujdak, Reference Bujdak2015; Flores et al. Reference Flores, Undabeytia, Morillo and Torres Sanchez2017). A well-known phenomenon is that OMntc hydrophobicity increases with increasing organo-cation chain number (Sun et al. Reference Sun, Park, Zheng, Ayoko and Frost2013; Flores et al. Reference Flores, Undabeytia, Morillo and Torres Sanchez2017; Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a) as well as with loadings of organo-cationic modifiers (He et al. Reference He, Ma, Zhu, Yuan and Qing2010; Wang et al. Reference Wang, Liu and Yu2015; Zhou et al. Reference Zhou, Zhu, Parker, Zhu, He and Molinari2015; Yang et al. Reference Yang, Yu and Liu2017). The hydrophobicity can be demonstrated directly by contact-angle tests. OMntc modified by single long alkyl chain cationic modifiers, such as octadecyltrimethylammonium chloride (OTAC) and N,N-dimethyl-N-octadecyl chloride, shared similar hydrophobicity. OMntc modified by dimethyldioctadecylammonium chloride (DDAC) had greater hydrophobicity than OMntc modified by OTAC, because of the hydrophobic interaction resulting from the long alkyl chain within the Mnt layer (Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a).
OMntc displayed superior catalytic properties (Wallis et al. Reference Wallis, Chaffee, Gates, Patti and Scott2010; Wang et al. Reference Wang, Song, Chen and Peng2010; Qin et al. Reference Qin, Troya, Shang, Hildreth, Helm and Xia2014). Fe3+-Mnt modified by hexadecyltrimethylammonium (HDTMA+) enhanced catalytic activity for oxidative coupling reactions, which can enhance the development of substrate-specific clay catalysts (Wallis et al. Reference Wallis, Chaffee, Gates, Patti and Scott2010). Similar materials have been made from Fe3+-Mnt modified by choline cations, which was shown to enhance catalytic activity for certain oxidative coupling reactions and a conjugate addition reaction (Wallis et al. Reference Wallis, Gates, Patti and Scott2011). The study by Wallis and colleagues indicated that maximum catalytic activity could be achieved by varying the ratios of Fe3+/choline+ in specific reaction types for specific substrates.
OMntc can provide improved rheological properties, such as viscosity and gel strength, due to the interfacial interactions between OMntc and organic solvent and interparticle interaction (Yu et al. Reference Yu, Ren, Tong, Zhou and Wang2014; Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a, Reference Zhuang, Zhang and Jaber2019a). The van der Waals forces between the alkyl chains of OMntc are weakened by certain organic solvents, leading to more organic solvent intercalating into the interlayer space of OMntc and enhancing interlayer swelling of OMntc (Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a, Reference Zhuang, Zhang and Jaber2019a). The rheological properties (such as viscosity and thixotropy) of OMntc in organic solvents were found by Zhuang et al. (Reference Zhuang, Zhang, Wu, Zhang and Liao2017a, Reference Zhuang, Zhang, Peng, Gao, Pereira and Jaber2019b) to be influenced by the ability of OMntc to swell and/or exfoliate. Cationic modifiers with larger molecular size generally led to easier exfoliation of OMntc in organic solvents (Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang and Liao2017a, Reference Zhuang, Zhang, Peng, Gao, Pereira and Jaber2019b). Exfoliation of OMntc results in more layers contributing to a strong gel structure, resulting in the improved viscosity and thixotropy of OMntc. Excessive exfoliation leading to dispersion and loss of gel structure can affect viscosity negatively, however, due to the decrease in connection among OMntc units.
OMntc can be used as a biosensor because the ion-exchange process in Mnt can incorporate charged molecules and facilitate the transfer between the electrode and biomolecules (Shumyantseva et al. Reference Shumyantseva, Bulko, Rudakov, Kuznetsova, Samenkova, Lisitsa, Karuzina and Archakov2007; Seleci et al. Reference Seleci, Ag, Yalcinkaya, Demirkol, Guler and Timur2012; Demir et al. Reference Demir, Seleci, Ag, Cevik and Timur2013; Yilmaz et al. Reference Yιlmaz, Yalçιnkaya, Demirkol and Timur2020). 4-aminothiophenol intercalated montmorillonite (4ATP-Mt) can be used as an immobilization layer for the pyranose oxidase (PyOx) enzyme on a glassy carbon electrode (Yilmaz et al. Reference Yιlmaz, Yalçιnkaya, Demirkol and Timur2020). Glucose in artificial body fluids and drinks can be analyzed by the compounded 4ATP-Mt/PyOx. Similar materials have been made from Mnt modified with dimethylamine (Seleci et al. Reference Seleci, Ag, Yalcinkaya, Demirkol, Guler and Timur2012). Trimethylamine (TM)-intercalated Mnt can be used as the immobilization matrix for microbial biosensors (Demir et al. Reference Demir, Seleci, Ag, Cevik and Timur2013). Gluconobacter oxydans cells were immobilized on the TM/Mnt matrix, and the consumption of oxygen was monitored at electrodes by using glucose as a substrate to monitor the respiratory activity of the cells.
ORGANIC ANION-MODIFIED MONTMORILLONITE
Interest in modifying Mnt with organic anionic modifiers is a result of the excellent thermal stability of anionic modifiers compared to traditional cationic modifiers. Some researchers assert that anions cannot intercalate effectively into the interlayer space of Mnt due to the electrostatic repulsion between negatively charged Mnt layers and negatively charged heads of the anionic modifier (Zheng et al. Reference Zheng, Li, Hao and Yao2013; Wu et al. Reference Wu, Zhang, Wang, Liao and Zhang2014b; Fu et al. Reference Fu, Zhang, Wu, Zhuang, Zhang, Yuan and Liao2016). Others, however, have presented alternative viewpoints, based on experimental results. Anions can enter the interlayer spaces of Mnt through interaction with hydroxyl groups at the Mnt layer edges (Yu et al. Reference Yu, Ke, Deng, Lu, Ji, Hu and Zhao2018) or ion–dipole attraction to the exchangeable cations located within the interlayer (Sarier et al. Reference Sarier, Onder and Ersoy2010; Zhang et al. Reference Zhang, Liao and Xia2010).
The mechanism for the intercalation of anionic modifiers into Mnt, however, is still unsettled. Mnt modified by an anionic modifier (OMnta) is best prepared in an acidic medium (Zhang et al. Reference Zhang, Liao and Xia2010; Cao et al. Reference Cao, Wang, Liu, Chen, Cao and Yu2015; Yu et al. Reference Yu, Ke, Deng, Lu, Ji, Hu and Zhao2018). Acidic conditions promote H+ exchange as well as acid attack at charge sites in the layer structure, which leads to partial dissolution (Wallis et al. Reference Wallis, Gates, Patti, Scot and Teoh2007; Liu et al. Reference Liu, Gates and Bouazza2013; Yi et al. Reference Yi, Yang, Zhao, Huang, Camino, Frache and Yang2017). The attack of acidic Mnt may help the anions to penetrate between the layers.
Ca2+-Mnt was modified by sodium dodecylsulfate (SDS) and octadecylcarboxylate (also known as sodium stearate, SSTA) in acidic medium by sonication (Zhang et al. Reference Zhang, Liao and Xia2010). The anionic modifiers expanded the basal spacing to 3.87 nm (Mnt-DS–) and 4.80 nm (Mnt-STA–) from 1.53 nm, thus proving that intercalation of anions into Mnt interlayer space was possible. A possible intercalation mechanism for anionic modification under acidic conditions is depicted in Fig. 3. The anions and the hydronium ions or the exchangeable cations in the interlayer of Mnt probably form ion pairs that allow the anions to enter the interlayer of Mnt. Ca2+-Mnt was modified by Yu et al. (Reference Yu, Ke, Deng, Lu, Ji, Hu and Zhao2018) using sodium laurylsulfonate/dodecylsofonate (SLS) in acidic medium (pH = 1) with vigorous stirring. The interlayer distance of the OMnta increased to 1.52 nm from 1.21 nm. The chain of organic molecules may prefer a horizontal orientation relative to the Mnt surface. The anion may also have intercalated into the Mnt interlayer spaces by replacement of, or interaction with, hydroxyl groups at the Mnt layer edges. In acidic media, many hydroxyl groups can be present at the edges of Mnt due to electrostatic interactions with hydronium ions. The anion could, therefore, intercalate into the Mnt by replacing or interacting with hydroxyl groups in acidic media. 50 wt.% naphthalene sulfonic acid (NSA) was intercalated into Mnt using microwave irradiation (Riaz et al. Reference Riaz, Ashraf and Khan2011). Mnt modified with linear alkylbenzene sulfonic acid (LABSA) or sodium lauryl ether sulfate (SLES) had better hydrophilicity. The hydrophilicity of 5 wt.% LABSA/Mnt was less than that of 5 wt.% SLES/Mnt (Akbhyulut et al. Reference Akbulut, Kurt and Arasan2012). The d 001 values of OMnta with 5 wt.% LABSA and 10 wt.% SLES were 1.501 nm and 1.496 nm, respectively (Akbulut et al. Reference Akbulut, Kurt, Arasan and Pekdemir2013).

Fig. 4. Schematic diagram of the intercalation processes and mechanism of the zwitterionic modified Mnt (reproduced from Zhu et al. (Reference Zhu, Zhang, Qing, Wen, Su, Ma, Wei, Liu, He and Xi2017), copyright 2017, with permission from Elsevier)
OMnta could be used for synthesis of OMnt/polymer nanocomposites (Sarier et al. Reference Sarier, Onder and Ersoy2010; Yu et al. Reference Yu, Ke, Deng, Lu, Ji, Hu and Zhao2018) as a scaffold for composites (Cao et al. Reference Cao, Wang, Liu, Chen, Cao and Yu2015) or to assist in the avoidance of virus infection (Liang et al. Reference Liang, Wei, Lee, Hsu, Lin and Lin2014). OMnta for the synthesis of OMnt/polymer nanocomposites possessed better dispersibility, greater antiwear property, and greater stability to thermal decomposition (Sarier et al. Reference Sarier, Onder and Ersoy2010; Yu et al. Reference Yu, Ke, Deng, Lu, Ji, Hu and Zhao2018). The onset degradation temperature of Mnt-3 wt.% SLS was increased by 30.1°C compared to pure Polystyrene (PS) (Yu et al. Reference Yu, Ke, Deng, Lu, Ji, Hu and Zhao2018). The coefficient of friction of the Mnt-3 wt.% SLS/PS particles as an additive to polyalphaolefin was 0.09, which means Mnt-3 wt.% SLS/PS nanocomposites are effective friction reduction and antiwear substances. OMnta, formed by modification by sodium salts of octadecanoic acid, would enhance thermal stability and dispersivity in the production of polyurethane (PU) nanocomposite foam (Sarier et al. Reference Sarier, Onder and Ersoy2010). The temperature of onset of thermal decomposition of 2 wt.% OMnt-SOD/PU moved to 238°C from 200°C for pure PU. OMnta made by modification by sodium stearate (SST) enhanced the thermal stability and flame retardancy of polyurethane (Yi et al. Reference Yi, Yang, Zhao, Huang, Camino, Frache and Yang2017). The addition of Mnt-SST to PU reduced the peak heat-release rate of PU by 38%; this means that Mnt-SST can be used in fire-proof material. OMnta modified by sodium dodecyl sulfate interfered with viral binding and blocked infection by Japanese encephalitis, dengue virus, and influenza A virus through electrostatic interaction; this may provide a new way of developing novel antiviral nanomaterials (Liang et al. Reference Liang, Wei, Lee, Hsu, Lin and Lin2014). OMnta can also improve the mechanical behavior, biodegradation, and biomineralization properties of chitosan-collagen/OMnt scaffold (Cao et al. Reference Cao, Wang, Liu, Chen, Cao and Yu2015).
ORGANIC ZWITTERION-MODIFIED MONTMORILLONITE
Harnessing organo-cationic modifiers in biomedical applications is often hampered by the generally greater toxicity of cationic organic species, and the lack of biocompatibility evidence in biological fields (Wicklein et al. Reference Wicklein, Darder, Aranda and Ruiz-Hitzky2010). Modification of Mnt by zwitterions (OMntz) enhances the biocompatibility of OMnt and expands their applications into biological fields (Wicklein et al. Reference Wicklein, Darder, Aranda and Ruiz-Hitzky2010; Wang et al. Reference Wang, Lian, Xi, Sun and Zheng2018). Zwitterion modifiers contain both an organo-cationic hydrophobic group and an anionic hydrophilic group simultaneously. OMntz can be obtained by cation exchange (Qi et al. Reference Qi, Fang, Wang, Ma, Jiang and Wang2008; Zhu et al. Reference Zhu, Qing, Wang, Zhu, Wei, Tao, Yuan and He2011), ion-dipole interaction (Zhu et al. Reference Zhu, Zhang, Qing, Wen, Su, Ma, Wei, Liu, He and Xi2017; Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019), or hydrogen bonding between end hydroxyl groups of the zwitterion and the siloxane surface of montmorillonite.
Different views exist on the reaction mechanism for modifiying Mnt with zwitterionic species. Some researchers hold that zwitterions can probably enter the interlayer of Mnt by exchanging with the inorganic cations in the interlayer space, or adsorbing on the surface due to their generally alkylammonium-based cationic group (Qi et al. Reference Qi, Fang, Wang, Ma, Jiang and Wang2008; Zhu et al. Reference Zhu, Qing, Wang, Zhu, Wei, Tao, Yuan and He2011). Zwitterions may also simply be adsorbed onto the Mnt surface due to electrostatic attraction between the positively charged group of zwitterions and the negative surface of Mnt, and not intercalate into the interlayer of Mnt (Ghafar et al. Reference Ghafar, Radwan and El-Wakee2020). According to Zhu et al. (Reference Zhu, Zhang, Qing, Wen, Su, Ma, Wei, Liu, He and Xi2017) and Wang et al. (Reference Wang, Xi, Lian, Sun and Zheng2019), zwitterions are more likely to undergo bridging between Ca2+-Mnt and zwitterion through ion–dipole interactions than through an exchange reaction. The ion–dipole interactions are generated between the hydrophilic head group and the negatively charged Mnt surface as well as the metal cations and the negatively charged component of the zwitterions (Fig. 4①). With small concentrations of 3-(N, N-dimethypalmitylammonio)propane sulfonate (SB16), –SO3 groups of zwitterionic modifiers are closer to the interlayer surface of Mnt (Zhu et al. Reference Zhu, Zhang, Qing, Wen, Su, Ma, Wei, Liu, He and Xi2017). Due to negative –SO3 groups not balancing all the Ca2+, some Ca2+ ions are still bound to the negatively charged interlayer surface. With the zwitterionic modifier concentration increased, the terminating –SO3 groups may be in the interlayer space. With the high zwitterionic modifier concentration, Ca2+ ions are removed from the interlayer surface due to two –SO3 groups balancing one Ca2+ ion.

Fig. 5. The basal spacing of zwitterion-modified Mnt. a, c: (Zhu et al. Reference Zhu, Qing, Wang, Zhu, Wei, Tao, Yuan and He2011); b, d: (Zhu et al. Reference Zhu, Zhang, Qing, Wen, Su, Ma, Wei, Liu, He and Xi2017); e, f: (Zhu et al. Reference Zhu, Qing, Ma, Zhu and He2014). SB12: 3-(N,N-dimethyldodecylammonio)propane sulfonate; SB14: 3-(N, N-dimethylmyristylammonio)propane sulfonate; SB16:3-(N,N-dimethylpalmityl-ammonio)propane sulfonate
Zwitterionic modification also changes the physical characteristics of Mnt, such as the basal spacing (Zhu et al. Reference Zhu, Qing, Wang, Zhu, Wei, Tao, Yuan and He2011, Reference Zhu, Qing, Ma, Zhu and He2014, Reference Zhu, Zhang, Qing, Wen, Su, Ma, Wei, Liu, He and Xi2017), hydrophobicity (Ma et al. Reference Ma, Chen, Zhu, Xi, He, Zhu, Tao and Ayoko2016; Silva et al. Reference Silva, Stefanichen Monteiro, Chaparro, Silva Hardt, Giudici, Barros-Timmons, Bourgeat-Lami and Martins Dos Santos2017; Lazorenko et al. Reference Lazorenko, Kasprzhitskii and Yavna2018), adsorption capacity (Eyama et al. Reference Eyama, Yogo, Fujimura, Tsukamoto, Masui, Shimada, Tachibana, Inoue and Takagi2012; Gu et al. Reference Gu, Gao, Lu, Liu and Yang2015; Liu et al. Reference Liu, Wu, Zhu and Tran2016), and thermal stability (Zhu et al. Reference Zhu, Qing, Wang, Zhu, Wei, Tao, Yuan and He2011; Ma et al. Reference Ma, Zhu, He, Xi, Zhu, Tao and Liu2015).
Zwitterions can be introduced into the interlayer space of Mnt (Soares et al. Reference Soares, Ferreira and Livi2016; Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019). OMntz was formed by modifying Mnt with dodecyldimethylbetaine (BS-12) or lauramidopropylbetaine (LAB-35) in an ultrasonic bath for 0.5 h and then transferred to a water bath at room temperature for 24 h (Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019). The arrangement of BS-12 in the interlayer of OMntz was as a lateral monolayer and flat-lying bilayer; while the arrangements of LAB-35 in the interlayer of OMntz was in a tilted way, perpendicular to the basal plane of the Mnt. Ciprofloxacin can be intercalated in the interlayer space of fluorohectorite, a synthetic smectite, at acidic pH (dos Santos et al. Reference dos Santos, Gates, Michels, Juranyi, Mikkelsen, da Silva, Fossum and Bodallo2018).
The maximum basal spacing of OMntz increased with increasing alkyl chain length of the zwitterionic modifier. OMntz made by 0.8 CEC SB16 modifier had a maximum basal spacing of 4.37 nm. OMntz made by 3.0 CEC 3-(N,N-dimethyldodecylammonio)propane sulfonate (SB12) and 2.5 CEC 3-(N, N-dimethylmyristylammonio)propane sulfonate (SB14) modifiers had maximum basal spacings of 4.13 nm and 4.23 nm (Fig. 5). OMntz can achieve a larger interlayer spacing than OMntc at the same modifier concentration. The maximum basal spacing of OMntz (4.13 nm) was almost twice the maximum basal spacing of OMntc made by dodecyl trimethylammonium bromide modifier (Zhu et al. Reference Zhu, Qing, Wang, Zhu, Wei, Tao, Yuan and He2011). The basal spacing of histidine-modified Mnt increased to 1.38 nm for an amino acid substituent with a ring structure from 1.14 nm for Na+-Mnt (Songurtekin et al. Reference Songurtekin, Yalcinkaya, Ag, Seleci, Demirkol and Timur2013).

Fig. 6. a A conceptual model of major bonding between a polyacrylamide (PAM) chain and exchangeable cations in the interlayer of smectite; b A conceptual model of a non-ionic PAM-smectite complex with d 001 spacing of ~1.5 nm. Water molecules are not shown (reproduced from Deng et al. (Reference Deng, Dixon, White, Loeppert and Juo2006), copyright 2006, with permission from Elsevier). PAM: polyacrylamide
Zwitterionic modification also transforms the surface properties of Mnt from hydrophilic to hydrophobic (Ma et al. Reference Ma, Chen, Zhu, Xi, He, Zhu, Tao and Ayoko2016; Lazorenko et al. Reference Lazorenko, Kasprzhitskii and Yavna2018). Modification of Na+-Mnt by hydroxyethylalkyl imidazoline (HEAI) and oleylmidopropylbetaine (OAPB) resulted in hydrophobization of the mineral surface. HEAI displaced more hydrated cations from Na+-Mnt than OAPB, thereby promoting greater hydrophobicity of the Mnt surface. The wetting contact angles of OMntz formed by HEAI modification increased from 37° to 83°, while the wetting contact angles of OMntz formed by OAPB modification increased to only 47° from 37° (Lazorenko et al. Reference Lazorenko, Kasprzhitskii and Yavna2018). OMntz formed by hexadecyldimethyl(3-sulphonatopropyl)ammonium modification, however, had lower hydrophobicity than OMntc formed by hexadecyltrimethylammonium bromide modification (Ma et al. Reference Ma, Chen, Zhu, Xi, He, Zhu, Tao and Ayoko2016). The mechanisms by which zwitterionic phospholipids phosphatidylglycerol (PG) and phosphatidylethanolamine (PE) altered the hydrophobicity of montmorillonite were investigated by Kessenich et al. (Reference Kessenich, Pokhrel, Kibue, Flury and Yoreo2020). The PE can sorb onto the negatively charged montmorillonite surface, whereas negatively charged PG lipids cannot. On comparing PE and PG Mnt/lipid films, three characteristics were found to change Mnt/lipid film wettability: they must bind to the mineral surface, be solid at room temperature, and have a relatively continuous distribution throughout the film.
The alkyl chain length and the loading of zwitterionic modifier both influence the adsorptivity of the resulting OMntz (Zhu et al. Reference Zhu, Qing, Ma, Zhu and He2014; Gu et al. Reference Gu, Gao, Lu, Liu and Yang2015; Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019). SB12-, SB14-, and SB16- Mnt were used by Zhu et al. (Reference Zhu, Qing, Ma, Zhu and He2014). When these modifier loadings were >1.8 CEC, the adsorbing ability of SB14-Mnt for nitrophenol and nitrobenzene was better than SB12-Mnt, but poorer than SB16-OMnt. The adsorption depends mainly on the distribution of the hydrophobic interaction between the zwitterionic alkyl chains. Further increase in the loading of zwitterionic modifier caused the adsorption of OMntz formed by SB12 modification to increase with modifier levels. The adsorption of OMntz formed by SB14 and SB16 modification, however, increased with modifier loading levels to a maximum then decreased. When the interlayer space of OMntz was occupied by zwitterions, the adsorption capacity decreased because the steric effect of large zwitterions precluded further adsorption.
OMntz could be used for the synthesis of OMnt/epoxy polymer nanocomposites (Soares et al. Reference Soares, Ferreira and Livi2016), mycotoxin adsorbents (Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019), and contaminant adsorbents (Liu et al. Reference Liu, Wu, Zhu and Tran2016; Ma et al. Reference Ma, Chen, Zhu, Xi, He, Zhu, Tao and Ayoko2016; Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019). The viscosity of the OMntz/epoxy polymer nanocomposites was increased significantly due to the dispersion of the OMntz modified by zwitterionic imidazolium-based ionic liquid into the epoxy matrix (Soares et al. Reference Soares, Ferreira and Livi2016). Moreover, the OMntz/epoxy polymer nanocomposites also presented very good transparency. OMntz formed by BS-12 or LAB-35 modification used as adsorbents for mycotoxins were shown to simultaneously detoxify multiple polar and non/weak polar mycotoxins (Wang et al. Reference Wang, Xi, Lian, Sun and Zheng2019). This provides new insights into development of versatile mycotoxin adsorbents. OMntz, formed by modification of 3-(N,N-dimethylhexadecylammonio)propane sulfonate, had significant capacity to adsorb to anionic dye (methyl orange) and cationic herbicides (paraquat and amitrole) (Gu et al. Reference Gu, Gao, Lu, Liu and Yang2015). It also indicated that OMntz has potential in treating wastewater-containing anionic and cationic contaminants. OMntz has simultaneously adsorptive properties for heavy metals and organic pollutants (Gu et al. Reference Gu, Gao, Lu, Liu and Yang2015; Liu et al. Reference Liu, Wu, Zhu and Tran2016; Ma et al. Reference Ma, Chen, Zhu, Xi, He, Zhu, Tao and Ayoko2016). The hydrophobic group of zwitterionic modifiers is beneficial in the adsorption of organic pollutants. The hydrophilic group of zwitterionic modifiers is beneficial in the adsorption of heavy metals. Mnt modified by amino acid, such as glycine and histidine, can immobilize the glucose oxidase on a glassy carbon electrode, applied to a biosensor to analyze catechol (Demir et al. Reference Demir, Demir, Yalcinkaya, Cevik, Demirkol, Anik and Timur2014; Songurtekin et al. Reference Songurtekin, Yalcinkaya, Ag, Seleci, Demirkol and Timur2013). The synthesis of hydroxylapatite (HAP) in Mnt-modified 5-aminovaleric acid was presented by Ambre (Reference Ambre, Katti and Katti2011). The nucleation mechanism of HAP may be due to chelation of the dissociated carboxylate groups with the calcium ions. The prepared material can be compounded with the chitosan to form chitosan/polygalacturonic acid (ChiPgA) composite films for culturing human osteoblast cells. Human osteoblasts were able to adhere to these films, form clusters on them, and exhibit good biocompatibility with them.
ORGANIC MOLECULE-MODIFIED MONTMORILLONITE
Mnt modified by organic, non-ionic species (OMntn) retain their original cation exchange capacity (CEC), resulting in OMntn which can adsorb cationic compounds by cation exchange and polar molecules by ion–dipole and H-bonding interactions (Guégan et al. Reference Guégan, Giovanela, Warmont and Motelica-Heino2015). Organo-non-ionic modifiers adsorb onto surfaces and in the interlayer space of Mnt by hydrogen bonds, ion–dipole interaction, and electrostatic interactions (Guégan et al. Reference Guégan, Oliveira, Gleuher and Sugahara2019; Borrego-Sánchez et al. Reference Borrego-Sánchez, Gómez-Pantoja, Morillo, Undabeytia and Sainz-Díaz2018; Wang et al. Reference Wang, Lian, Xi, Sun and Zheng2018; Hojiyev et al. Reference Hojiyev, Ulcay and Celik2017a).
Some non-ionic modifiers (e.g. nonpolar solvents) can be introduced easily into the interlayer space of Mnt due to their large dielectric values (Silva et al. Reference Silva, Sousa, Menezes, Neves, Santana and Ferreira2014; Gates et al. Reference Gates, Shaheen, Turney and Patti2016; Hojiyev et al. Reference Hojiyev, Ulcay and Celik2017a). The aqueous solution of OP-10 to Mnt suspension was introduced slowly using a peristaltic pump (Wang et al. Reference Wang, Lian, Xi, Sun and Zheng2018). The mixtures were treated for 0.5 h in an ultrasonic bath at 60°C, and then stirred for 24 h at room temperature. Non-ionic species were introduced successfully into the Mnt interlayer space. Hydrogen bonds can be formed between the polyoxyethylene ether chain of OP-10 and siloxane surfaces of Mnt. Non-ionic modifier octylphenolpolyoxyethylene ether (OP-10) can be intercalated into the interlayer of Mnt through hydrogen bonding. OMntn modified by reacting Mnt with glycerol carbonate (GC) was prepared by Gates et al. (Reference Gates, Shaheen, Turney and Patti2016). First, oriented films of Na+-Mnt were deposited under suction onto ceramic tiles from Mnt suspensions, followed by addition of GC solution to the wet Na+-Mnt film and air-drying under ambient conditions for 3 h. GC interacted with the interlayer cations of Mnt through the carbonyl group, interacted with the interlayer surfaces of Mnt through its hydroxyl group. A loading of 100 wt.% GC (oven-dried Mnt basis) provided a highly ordered GC-Na+-Mnt complex, but larger loadings resulted in layer stacking disorder. Loadings of <100 wt.% resulted in two distinct d values. A wide variety of OMnt complexes are possible with GC and its derivatives (Fehervari et al. Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016a, Reference Fehervari, Gates, Patti, Turney, Bouazza and Roweb) including polymeric-GC Mnt complexes (Shaheen et al. Reference Shaheen, Turney, Saito, Gates and Patti2016). Na+-Mnt was modified with octylphenylpolyoxyethylene ether (OPP) via wet ball-milling (Yan et al. Reference Yan, Zhang, Chen, Bao, Zhao, Hu, Liu and Lin2020b) . During the wet ball-milling process, the Na+-Mnt formed lattice defects easily under the forces of extrusion, collision, and friction, which favored the intercalation modification of OPP. The interlayer spacing of OMnt-OPP increased to 1.76 nm from 1.24 nm of Na+-Mnt. Mnt was modified with tri-ethyleneglycolmono-n-decyl ether (C10E3) by Balme et al. (Reference Balme, Guegan, Janot, Jaber, Lepoitevin, Dejardin, Bourrat and Motelica-Heino2013). The interlayer spacing of OMnt-C10E3 increased to 3.6 nm from 1.2 nm of Na+-Mnt.
The probable bonding mechanisms between non-ion polyacrylamides (PAM) and Mnt were investigated by Deng et al. (Reference Deng, Dixon, White, Loeppert and Juo2006). Their research found the ion–dipole interaction between the amide groups of the PAM and the exchangeable cations. Hydrogen bonding existed between the amide groups and water molecules in the hydration shells of exchangeable cations (Fig. 6). Similar observations by Gates et al. (Reference Gates, Shaheen, Turney and Patti2016), when intercalating Na+-Mnt with glycerol carbonate (GC), indicated that strong ion–dipole interactions between the solvating GC and the interlayer cation were stable up to high salt concentrations but, at lower salt concentrations, H-bonding with interlayer water and the interlayer surface were important mechanisms. At very high salt concentrations, the OMntn-GC maintained a more swollen state and better hydraulic barrier performance (Fehervari et al. Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016a, Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016b). The adsorption of Ethomeen T/15 in the interlayer space of Mnt was modeled at the molecular level by Borrego-Sánchez et al. (Reference Borrego-Sánchez, Gómez-Pantoja, Morillo, Undabeytia and Sainz-Díaz2018) by computation. Hydrogen bonds formed between the H atoms of the modifier and the tetrahedral basal O atoms of the Mnt interlayer. These hydrogen bonds and the electrostatic interactions between cations and the phyllosilicate surface are the main driving forces of the adsorption.

Fig. 7. Schematic illustration of the formation of CTA+ and DS– co-intercalated OMnt by the two-step method (reproduced from Fu et al. (Reference Fu, Zhang, Wu, Zhuang, Zhang, Yuan and Liao2016), copyright (2016), with permission from Elsevier). CTA+: cetyltrimethylammonium; DS–: dodecyl sulfonate
Not all non-ionic modifiers can enter the interlayer space of Mnt. The basal spacing of OMntn, caused by OP-10 modification, increased to 4.26 nm from 1.45 nm (Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang, Zhang and Liao2017b). The basal spacings of OMntn made using Span-80 and Span-20 modifiers, however, were similar to Mnt (Table 2). This may mean that Span-80 and Span-20 fail to intercalate Mnt. The interlayer spacings of Mnt made by polypropylene glycol (PPG) 1200 and PPG 2000 modifiers remain unchanged within the range 0.25–2.00 CEC and remained below 2 nm (Ouellet-Plamondon et al. Reference Ouellet-Plamondon, Stasiak and Al-Tabbaa2014). This is probably because some non-ionic modifiers inhibited intercalation. OMntz shows a dual hydrophilic/hydrophobic character (Guegan et al. Reference Guégan, Giovanela, Warmont and Motelica-Heino2015). The contact angles of OMntn formed by Span-80, Span-20, and OP-10 were 52, 50, and 33°, respectively (Zhuang et al. Reference Zhuang, Zhang, Wu, Zhang, Zhang and Liao2017b). However, the surface of OMntn made using polyvinylpyrrolidone as a modifier became more hydrophilic (Hojiyev et al. Reference Hojiyev, Ulcay and Celik2017a).
Table 2. Recent, typical methods and results of Mnt modified by nonionic organic molecules

*EA: erucamide; OA: oleamide; OP-10: octylphenolpolyoxyethylene ether.
OMntn can be used in drug carriers (Yan et al. Reference Yan, Zhang, Chen, Bao, Zhao, Hu, Liu and Lin2020b), as adsorbents of cationic compounds (Wang et al. Reference Wang, Wang, Sun, Zheng and Xi2017a), in the oil industry (Cardoso et al. Reference Cardoso, Ferreira, da Silva, Ferreira and Neves2012; Ouellet-Plamondon et al. Reference Ouellet-Plamondon, Stasiak and Al-Tabbaa2014; Silva et al. Reference Silva, Sousa, Menezes, Neves, Santana and Ferreira2014), and in geosynthetic clay liners (GCLs) (Fehervari et al. Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016a, Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016b; Shaheen et al. Reference Shaheen, Turney, Saito, Gates and Patti2016). Plysorbate 20-modified Mnt nanocomposite material was applied as a drug carrier for the inhibition of intestinal efflux transporters. OMnt-OPP can enhance adsorption capacity for ibuprofen and exhibit well controlled release properties (Yan et al. Reference Yan, Zhang, Chen, Bao, Zhao, Hu, Liu and Lin2020b). OMntn formed by OP-10-modified OMnt had a significant adsorptivity for the cationic organic dye methylene blue (MB) due to the remaining cation exchangeability (Wang et al. Reference Wang, Zhang, Hua, Su, Ma, Wang, Tao, Wang and Komarneni2017b). The polyoxyethylene ether chains of OP-10 can form hydrogen bonding with MB molecules, further improving the adsorptivity of MB. OMntn is a potential adsorbent for simultaneous detoxification of polar and non-polar mycotoxins. OMntn formed by ultramine 50 (TA50) had 100% swelling in diesel oil and kerosene (Silva et al. Reference Silva, Sousa, Menezes, Neves, Santana and Ferreira2014). This means that OMntn made in this way is suitable for use in organic-based drilling fluids. In addition, OMntn is used as a swelling inhibitor in the oil industry (Cardoso et al. Reference Cardoso, Ferreira, da Silva, Ferreira and Neves2012; Silva et al. Reference Silva, Sousa, Menezes, Neves, Santana and Ferreira2014). OMntn formed by PPG modification acts as an intercalation inhibitor, limiting further expansion of the basal spacing (Ouellet-Plamondon et al. Reference Ouellet-Plamondon, Stasiak and Al-Tabbaa2014). OMntn has been considered for use in geosynthetic clay liners (GCLs) of hypersaline leachates (Fehervari et al. Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016a, Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016b; Shaheen et al. Reference Shaheen, Turney, Saito, Gates and Patti2016). OMntn formed by GC minimizes adverse effects associated with the loss of water from the interlayer space of Mnt as well as Ca2+-for-Na+ exchange in the hypersaline leachates (Fehervari et al. Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016a, Reference Fehervari, Gates, Patti, Turney, Bouazza and Rowe2016b). OMntn formed by modification of OMnt by cyclic carbonate polyether, Poly(4-((oxiran-2-ylmethoxy)methyl)21, 3-dioxolan-2-one) (POMD), strengthened the hydraulic barrier capabilities of sodium bentonite to 3 M sodium chloride solution (Shaheen et al. Reference Shaheen, Turney, Saito, Gates and Patti2016).
TWO MODIFIERS IN CO-MODIFIED MONTMORILLONITE
Mnt can be co-modified by two (or more) organic modifiers. The mixtures of various types of modifiers exhibit different properties compared with the corresponding single modifiers, and can cause synergistic effects in both the liquid and solid systems (Sehgal et al. Reference Sehgal, Kosaka, Doe and Otzen2009; Zhang et al. Reference Zhang, Zhao, Zhu, Wu, Wang and Lu2012b). The types of modifiers used for Mnt co-modification are usually combinations of cations and anions (Zhang et al. Reference Zhang, Zhao, Zhu, Wu, Wang and Lu2012b, Reference Zhang, Zhang, Liao and Xia2013), of cations and non-ionic species (Fan et al. Reference Fan, Zhu, Li and Chen2015; Yin et al. Reference Yin, Zhang, Wu, Tan and Ke2015), and of anions and non-ions species (Zhang et al. Reference Zhang, Long, Zhang, Zhu, Wang, Wu and Lu2012a).
Cation and anion co-modified OMnt can be prepared by one-step (Fu et al. Reference Fu, Zhang, Wu, Zhuang, Zhang, Yuan and Liao2016) or two-step methods (Chen et al. Reference Chen, Chen, Luan, Ji and Xia2011; Zhang et al. Reference Zhang, Zhang, Liao and Xia2013; Wu et al. Reference Wu, Zhang, Wang, Liao and Zhang2014b; Fu et al. Reference Fu, Zhang, Wu, Zhuang, Zhang, Yuan and Liao2016). A one-step method can be used to mix an aqueous solution of cationic and anionic modifiers in Mnt dispersions. A two-step method involves adding aqueous solutions of one modifier to aqueous dispersion of Mnt first and reacting for some time, then adding aqueous solution of another modifier. The addition sequence of CTAC and SDS had an impact on the intercalation reaction, as did the choice of method. The simultaneous intercalation of CTA+ and DS– resulted in a sample with the largest basal spacing using the one-step method. The intercalation in which CTAC was added first resulted in a sample with larger basal spacing than that where SDS was added first. A co-modified mechanism resulting from the two-step method was proposed by Fu et al. (Reference Fu, Zhang, Wu, Zhuang, Zhang, Yuan and Liao2016) (Fig. 7). Firstly, cations entered the interlayer of Mnt by ion exchange and formed an organic environment which then allowed CTA+-DS– ion-pairs to form within the Mnt layer. DS is presumed to enter the interlayer as neutralized molecules (e.g. DS–-Na+) due to the concentration difference. The addition of CTAB to SDS was considered by Zheng et al. (Reference Zheng, Li, Hao and Yao2013) to decrease the negative charge density and charge repulsion of the anion. Thus, CTAB-SDS could interact with Mnt and intercalate into the interlayer space of Mnt.

Fig. 8. Schematic illustration of the formation of a HDPB and TX100 co-intercalated Mnt (reproduced from Zhang et al. (Reference Zhang, Zhao, Zhu, Wu, Wang and Lu2012b), copyright 2012, with permission from Elsevier); b ODTMA+ and EA co-intercalated Mnt (reproduced from Zhou et al. (Reference Zhou, Li, Gates, Zhu and Yu2019), copyright 2019, with permission from Elsevier). EA: erucamide; HDPB+: hexadecylpyridinium; ODTMA+: octadecyltrimethylammonium; TX100: Triton X-100
Cation and non-ionic co-modified Mnt was prepared by the one-step method (Zhang et al. Reference Zhang, Long, Zhang, Zhu, Wang, Wu and Lu2012a; Fan et al. Reference Fan, Zhu, Li and Chen2015; Yin et al. Reference Yin, Zhang, Wu, Tan and Ke2015; Zhou et al. Reference Zhou, Li, Gates, Zhu and Yu2019). CTAB and OP-10 were added to the Mnt dispersion (Yin et al. Reference Yin, Zhang, Wu, Tan and Ke2015) and then sonicated at 40–60°C for 1 h. The CTA+-OP-10-Mnt had a larger basal spacing than either CTA+-Mnt and OP-10-Mnt. The change in the order of addition of CTAB and OP-10 had an insignificant effect on the basal spacing of CTAB-OP-10-Mnt. The hydrophobicity decreased with increases in OP-10 at a fixed concentration of CTAB. The hydrophobicity of CTA+-OP-10-Mnt was greater than OP-10-Mnt and less than CTA+-Mnt. The contact angles of CTA+-OP-10-Mnt, OP-10-Mnt, and CTA+-Mnt were 61.5, 30.5, and 65.5, respectively. Zhang et al. (Reference Zhang, Zhao, Zhu, Wu, Wang and Lu2012b) presented a schematic diagram for the adsorption of cation-non-ionic mixed modifiers onto Mnt (Fig. 8a). At lower concentrations, both modifiers were adsorbed on the Mnt surface through electrostatic interaction and hydrogen bonding. Because the electrostatic attraction for the adsorption of cationic molecules is usually stronger than hydrogen bonding, a cationic modifier could be adsorbed more than a non-ionic modifier. At intermediate concentrations, the pre-adsorbed cationic molecules acted as anchors and more non-ionic species adsorbed through a hydrophobic chain–chain reaction and formed the mixed aggregates at the solid/liquid interface. At higher concentrations, the amount of non-ionic organic species adsorbed decreased with increases in concentration of cationic organic ions. Octadecyltrimethylammonium (ODTMA+) cations and the non-ionic erucamide (EA) were co-introduced by Zhou et al. (Reference Zhou, Li, Gates, Zhu and Yu2019) into OMnt. A two-stage intercalation was proposed: cation exchange of ODTMA+ to form a paraffin-type monolayer in the interlayer space of Mnt, followed by hydrophobic entropy-driven adsorption of non-ion EA by intertwining organic chains into the interlayer spaces of the OMnt (Fig. 8b).

Fig. 9. Simulation of the grafting process of APTES to Ca2+-Mnt (reproduced from Wu et al. (Reference Wu, Dai, Long, Zhu, Li, Wu and Dang2012), copyright 2010, with permission from Elsevier)
Anionic and non-ionic co-modified Mnt was produced following a one-step method. Non-ionic OP-10 and anionic sodium stearate (SSTA) were modified using a ball mill (Zhuang et al. Reference Zhuang, Zhang, Guo, Liao and Zhao2015). The basal spacing of Mnt modified by an anionic-non-ionic modifier increased with increasing dosage of anionic modifier, in the case of fixed non-ionic dosage. The basal spacing of anion-nonionic-Mnt decreased as dosage of anionic modifier increased when the dosage of anion modifier is more than 1.0 CEC. The hydrophobicity of 0.9 CEC STA–-0.7 CEC OP-10-Mnt was better than that of 0.7 CEC OP-10 –Mnt and Ca2+-Mnt (Table 3). Anionic modifiers were absorbed by OP-10 as the alkyl tails entwined with each other and formed molecular braids. The non-ionic modifier enters into the interlayer space of Mnt through the hydrogen bond with Mnt and thus expands the basal space of Mnt (Zhang et al. Reference Zhang, Long, Zhang, Zhu, Wang, Wu and Lu2012a; Zhuang et al. Reference Zhuang, Zhang, Guo, Liao and Zhao2015). The anionic-non-ionic modified Mnt can be used for the remediation of soils and groundwater contaminated by hydrophobic organic compounds (Chen et al. Reference Chen, Chen, Luan, Ji and Xia2011; Zhang et al. Reference Zhang, Long, Zhang, Zhu, Wang, Wu and Lu2012a). The mixture of anionic and cationic modifiers can form mixed micelles, which can synergistically solubilize organic compounds. This can improve the adsorption of organic compounds (Sehgal et al. Reference Sehgal, Kosaka, Doe and Otzen2009; Chen et al. Reference Chen, Chen, Luan, Ji and Xia2011). The 1.0 CEC CTMA+-0.1 CEC STA–-Mnt had greater adsorption of methyl orange than did 1.0 CEC CTMA+-Mnt and 0.1 CEC STA–-Mnt (Chen et al. Reference Chen, Chen, Luan, Ji and Xia2011). The cationic-non-ionic modified Mnt can be used as a fluid-control additive in oil-drilling engineering due to its excellent dispersibility in white oil (Fan et al. Reference Fan, Zhu, Li and Chen2015). The anionic-non-ionic modified Mnt also expanded the application range of OMnt in the areas of paint and ink because of better dispersion in highly polar solutions (Yin et al. Reference Yin, Zhang, Wu, Tan and Ke2015). Cation-non-ion-modified Mnt has been investigated as a carrier for loading and controlled-release of hydrophobic drugs (Yan et al. Reference Yan, Chen, Bao, Yi, Lei, Ke, Zhang and Lin2020a). CTA+-NPE-Mnt and alginate were constructed to provide OMnt/Alg composite hydrogel beads. These composite hydrogel beads improved the stability of the drug carrier and its sustained release effect. The OMnt/Alg composite hydrogel beads displayed superior sustained-release properties for Na-Mnt/Alg, mainly ascribed to the good affinity of OMnt for hydrophobic drugs which retarded the drug diffusion.
Table 3. Typical, recent methods and results of coupled modifier-modified Mnt

*CTAB: cetyltrimethylammonium bromide; HDTMAB: hexadecyltrimethylammonium bromide; CTAC: hexadecyltrimethylammonium chloride; HDTMAC: hexadecyltrimethylammonium chloride; DTAB: dodecyltrimethylammonium bromide; EA: erucamide; FTMA: (11-Ferrocenylundecyl)trimethylammonium bromide; OA: oleamide; STAC: octadecyltrimethylammonium chloride; ODTMAC: octadecyltrimethylammonium chloride; SDBS: sodium dodecylbenzene sulfonate; SDS: sodium dodecyl sulfonate.
ORGANIC GRAFTING-MODIFIED MONTMORILLONITE
OMnt is frequently used for the preparation of clay/polymer nanocomposites (CPN). Mnt modified by organosilane grafting (OMntg) enhanced the interfacial compatibility with a polymer. In addition, the modifiers in OMnt may leach into the surrounding solution when the OMnt are used in solution. This decreases the performance of OMnt and potentially limits its application in remediation of water pollution (Bertuoli et al. Reference Bertuoli, Piazza, Scienza and Zattera2014). Modification of Mnt by grafting is an effective method to solve this problem. OMntg is obtained by organosilane grafts bonding covalently to the surfaces as well as the interlayer space and the edges of Mnt (Bee et al. Reference Bee, Abdullah, Bee, Sin and Rahmat2018).
Mnt is modified commonly by grafting through the emulsion polymerization method. Mnt was dispersed (Thue et al. Reference Thue, Sophia, Lima, Wamba, de Alencar, dos Reis, Rodembusch and Dias2018) in a solution of ethanol and ammonia at pH 10–11.5. Then, 3-triethoxysilylpropylamine (APTES) was added and stirred under reflux for 24 h until a brown product was formed. The addition of ammonia solution to the reaction led to the rapid hydrolysis and polymerization of APTES onto the surface and broken edges of Mnt and into its interlayer space. The triethoxysilane of APTES hydrolyzed first in the solvent, and then –OH connected to Ca2+-Mnt (Fig. 9) (Wu et al. Reference Wu, Dai, Long, Zhu, Li, Wu and Dang2012).
The basal spacing of the OMntg is related to the location of grafted organosilane (Silva et al. Reference Silva, Dahmouche and Soares2011), the number of functional groups present in the organosilane (Shen et al. Reference Shen, He, Zhu, Yuan and Frost2007; Bruce et al. Reference Bruce, Lieber, Hua and Howarter2014), and chain length of the organosilane (Piscitelli et al. Reference Piscitelli, Posocco, Toth, Fermeglia, Pricl, Mensitieri and Lavorgna2010; Varadwaj et al. Reference Varadwaj, Parida and Nyamori2016). Grafting into the interlayer space of Mnt increased the basal spacing (Silva et al. Reference Silva, Dahmouche and Soares2011). Grafting on the broken edges and external surface shows less change in basal spacing. The number of functional groups also determined the basal spacing of silylated Mnt (Shen et al. Reference Shen, He, Zhu, Yuan and Frost2007; Bruce et al. Reference Bruce, Lieber, Hua and Howarter2014; Sepehri et al. Reference Sepehri, Rafizadeh, Hemmati and Bouhendi2014). Trifunctional APTES-grafted Mnt showed a larger basal spacing compared to the monofunctional trimethylchlorosilane-grafted Mnt (Shen et al. Reference Shen, He, Zhu, Yuan and Frost2007). However, Sepehri et al. (Reference Sepehri, Rafizadeh, Hemmati and Bouhendi2014) observed that the basal spacings of monofunctional dimethylchlorovinylsilane-grafted Mnt are larger than those of trifunctional trichlorovinylsilane-grafted Mnt. Trifunctional silanes may interact with each other and form siloxane bridges which prevent the penetration of other silane molecules into the interlayer space. Similarly, trifunctional silane was shown by Bruce et al. (Reference Bruce, Lieber, Hua and Howarter2014) to have a tendency to exhibit a pillaring effect between adjacent Mnt layers which fixed and restrained the interlayer height. This pillaring effect fixes the interlayer height so it does not change upon intercalation of other species. The basal spacing of OMntg also varies with the chain length of the organic silane moieties (Piscitelli et al. Reference Piscitelli, Posocco, Toth, Fermeglia, Pricl, Mensitieri and Lavorgna2010; Varadwaj et al. Reference Varadwaj, Parida and Nyamori2016). Increasing the chain length of the silane resulted in a decrease in the basal spacing of the host material (Piscitelli et al. Reference Piscitelli, Posocco, Toth, Fermeglia, Pricl, Mensitieri and Lavorgna2010). The basal spacing of Mnt grafted by 3-aminopropyltriethoxysilane with a short chain was greater than Mnt grafted by 3-[2-(2-aminoethylamino)ethylamino]-propyl-trimethoxysilane with a long chain. The long chains of silane interacted with each other to form siloxane bridges due to intermolecular hydrogen bonding and hydrophobic interactions. The siloxane bridge fixes the interlayer height of the Mnt-grafted by 3-aminopropyltriethoxysilane.
OMntg could be used for synthesis of OMnt/polymer nanocomposites (Theng Reference Theng2012; Daitx et al. Reference Daitx, Carli, Crespo and Mauler2015; Scarfato et al. Reference Scarfato, Incarnato, Di Maio, Dittrich and Schartel2016) and contaminant adsorbents (Silva et al. Reference Silva, Dahmouche and Soares2011; Parolo et al. Reference Parolo, Pettinari, Musso, Sánchez-Izquierdo and Fernández2014; Thue et al. Reference Thue, Sophia, Lima, Wamba, de Alencar, dos Reis, Rodembusch and Dias2018). Organosilanes serve as a bridge to enhance the interfacial interaction between the silylated-OMnt, thus enabling better dispersibility of the OMntg in the polymer matrix (Theng Reference Theng2012). For example, 3-(glycidyloxypropyl)trimethoxysilane is able to bond chemically with certain polymers such as epoxy matrices using their epoxy functional group (Scarfato et al. Reference Scarfato, Incarnato, Di Maio, Dittrich and Schartel2016). This can greatly enhance the mechanical property of OMnt/polymer nanocomposites (Bruce et al. Reference Bruce, Lieber, Hua and Howarter2014). In addition, OMntg modified by APTES adsorbed significant amounts of acid red 1 and acid green 25, 97.94% and 95.94%, respectively (Thue et al. Reference Thue, Sophia, Lima, Wamba, de Alencar, dos Reis, Rodembusch and Dias2018). Those authors proposed that this OMntg adsorbent presented good applicability for the treatment of synthetic wastewaters; they also proposed that this OMntg, with significant hydrophilic properties, can open a new window for applications in the coating, self-cleaning, or humidity control industries.
SUMMARY AND REMARKS
Great progress has been made in the preparation, property assessment, characterization, and application of OMnt over the past 10 years. Organic cations were the most frequently used materials for the modification of Mnt up to 15 years ago. For the past 10 years, organic anion, zwitterion, and non-ionic molecules have been used for modification of the surface properties of Mnt. The combination of two modifiers can exhibit synergistic effects providing new opportunities for OMnt. Organosilane can also modify Mnt by grafting.
Organic cations can be intercalated easily into Mnt interlayer spaces by a cation exchange reaction. Anionic species have been intercalated successfully into the interlayer space of Mnt through ion–dipole interaction in acidic medium. Zwitterions and non-ionic species can intercalate into the interlayer space of Mnt by ion–dipole, hydrogen bond, and van der Waals force interactions. The co-intercalation of Mnt by two modifiers is relatively complicated. The alkyl tails of anionic and non-ionic molecules become entangled with the cation alkyl tails and form ion-pairs. Thus, anionic and non-ionic species can co-intercalate with cations into the interlayer space of Mnt. Non-ionic and anionic modifiers can co-intercalate efficiently into the interlayer space of Mnt if non-ionic modifiers entwine with anionic modifiers and form molecular braids. OMntg is obtained by covalently binding organosilane to the surface and edge of Mnt. The modification mechanisms of OMnt continue to be debatable, especially non-ionic and zwitterion modification. The detailed interaction between organics and Mnt at the molecular or atomic level remain unclear or even unknown.
OMnt has been commercialized in applications such as drilling fluids, paints, and environmental adsorptive materials for more than 10 years. During the past decade, exfoliation of OMnt with nano-assembly has been used to create more functional materials. This clean, time-saving, non-toxic, and environmentally friendly way of producing such materials needs to be explored further. In addition to adsorbents, rheology controllers, and multifunctional materials, recent interests are in the uses of OMnt for catalysis, drug carrying, and biomaterials, and these areas remain open to exploration by means of organic modification of Mnt.
ACKNOWLEDGMENTS
The authors acknowledge financial support by the National Natural Scientific Foundation of China (41672033; 22072136); and from the open fund from the Engineering Research Center of Non-metallic Minerals of Zhejiang Province, Zhejiang Institute of Geology and Mineral Resource, China (ZD2020K09).
Funding
Funding sources are as stated in the Acknowledgments.
Compliance with Ethical Statements
Conflict of Interest
The authors declare that they have no conflict of interest.