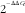Lactic acid bacteria (LAB) are defined as bacteria that produce lactic acid as their main fermentation product. They have been traditionally used in the manufacture of various types of food products such as pickles, cheese and yogurt and are recognised as safe and beneficial bacteria( Reference Hammes and Tichaczek 1 ). It has been recognised that some LAB can be used as probiotics, which have valuable effects on human health: the WHO has defined probiotics as ‘Live microorganisms that, when administered in adequate amounts, confer a health benefit to the host’( Reference Hill, Guarner and Reid 2 ). A large number of studies have reported that probiotic strains of LAB exhibit beneficial effects such as prevention of diarrhoea, allergic disease and infectious diseases( Reference Tsai, Cheng and Pan 3 , Reference Jan, Yeh and Hsieh 4 ); several strains of LAB have also been recently reported to have an anti-obesity effect( Reference Lee, Park and Seok 5 – Reference Tsai, Cheng and Pan 9 ).
Lactobacillus gasseri SBT2055 (LG2055) is a probiotic bacterium isolated from human faeces. It has been shown that oral administration of LG2055 beneficially changes the composition of human intestinal microflora and the physical characteristics of faeces( Reference Fujiwara, Seto and Hashiba 10 ), and that the administration prevents mice from influenza infection( Reference Nakayama, Moriya and Sakai 11 ). The anti-obesity effect of this bacterium has been demonstrated in both rats and humans( Reference Sato, Uzu and Yoshida 12 – Reference Kadooka, Sato and Ogawa 16 ). Miyoshi et al.( Reference Miyoshi, Ogawa and Higurashi 17 ) recently confirmed an anti-obesity effect of LG2055 in diet-induced obese mice, and also reported that the expression of the Ccl2 gene in adipose tissue was down-regulated by the administration of LG2055.
Under the obese state, adipocytes secrete free fatty acids and pro-inflammatory cytokines such as TNF-α, IL-6 and CC chemokine ligand 2 (CCL2, the translational product of the Ccl2 gene is also known as monocyte chemoattractant protein 1 (MCP-1))( Reference Chawla, Nguyen and Goh 18 ). CCL2 leads to infiltration of monocytes expressing CCR2 (CC chemokine receptor 2) into adipose tissue, where the infiltrated monocytes differentiate into classically activated inflammatory macrophages (M1 macrophage). Under the lean state, however, most macrophages in the adipose tissue show an alternatively activated anti-inflammatory macrophage (M2 macrophage) phenotype. Consequently, the ratio of the M1:M2 phenotype is increased with development of obesity( Reference Fujisaka, Usui and Bukhari 19 ). M1 macrophages also secrete TNF-α, IL-6 and CCL2: TNF-α can further induce the secretion of pro-inflammatory cytokines including CCL2( Reference Chawla, Nguyen and Goh 18 ). Inflammation is, thus, exacerbated by this ‘feed-forward loop’ in obese adipose tissue, and the augmented inflammation leads to the development of obesity-associated insulin resistance and type 2 diabetes( Reference Xu, Barnes and Yang 20 , Reference Lumeng and Saltiel 21 ). It was also reported that transgenic over-expression of CCL2 increased macrophage infiltration and inflammation( Reference Kamei, Tobe and Suzuki 22 ), whereas the knock down of CCL2 and CCR2 depressed migration of macrophages into adipose tissue and improved insulin sensitivity( Reference Kanda, Tateya and Tamori 23 , Reference Weisberg, Hunter and Huber 24 ).
Given that LG2055 has previously been shown to down-regulate the expression of the Ccl2 gene( Reference Miyoshi, Ogawa and Higurashi 17 ), the translational product of which is a crucial factor for macrophage infiltration, it is of interest to examine whether the administration of LG2055 actually suppresses macrophage invasion into adipose tissue, which has not been studied thus far. We investigated the effect of administration of LG2055 on macrophage infiltration and ratio of the M1:M2 phenotype in adipose tissue, as well as systemic insulin sensitivity, using HFD-induced obese mice.
Methods
LG2055 cells
LG2055 was cultured in deMan, Rogosa and Sharpe (MRS) broth (BD Bioscience) for 18 h at 37°C. LG2055 cells were collected by centrifugation at 3000 g for 10 min at 4°C and washed three times (twice with saline and once with distilled water). Finally, the cells were suspended in 5 volumes of 10 % (w/v) lactose solution and lyophilised using a FDU-2200 freeze-dryer (Tokyo Rikakikai Co. Ltd). The colony-forming units (CFU) of dried LG2055 powder, determined using an MRS agar plate, was 1×1011 CFU/g.
Diets and animals
A high-fat diet (HFD) containing 45 % energy fat (D12451) and a low-fat control diet containing 10 % energy fat (D12450H) were purchased from Research Diets Inc.; 1 % (w/w) of the dried LG2055 powder was mixed with the diet containing 45 % energy of fat to produce the HFD containing LG2055 (HFLGD). The CFU of this diet, determined using an MRS agar plate, was 1×109 CFU/g. The normal-fat diet (NFD) and HFD were prepared using 1 % (w/w) lactose mixed with the 10 % energy fat diet and 45 % energy fat diet, respectively. No LG2055 powder was mixed into the NFD and HFD.
Six-week-old male C57BL/6J mice (weaned at 3 weeks of age) were purchased from Charles River Japan Inc. Mice were individually housed in an air-conditioned, specific pathogen-free room (21–24°C, lights on 08.00–20.00 hours). After 1 week of acclimatisation, mice were divided into three groups (n 12 in each group), each fed the NFD, HFD or HFLGD. During the feeding period, mice were given free access to the experimental diet and water. Dietary amounts initially dispensed and residual diet after consumption were recorded every day, and the amount of food consumed was calculated by subtraction of the amount of residual diet from that initially dispensed. The amount of diet dispensed was daily adjusted by pair-feeding so that there were no differences in dietary intake between the HFD- and HFLGD-fed groups, whereas the amount dispensed for the NFD-fed group was not adjusted. At the start of the experiment, the amount of diet dispensed was 4·0 g/d; 1 week later, the amount was adjusted to 3·5 g/d, because the mean diet intake of both groups did not exceed 3·5 g. From weeks 1 to 6, the HFLGD group tended to consume more food compared with the HFD group, although there was no significant difference; subsequently, from week 6 to the end of the experiment, the amount of diet dispensed was reduced to 3·0 g/d according to the mean diet intake in the HFD group. Body weight of mice was monitored weekly.
At the end of the feeding period, mice were anaesthetised by diethyl ether and killed by cervical dislocation after fasting for 12 h. The visceral adipose tissue samples (mesenteric, perirenal/retroperitoneal and epididymal) were dissected and weighed. All mesenteric fat and part of the epididymal fat were frozen in liquid N2 and stored at –80°C for RNA preparation. The remaining section of epididymal fat was suspended in RPMI1640 medium (Life Technologies) containing 10 % (v/v) fetal bovine serum (FBS), 10 mm-HEPES, 2 mm-l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0·05 mm-2-mercaptoethanol and stored on ice before preparation of the stromal vascular fraction (SVF).
The protocol was in accordance with the animal experimentation regulations of the Milk Science Research Institute of Megmilk Snow Brand Co. which are based on the guidelines proposed by the Science Council of Japan.
Isolation of stromal vascular fraction and flow cytometry analysis
Isolation of SVF was performed using the method proposed by Nishimura et al.( Reference Nishimura, Manabe and Nagasaki 25 ), with slight modification. Epididymal fat was transferred into PBS containing 1 μg/ml heparin and minced into small pieces using a pair of scissors. They were vigorously shaken for 30 s to remove the circulating blood cells and then centrifuged at 370 g for 5 min. Floating pieces of adipose tissue were collected into collagenase solution (RPMI1640 containing 10 mm-HEPES, 1 mg/ml collagenase type II (Sigma-Aldrich) and 10 μg/ml DNase I (F. Hoffmann-La Roche)) and incubated for 20 min at 37°C. Adipose tissues were suspended by pipetting and were filtered using a 70-μm mesh (BD Bioscience). After centrifugation, precipitated cells were washed twice with RPMI1640 (containing 10 % (v/v) FBS and 10 mm-HEPES), then re-suspended into erythrocyte lysis buffer (168 mm-NH4Cl, 10 mm-KHCO3 and 0·08 mm-EDTA) and incubated for 3 min. The cells were washed and suspended in FCM buffer (PBS containing 1 % (v/v) FBS and 0·05 % (w/v) NaN3), and the number of cells was counted using a Celltac haematology analyser (Nihon Kohden).
Isolated cells were incubated with anti-mouse CD16/32 (eBioscience) for 30 min at 4°C to block non-antigen-specific binding via the Fc receptor. Cells were washed with FCM buffer and incubated with anti-mouse FITC-labelled CD11b, APC-labelled CD11c, PE-labelled CD206 (all from BD Bioscience) and Brilliant violet 421-labelled F4/80 (BioLegend) for 1 h at 4°C. The cells were then washed and suspended in FCM buffer before flow cytometry analysis (FACS Canto II; BD Bioscience). Isotype control antibodies were used to estimate the non-specific binding of target antibodies to cell surface antigens. The 7AAD (BD Bioscience) was used to exclude dead cells. Cells highly labelled with both anti-CD11b and anti-F4/80 antibodies (F4/80highCD11bhigh) were regarded as macrophages( Reference Cho, Morris and Lumeng 26 ). Macrophages labelled with the anti-CD11c antibody but not the anti-CD206 antibody were regarded as M1 macrophages, and those labelled with the anti-CD206 antibody but not the anti-CD11c antibody were regarded as M2 macrophages( Reference Fujisaka, Usui and Bukhari 19 ).
Gene expression analysis
Total RNA were isolated from epididymal fat tissue using an RNeasy lipid tissue kit (Qiagen). The quality of RNA was confirmed using an Agilent 2100 Bioanalyzer (Agilent), and RNA with an RNA integrity number above 7 were regarded as intact RNA. Intact total RNA (2 μg) was reverse-transcribed to cDNA using a High-Capacity RNA-to-cDNA kit (Life Technologies). Real-time PCR analysis was performed using a TaqMan Fast Advanced Master Mix and Viia7 system (both from Life Technologies). TaqMan primer and probe sets for CC chemokine ligand 2 (Ccl2; Mm00441242_m1), CC chemokine receptor 2 (Ccr2; Mm00438270_m1), CD68 antigen (Cd68; Mm03047340_m1), IL-6 (Il6; Mm00446190_m1), nitric oxide synthase 2, inducible (Nos2; Mm00440502_m1), TNF (Tnf; Mm00443258_m1) and leptin (Lep; Mm00434759_m1) were purchased from Life Technologies. The expression levels of target genes were normalised with the endogenous control genes(
Reference Vandesompele, De Preter and Pattyn
27
), β-actin (Actb; Mm01205647_g1) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh; Mm99999915_g1), and the data were analysed according to the
![]() $${\rm 2}^{{^{{{\minus}\Delta \Delta C_{t} }} }} $$
method using DataAssist v3.0 software (Life Technologies).
$${\rm 2}^{{^{{{\minus}\Delta \Delta C_{t} }} }} $$
method using DataAssist v3.0 software (Life Technologies).
Serological analysis
Blood was obtained from the inferior vena cava at the end of the feeding period, incubated at room temperature for 30 min to form blood clots and then centrifuged at 13 000 g for 10 min to obtain serum samples. Serum samples collected were stored at –80°C until assay. Serum glucose levels were analysed using the DRI-CHEM 7000V and GLU-P-III slide (both from Fujifilm Corporation). Serum insulin was analysed using a Mouse Insulin ELISA KIT (Shibayagi Co. Ltd). The index of homoeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: fasting glucose (mmol/l)×fasting insulin (mU/l)/22·5( Reference Matthews, Hosker and Rudenski 28 ).
Statistical analysis
Statistical analysis was performed using StatView software package 5.0 (SAS Institute Inc.) and PASW Statistics 17 (SPSS Inc.). Significant differences among experimental groups were analysed using ANOVA, followed by Dunnett’s test. In this study, to investigate the effect of administration of LG2055 in mice fed a HFD, the group of mice fed HFD only was regarded as the control group. A value of P<0·05 was considered to be statistically significant. Correlation analysis was assessed by the Pearson’s test using StatView software.
Results
Diet intake, body weight and visceral fat weight
Mice were divided into three groups and fed NFD, HFD or HFLGD for 12 weeks. Morphometric and biochemical data of mice are shown in Table 1. Although the dietary intake of the NFD-fed group was significantly higher than that of the HFD-fed group, energy intake was vice versa, which was due to the high energy content of HFD. There were no differences in diet and energy intakes between the HFD and the HFLGD groups. Body weight and adipose tissue weight were significantly increased by the feeding HFD compared with feeding NFD. No significant differences in body and adipose tissue weights were observed between the HFD and HFLGD groups.
Table 1 Morphometric and biochemical markers of mice (Mean values and standard deviations)

NFD, normal-fat diet; HFD, high-fat diet (45 % energy); HFLGD, high-fat diet (45 % energy) containing LG2055.
* P values were calculated using Dunnett’s test.
Flow cytometric analysis of macrophages in epididymal fat
The gating strategy to identify M1 and M2 macrophages is shown in Fig. 1. The number of SVF cells/1 g of epididymal fat did not differ between the groups (Fig. 2(a)). The percentage of F4/80highCD11bhigh macrophages in the SVF collected from epididymal fat tissue was significantly increased by feeding HFD compared with feeding NFD (Fig. 2(b)). The feeding of HFLGD suppressed the increase in F4/80highCD11bhigh macrophages compared with feeding HFD. The percentage of CD11c+CD206– cells (M1 macrophages) to the F4/80highCD11bhigh macrophages was also significantly increased in mice fed HFD compared with mice fed NFD (Fig. 2(c)), and it was significantly suppressed by feeding HFLGD compared with feeding HFD. The percentage of CD11c–CD206+ cells (M2 macrophages) significantly decreased by feeding HFD compared with feeding NFD, and it was increased by feeding HFLGD compared with feeding HFD (Fig. 2(d)). The M1:M2 macrophage ratio was significantly increased by feeding HFD compared with feeding NFD, and it was significantly suppressed in mice fed HFLGD compared with mice fed HFD (Fig. 2(e)).

Fig. 1. Gating strategy to identify M1 and M2 macrophages. Stromal vascular fraction cells labelled with anti-F4/80, anti-CD11b, anti-CD11c and anti-CD206 antibodies were analysed using a FACS Canto II flow cytometer. Cell debris and dead cells (7AAD+) were excluded in the first step. F4/80highCD11bhigh cells were identified as macrophages and gated to expand on a new cytogram presenting CD11c and CD206 as axes. CD11c+CD206– cells and CD11c–CD206+ cells were identified as M1 and M2 macrophages, respectively. Isotype controls were used to set the lines separating positive cells from negative cells.

Fig. 2. Population analysis of macrophages in epididymal fat tissue. (a) Stromal vascular fraction (SVF) cells were counted using a Celltac haematology analyser and the number of cells in 1 g of epididymal fat tissue was calculated. Figures show the percentage of (b) macrophage population in live SVF cells; (c) M1 macrophage and (d) M2 macrophage population from all macrophages; (e) shows the ratio of M1:M2 macrophages. ![]() , Values of the normal-fat diet (NFD) fed group;
, Values of the normal-fat diet (NFD) fed group; ![]() , values of the high-fat diet (HFD) fed group; and
, values of the high-fat diet (HFD) fed group; and ![]() , values of the HFD containing LG2055 (HFLGD) fed group. Data are shown as box plots (n 12); the centre line of each box represents the median; the top and bottom of the boxes represent the 25th and 75th percentiles of the data, respectively; and the top and the bottom of the error bars represent the 10th and 90th percentiles of the data, respectively. Statistically significant difference: * P<0·05, ** P<0·01 (Dunnett’s test). Borderline significant difference: † P=0·08, †† P=0·06 (Dunnett’s test).
, values of the HFD containing LG2055 (HFLGD) fed group. Data are shown as box plots (n 12); the centre line of each box represents the median; the top and bottom of the boxes represent the 25th and 75th percentiles of the data, respectively; and the top and the bottom of the error bars represent the 10th and 90th percentiles of the data, respectively. Statistically significant difference: * P<0·05, ** P<0·01 (Dunnett’s test). Borderline significant difference: † P=0·08, †† P=0·06 (Dunnett’s test).
Real-time PCR analysis of pro-inflammatory gene expression in epididymal fat tissue
Feeding HFD elevated the expression levels of macrophage marker genes (Ccr2 and Cd68) and pro-inflammatory genes (Ccl2, Il6, Nos2, Tnf and Lep) compared with feeding NFD (Fig. 3). In mice fed HFLGD, the expressions of Ccr2 and Lep were significantly down-regulated compared with HFD. Although not significant, expression levels of the Ccl2 gene was down-regulated by feeding HFLGD (P=0·06), and the other genes examined (Cd68, Il6, Tnf and Nos2) tended to be down-regulated in mice fed HFLGD.

Fig. 3. Relative RNA expression in epididymal fat tissue. Gene expression levels in each diet group were determined by real-time PCR analysis. The expression levels of target genes were normalised with endogenous control genes, β-actin and glyceraldehyde-3-phosphate dehydrogenase, and the data were analysed according to the
![]() $${\rm 2}^{{^{{{\minus}\Delta \Delta C_{t} }} }} $$
method.
$${\rm 2}^{{^{{{\minus}\Delta \Delta C_{t} }} }} $$
method. ![]() , Values of the normal-fat diet group;
, Values of the normal-fat diet group; ![]() , values of the high-fat diet (HFD) fed group;
, values of the high-fat diet (HFD) fed group; ![]() , HDF containing LG2055-fed group. The data are shown as relative values based on the expression levels in the HFD-fed group. Values are means (n 12), with standard deviations represented by vertical bars. Statistically significant difference: * P<0·05, ** P<0·01 (Dunnett’s test). Borderline significant difference: † P=0·10, †† P=0·06 (Dunnett’s test).
, HDF containing LG2055-fed group. The data are shown as relative values based on the expression levels in the HFD-fed group. Values are means (n 12), with standard deviations represented by vertical bars. Statistically significant difference: * P<0·05, ** P<0·01 (Dunnett’s test). Borderline significant difference: † P=0·10, †† P=0·06 (Dunnett’s test).
Serological analysis aimed to assess insulin resistance
Mouse serum was collected at the end of the feeding period. Fasting blood glucose levels were slightly increased in the HFD-fed group compared with the NFD-fed group, although it was not significant (Fig. 4(a)). Feeding HFLGD significantly decreased fasting glucose levels compared with feeding HFD. Fasting blood insulin levels and the HOMA-IR index were significantly increased by HFD feeding compared with NFD feeding (Fig. 4(b) and (c)). HFLGD feeding did not suppress blood insulin levels and the HOMA-IR index compared with HFD feeding. The HOMA-IR index was significantly correlated with the M1:M2 macrophage ratio (Fig. 4(d)).

Fig. 4. Serological analysis of mouse serum. Mouse serum was collected after 12 weeks of feeding. Fasting glucose (a) and insulin (b) levels were analysed. The index of homoeostasis model assessment for insulin resistance (HOMA-IR) (c) was calculated using the following formula: fasting glucose (mmol/l)×fasting insulin (mU/ml)/22·5. ![]() , Values of the normal-fat diet (NFD) group;
, Values of the normal-fat diet (NFD) group; ![]() , values of the high-fat diet (HFD) fed group;
, values of the high-fat diet (HFD) fed group; ![]() , values of the HDF containing LG2055 (HFLGD) fed group. Data are shown as box plots (n 12), as described in Fig. 2. Correlation between the HOMA-IR index and the M1:M2 macrophage ratio was plotted (d) with Pearson’s r correlation and the corresponding P value. Statistically significant difference: * P<0·05 (Dunnett’s test).
, values of the HDF containing LG2055 (HFLGD) fed group. Data are shown as box plots (n 12), as described in Fig. 2. Correlation between the HOMA-IR index and the M1:M2 macrophage ratio was plotted (d) with Pearson’s r correlation and the corresponding P value. Statistically significant difference: * P<0·05 (Dunnett’s test).
Discussion
In a diet-induced obese model, some LAB have been shown to improve obesity and insulin resistance that closely relate to chronic inflammation, to which macrophage infiltration largely contributes( Reference Lee, Park and Seok 5 – Reference Tsai, Cheng and Pan 9 ). The present study demonstrates that, on a cell basis by flow cytometry, LG2055 administration prevented infiltration of macrophages into the adipose tissue of mice fed an HFD, which had been suggested by a previous study( Reference Miyoshi, Ogawa and Higurashi 17 ) showing that expression of the Ccl2 gene, a crucial factor for macrophage infiltration, was down-regulated in diet-induced obese mice.
A number of studies have reported that obesity promotes macrophage infiltration into adipose tissue( Reference McNelis and Olefsky 29 ): invading macrophages then polarise to pro-inflammatory M1 macrophages and exacerbate inflammation by pro-inflammatory cytokines produced there; the cytokines then activate the JNK and IKKβ signalling pathways, leading to exacerbation of insulin resistance( Reference Solinas and Karin 30 , Reference Gregor and Hotamisligil 31 ). It is reported that an increase in the M1:M2 ratio is associated with an increase in systemic inflammation and induction of insulin resistance( Reference Fujisaka, Usui and Bukhari 19 ). Chronic inflammation and insulin resistance are critical in exacerbating the symptoms of obesity-induced metabolic diseases such as CVD and type 2 diabetes( Reference Lumeng and Saltiel 21 , Reference McNelis and Olefsky 29 ). Inhibition of macrophage infiltration into adipose tissue and down-regulation of pro-inflammatory M1 macrophages must contribute to amelioration of these diseases. It has been reported that a CCR2 antagonist, an inhibitor of macrophage infiltration, ameliorated obesity-associated inflammation and insulin resistance in mice( Reference Kang, Lee and Song 32 ). Furthermore, positive effects of CCR2 antagonist have been recently shown in human diabetic patients( Reference McNelis and Olefsky 29 , Reference Di Prospero, Artis and Andrade-Gordon 33 ).
In the present study, gene expression and flow cytometric analyses revealed that an increase in macrophage invasion into adipose tissue caused by feeding an HFD was suppressed by the additional feeding of LG2055 (Fig. 2 and 3). Flow cytometric analysis also revealed that the abundance in M1 macrophages and the M1:M2 macrophage ratio were significantly increased in the adipose tissue after feeding the HFD and significantly suppressed by the additional feeding of LG2055 (Fig. 2). Some studies on the abundance of M1 macrophages in the adipose tissue of diet-induced obese mice reported that approximately 25–70 % of adipose tissue macrophages were M1 macrophages( Reference Fujisaka, Usui and Bukhari 19 , Reference Cho, Morris and Lumeng 26 , Reference Ying, Kanameni and Chang 34 ). In our study, the mean percentage of M1 macrophages in the HFD-fed group was approximately 15 %, which could be low compared with other reports. Nevertheless, the suppressive effect of LG2055 on macrophage invasion was clearly demonstrated.
The present study minimised an influence of granulocytes contamination on the flow cytometric analysis of macrophages. Cho et al.( Reference Cho, Morris and Lumeng 26 ) reported that the F4/80midCD11bmid/high population in SVF cells from adipose tissue contains neutrophils and eosinophils; therefore, only the F4/80highCD11bhigh population should be gated to minimise contamination. In our study, the F4/80highCD11bhigh population was defined as macrophages according to the method of Cho et al. In addition, even if the F4/80+CD11b+ cells were defined as macrophages, the results of this study were not affected: the percentage of M1 macrophages and the M1:M2 ratio in the HFD-fed group were higher than those in the HFLGD-fed group (data not shown).
It is known that the gene expression levels of pro-inflammatory cytokines increase together with an increase in macrophage invasion in the adipose tissue of diet-induced obese mice( Reference Chawla, Nguyen and Goh 18 , Reference McNelis and Olefsky 29 ). In our study, the expression levels of pro-inflammatory cytokine genes such as Tnf and Lep were also increased by feeding a HFD, and the levels of lep were down-regulated by the additional feeding of LG2055 (Fig. 3). Leptin, the translational product of the Lep gene, known to be a hormone produced by adipocytes, which influences energy homoeostasis, is also reported to be a cytokine that induces pro-inflammatory cytokine secretion from macrophages and monocytes( Reference Carbone, La Rocca and Matarese 35 ). Dib et al.( Reference Dib, Ortega and Fleming 36 ) showed that obesity-associated inflammation in adipose tissue was suppressed when the leptin receptor was absent on the cells derived from the bone marrow, suggesting the importance of leptin signal in the exacerbation of obesity-associated inflammation. Our results showing down-regulation of Lep also suggested that inflammation in adipose tissue was ameliorated by the administration of LG2055.
Based on flow cytometric and gene expression analysis data, it was suggested that administration of LG2055 might improve insulin resistance, which is closely related to the status of inflammation in adipose tissue( Reference Fujisaka, Usui and Bukhari 19 , Reference McNelis and Olefsky 29 ). Our results show that fasting glucose was significantly reduced by the administration of LG2055, although the HOMA-IR index was not significantly decreased (Fig. 4). Interestingly, a significant correlation was observed between the HOMA-IR index and the M1:M2 macrophage ratio (Fig. 4(d)), suggesting that the reduced M1:M2 ratio by feeding LG2055 might lead to the improvement of the insulin resistance.
Although much remains to be elucidated regarding how LG2055 suppresses macrophage invasion and adipose tissue inflammation, a compositional change in the gut microbiota could be a factor for the suppression. Wang et al.( Reference Wang, Tang and Zhang 37 ) showed that the administration of probiotic Lactobacillus and Bifidobacterium modulated the gut microbiota and attenuated adipose tissue inflammation, macrophage invasion and insulin resistance in HFD-fed mice. A number of studies have demonstrated that the composition of the gut microbiota is altered in obese mice and humans as well as in type 2 diabetes patients. The compositional change in the gut microbiota is known to affect the amount of SCFA and secondary bile acids in the intestine, as well as lipopolysaccharide levels in the blood, which induce inflammatory immune responses and exacerbate inflammation( Reference Musso, Gambino and Cassader 38 , Reference Belkaid and Hand 39 ). Although we have not assessed the effect of LG2055 on the gut microbiota, Sakai et al.( Reference Sakai, Hosoya and Ono-Oumachi 40 ) reported that the administration of LG2055 increased the IgA antibody production in the intestine of mice. Given that the secreted IgA antibody has the potential to modify the composition of microbiota( Reference Kawamoto, Tran and Maruya 41 ), the administration of LG2055 could affect the state of inflammation through the compositional alteration.
In this study, body weight and adipose tissue weight were not significantly decreased by feeding 45 % energy fat (24 %, w/w fat) diet plus LG2055 for 12 weeks (Table 1). In contrast, Miyoshi et al.( Reference Miyoshi, Ogawa and Higurashi 17 ) reported reduced body and adipose tissue weights after the consumption of LG2055 using a diet-induced obese mouse model, in which the diet used contained 10 % (w/w) fat and the feeding period was 24 weeks. Thus, the difference in fat content in the diet and the feeding period might produce the discrepancy in the body and adipose tissue weight results between the studies. The present study used a 45 % energy fat-containing diet, because Thomas et al.( Reference Thomas, Dunn and Oort 42 ) demonstrated that the intake of a 45 % energy fat diet significantly induced macrophage invasion and adipose tissue inflammation in mice, which were not significantly induced by the feeding of 10 % (w/w) fat diet according to Miyoshi et al.( Reference Miyoshi, Ogawa and Higurashi 17 ).
In summary, administration of LG2055 to mice fed an HFD significantly suppressed infiltration of pro-inflammatory M1 macrophages, chemokine receptor gene (Ccr2) expression, which is responsible for macrophage infiltration and adipokine gene (Lep) expression, which is capable of inducing pro-inflammatory cytokines from macrophages, in adipose tissue. A significant reduction in fasting glucose levels in the blood was also observed. Taken together, LG2055 ingestion is suggested to bring about beneficial properties such as anti-inflammatory tendencies and a possible involvement in improving insulin resistance.
Acknowledgements
All authors are employed by Megmilk Snow Brand Co. Ltd. The authors are grateful to Ken Kato, Yuko Ishida, Akihiro Ogawa, Maya Yamashita, Michio Kawano, Kurumi Takagi, Hiroshi Uenishi and Satoshi Higurashi (Megmilk Snow Brand Co. Ltd) for their skilful collaboration and useful suggestions. The authors also appreciate the work of Emiko Yamaguchi (YBS Co. Ltd) in preparing the experimental diets and technical assistance, and Masaki Kumai, Shigeki Kanoh and Mie Kasuga (YBS Co. Ltd) for animal care.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
K. U. designed the study, carried out experimental work, analysed data and wrote the manuscript. M. M. designed the study and carried out experimental work. Y. K. designed the study.
The authors declare no conflicts of interest.