INTRODUCTION
Influenza is recognized as a cause of a broad spectrum of morbidity and mortality, and the true burden is thought to be much greater than is recorded in hospital discharge records or identified as the underlying cause of death [Reference Simonsen1–Reference Lui and Kendal3]. Laboratory confirmation of viral aetiology of a respiratory illness is often not sought during hospital admission, and various pre-existing morbidities, such as heart disease, increase the risk of complications and death. Influenza-associated mortality has, therefore, been estimated statistically, particularly in the United States [Reference Lui and Kendal3–Reference Simonsen5]. A need to assess various target groups for immunization recommendations as well as planning for the next pandemic [Reference Schopflocher6] has renewed interest in new models for estimating the disease impact [Reference Thompson7]. The classical epidemic-threshold model of Serfling, introduced in 1963 [Reference Serfling8] is still in use [Reference Simonsen4, Reference Simonsen9]. This methodology assumes that all deaths in excess of a seasonal baseline based on years of low influenza activity are attributable to influenza. With various markers for influenza activity and weekly data now available, Poisson regression models provide an analytical tool that can make use of all available data, and provide good control for trends and seasonality. Residual analyses also help assess biases due to potential confounders. Surrogate markers for influenza activity in various Poisson regression models have included influenza-certified deaths [Reference Sprenger10, Reference Baltussen11] physician visits for influenza-like illnesses [Reference Scuffham12, Reference Clifford13], laboratory confirmations of influenza [Reference Fleming, Cross and Pannell14] and proportion of samples that tested positive by influenza strain and respiratory syncytial virus (RSV) [Reference Thompson7].
We sought to establish a model for estimating influenza-attributable deaths using population-based vital statistics data, to describe influenza-attributable deaths by age, place of death, and certified underlying cause of death, and to assess the robustness of model estimates.
METHODS
Mortality data
Records of deaths occurring in Canada from 1989 to 1999 were obtained from the Canadian Vital Statistics database, Statistics Canada [15]. Underlying cause of death was coded using the ninth revision of the International Classification of Disease (ICD-9) [16]. For this study, deaths were aggregated to weekly levels by 10-year age groups, cause of death, region, and place of death (in hospital or other). As the coding system for cause of death changed from the 9th to 10th revision in 2000, 1989–1999 is the most recent 10-year period with a consistent coding of cause of death. Population denominators for mortality rates were obtained from Statistics Canada census and inter-census estimates [17].
Influenza activity
Influenza-certified deaths were obtained from the vital statistics database. These are deaths for which the underlying cause of death was coded to ICD-9 487. Laboratory confirmations for influenza were provided by the Respiratory Virus Detection Surveillance System, Public Health Agency Canada [Reference Li18], and weekly hospital admissions with an influenza diagnosis were provided by the Canadian Institute of Health Information. All were considered as surrogate measures for influenza activity, although influenza-certified deaths provided the best model fit.
Statistical model
In order to predict the weekly number of deaths, we developed a Poisson regression model to explain deaths as a function of influenza activity, seasonality and trend (SAS [19], proc genmod). A linear link function was chosen to maintain a linear relationship between influenza activity and influenza-attributed deaths. (For example, a doubling of influenza activity from one week to the next should result in a doubling of influenza-attributable deaths rather than a doubling of all pneumonia deaths.) Influenza-attributed deaths were calculated as the model-predicted number of deaths minus the baseline-predicted number in the hypothetical absence of influenza (surrogate for influenza activity was set to zero). Separate models were developed for age-specific, cause-specific and region-specific weekly deaths as well as for national total weekly deaths. The total number of weekly deaths certified as due to influenza as the underlying cause was chosen as the best proxy for weekly influenza activity. Seasonality was modelled using monthly indicator variables. Twelve indicator variables, rather than weekly indicator variables or a sinusoidal function, provided a reasonable level of parameterization to capture seasonality. To capture the complete influenza season in a 1-year period, the year was designated to start in September. To capture trends in mortality rates and population growth, a separate indicator variable was included for each year. Models with other explanatory variables, such as other surrogate measures of influenza activity with various lags between influenza activity and death, along with various trade-offs between the number of parameters were explored. Results are presented for models at a level of parameterization that produced consistent and robust estimates. The proportion of influenza-attributable deaths that were certified in the mortality database as due to influenza was also expected to vary with the particular strain, and hence each influenza season. Deaths were well explained by the following model, which included parameters for seasonality, trend, percent certified by influenza season and percent certified by month:
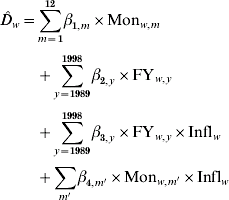
where 
The ratio of influenza-attributed deaths to influenza-certified deaths was calculated to indicate the hidden mortality burden, and corresponding 95% CIs were calculated from the 95% CIs of the estimated parameters. The proportion certified as influenza is the reciprocal of this ratio.
Assessment of model validity included checks for consistency among the estimates of the proportion certified, particularly the separate monthly estimates of proportion certified. The estimated average annual number of influenza-attributed deaths were observed for robustness to variation in model form as described above, as well as additivity across disease groups, age groups, regions (Canadian provinces, with smaller provinces grouped into regions, geographically running east to west) and place of death. Model fit was viewed graphically using plots similar to Figure 1 for each subgroup estimate, while residuals were also superimposed on the estimated influenza-attributed deaths to check for auto-correlation, outliers or other indications of poor fit. Poor model fit over a period of 4–10 weeks would be suggestive of potential bias due to confounders. All models exhibited some extra Poisson variation and some correlation of residuals across subgroups, although model parameterization was guided in reducing these.

Fig. 1. Estimated influenza-attributable deaths and deaths due to all causes, weekly, Canada, 1990–1999. 

RESULTS
The estimated number of influenza-attributable deaths averaged approximately 4000 annually (13/100 000), or 2% of all deaths, and varied from undetectable levels to up to 8000 annual deaths. Of these influenza-attributable deaths, only 15% were certified as pneumonia (Table 1). The ratio of influenza-attributable deaths to certified influenza deaths averaged 12·5 times (95% CI 9·9–15·1). Equivalently, influenza was determined to be the underlying cause of death in only 8% (95% CI 6–10) of the estimated influenza-attributable deaths, varying annually from 4% to 12% with the exception of the mild influenza season of 1990/1991.
Table 1. Annual average influenza-attributable deaths, 1989/1990 to 1998/1999 influenza seasons by cause of death, sorted by no. attributed deaths

COPD, Chronic obstructive pulmonary disease.
The good model fit is illustrated in Figure 1, where total deaths by week are plotted, along with the estimated baseline- and model-predicted number of deaths. Influenza-attributable deaths, calculated as the difference between predicted deaths and the seasonal baseline, are plotted against the second axis. At the peak of the influenza period, influenza contributed heavily to the weekly mortality, typically accounting for 10–20% of deaths. As part of the model validation process, excess mortality, calculated as weekly deaths minus baseline, was plotted against the influenza-attributed weekly deaths (not shown). From assessing alternative model forms, as well as residual analyses, no obvious lags were detected between influenza-certified deaths and the excess mortality for other causes. Influenza-certified deaths, which lagged laboratory-confirmed influenza by approximately 1 week, were found to be a slightly better proxy variable than influenza-positive laboratory confirmations. Confirmations were assigned to the week of specimen collection, and would include specimens taken under a variety of clinical conditions. The level of statistical significance for all surrogate measures was very high. Influenza-certified deaths seemed to capture the effects of variation in both the underlying attack rate and case-fatality rate best, as evident in the model residuals.
Of the leading causes of death, pneumonia and chronic obstructive pulmonary disease (COPD; ICD-9 490-496) had the highest percentage of deaths likely attributable to influenza at 8% and 6% respectively, although these percentages varied by year depending on the severity of the influenza season (Table 1, Fig. 2). Ischaemic heart disease (IHD; ICD-9 410-414) accounted for the largest proportion of influenza-attributable deaths. More than 60% of the influenza-attributed deaths were certified as either IHD, pneumonia, COPD or other heart disease. The association between influenza and cancer was weaker, although still statistically significant. Only 0·5% of cancer deaths were estimated to be attributable to influenza, thus this relationship was not visible graphically nor would it be detectable using a threshold methodology. Although a highly statistically significant association with influenza deaths was found for each of the other causes of death in Table 1, these series did not exhibit the same strong peaks that are required for estimation of excess deaths by the Serfling-type models. These other causes of death, with the exclusion of cancer and external injuries, were combined and modelled again as a group. Results were nearly identical to the sum of the individual results, and, in aggregate, exhibited the same strong peaks of excess mortality (not shown).

Fig. 2. Average annual influenza-attributable deaths by underlying causes of death, Canada, 1990–1999.
External injuries, in general, were not related to influenza deaths. A few minor exceptions were noted: transportation accidents, including primarily motor vehicular traffic accidents, were negatively related to influenza deaths, with the December coefficient statistically significant (P value=0·005); accidental poisoning deaths; and accidental falls. For all of these causes of death, the number of influenza-attributable deaths was very small. The ill-defined category gave unusual results, as deaths coded to ill-defined regularly rose towards the end of the calendar year, causing spurious correlation. In summary, the estimation of influenza-attributable deaths from all-cause mortality is feasible, with only minor differences if external injuries were to be excluded.
Age-specific models produced results that were consistent with the aggregate model (Table 2). Figure 3 shows that the influenza-attributed mortality rate increased steadily with age, although more deaths occurred among 70- and 80-year-olds than those aged ⩾90 years. The estimated percent certified (or identified as due to influenza) was highest for the ⩾90 years age group at 14%. For children and young adults, the annual number of deaths due to influenza was small, and we found no evidence of additional deaths attributable to influenza. Hence, results for ages <50 years were grouped. The estimated percent of influenza-attributable deaths certified as influenza varied regionally, with less regional variation in the influenza-attributed mortality rate than in the influenza mortality rate. Again, the sum of the regional results agreed closely with the national results. Sixty-five percent of the influenza-attributable deaths occurred in hospital.
Table 2. Annual average influenza-attributable deaths, 1989/1990 to 1998/1999 influenza seasons by age, region and place of death


Fig. 3. Average annual influenza-attributable deaths and mortality rate by 10-year age group, Canada, 1990–1999. 

The percentage of deaths certified as being due to influenza varied significantly with each influenza season and were lower for the month of December compared to January–April, although estimates of the average annual number of influenza-attributed deaths were robust to the exclusion of these additional parameters. That the estimated proportion certified was similar for January–April shows remarkable reproducibility and strongly supports the model's validity. For most influenza seasons, the percent certified ranged from 4% to 12%. The simple model tended to estimate a lower burden due to influenza (3000 deaths attributed to influenza per year vs. 4000 when the proportion certified was allowed to vary).
DISCUSSION
There are two main findings of this study: that there is a large ‘hidden’ mortality burden due to influenza; and that this burden is quantifiable. For most influenza seasons, influenza-attributed deaths should be considered a leading cause of death, and in the more severe years, influenza-attributed deaths were surpassed only by the five major causes of death: cancer, heart disease, stroke, COPD and pneumonia. A few model refinements have facilitated the estimation of influenza-attributed deaths at smaller sub-categorical levels. Hence, we were able to conclude: that mortality for most causes of death was statistically elevated during influenza outbreaks; that there was no evidence of hidden excess mortality associated with periods of influenza activity for children; that 65% of influenza-attributed deaths occurred in hospital; and that the relationship between any of the available surrogate measures for influenza activity and the influenza burden varied with the influenza season.
An important objective of this study was to assess the potential biases in parameter estimates due to potential confounders. Hence, model validity was assessed along with model fit, correlation of residuals, and consistency of parameter estimates. Parameters included in the model, such as monthly indicator variables for seasonality, rather than a sinusoidal curve, were important in reducing extra-Poisson variation, as well as reducing uncertainty of the estimated number of influenza-attributable deaths. The use of a weekly level of aggregation facilitated model validation, and particularly the assessment of potential confounders.
Influenza-certified deaths provided the best surrogate measure of influenza activity for predicting excess mortality, although this was strongly correlated with laboratory confirmations of influenza. The relationship between laboratory confirmations and excess deaths appeared to be lagged by 1 week. The timing of peak mortality rates was the same for influenza-certified deaths and other causes of death. Other studies [Reference Scuffham12, Reference Fleming, Cross and Pannell14, Reference Reichert20] that have looked at the timing of death have likewise found general agreement in the timing of peak other cause mortality and pneumonia and influenza (P&I) deaths. The proportion of tests that were positive for influenza was generally correlated with the number of positive tests. While correcting for any possible seasonal intensity of testing, proportions can be misleading, as co-circulation of another virus would reduce the proportion and hence imply that each case of influenza is less serious if other viruses were co-circulating. The proportion certified as influenza was found to be slightly lower in December than January–April. This could be due to the Canadian medical system slow-down over 2 weeks for the Christmas break. The main purpose of estimating the proportion certified by month was to provide a consistency check, and the close agreement of the proportion certified for January–April shows strong repeatability and hence strengthens model credibility. Moreover, in the Canadian data, the percent certified was found to vary annually. In various analyses of the residuals, the residuals showed some correlation across disease and age groups, and even individual annual estimates for the percent certified did not completely eliminate the extra Poisson variation, nor correlation among residuals. Model fit appeared poorest for years dominated by more than one viral strain, as deaths attributable to influenza B (or H1N1 vs. H3N2) were generally less likely to be certified as influenza.
The relationship between influenza-certified deaths and deaths due to some external injury categories was statistically significant but nearly negligible in terms of the number of estimated deaths. The risk of accidental falls in the elderly and the risk of accidental poisoning (from medications) may be slightly elevated as a result of influenza, or these could be spurious results. More importantly, these results by cause of death demonstrate a strong level of consistency, and minimal potential for bias due to omitted confounders.
The estimate of an average of 4000 deaths per year attributable to influenza was found to be reasonably robust to variations in model form, suggesting that estimates of influenza-attributed deaths in other studies, including some threshold studies [Reference Simonsen4], should generally be comparable, despite concerns about model form. Like Reichert et al. [Reference Reichert20], we tend to agree that excess mortality is primarily attributable to influenza. However, our Poisson regression model was sensitive enough to identify some clusters of correlation in the residuals that would suggest that other viruses also contribute to mortality. Methods that use an epidemic threshold would tend to result in underestimation of the actual extent of excess mortality. Because the coefficient of variation decreases with increasing population counts, the relative bias introduced would be larger for a smaller country like Canada than for the United States. Threshold methods cannot assess whether all excess mortality during periods when influenza is in general circulation is influenza related.
Our findings are in reasonable agreement with other estimates of the mortality burden associated with influenza. Thompson et al. [Reference Thompson7] estimated an influenza-attributable mortality rate of 20/100 000, which is higher than the estimated 13/100 000 in this paper. Influenza-attributable mortality rates for seniors, aged ⩾65 years were 133 and 108/100 000 in Thompson et al.'s and our study respectively. Simonsen et al. [Reference Simonsen4] found that all-cause excess mortality was 3·8 times larger than the P&I excess mortality for a period covering the 1980s in the United States. In comparison, Thompson et al. estimated all-cause influenza-attributable deaths at six times the P&I rate, and our ratio was 4·5. Our rate is also comparable to the excess mortality rate Schopflocher et al. [Reference Schopflocher6] estimated for senior respiratory deaths of 57/100 000. Since the 2003/2004 season, during which 63 influenza-associated deaths in otherwise healthy children were confirmed in the United States [Reference Bhat21], influenza-associated deaths in children has been the subject of active surveillance in the United States and in Canada. While an excess burden of death attributable to influenza was not detected in children in the Canadian population, Thompson et al. estimated an influenza-attributable mortality rate for infants aged <1 year of 2·2/100 000 person-years, or approximately three times the rate of for 2003/2004 found by Bhat et al. [Reference Bhat21] for the same age group.
In conclusion, influenza deaths appear to be a good predictor of excess mortality, and a similar relationship between influenza-certified deaths and influenza-attributable deaths under ICD-10 coding is anticipated. This study confirms that for pandemic planning it is important to consider the influenza burden hidden in all causes of death, not just respiratory deaths, and that in the event of a pandemic, we will probably be faced with a large surge of sudden deaths. The identification of influenza-certified deaths as a proxy for influenza severity greatly facilitated the use of relatively simple models for estimating the burden of influenza.
DECLARATION OF INTEREST
None.







