Introduction
With over 10 million traumatic brain injuries (TBIs) resulting in death or hospitalization occurring annually,Reference Schurman and Lichtman1,Reference Langlois, Rutland-Brown and Wald2 there is a need for an effective treatment strategy to recover from these injuries. TBIs can cause difficulty in performing day-to-day activities and even return to normal physiological functioning does not assure that symptoms will clear.Reference McGeown, Hume, Kara, Neary and Gardner3 The majority of head injuries, such as concussions, are considered mild traumatic brain injuries (mTBIs) and rarely receive full medical treatment.Reference Corrigan, Selassie and Orman4 Alongside TBIs, there has been an enormous increase in research done in the area of endogenous cannabinoids (endocannabinoids) and the Cannabis sativa and Cannabis indica plants.Reference Bridgeman and Abazia5 Research into phytocannabinoids (plant based), endocannabinoids, and the synthetic development of cannabinoids has hinted at TBI as a potential therapeutic target.Reference Schurman and Lichtman1,Reference Shohami, Cohen-Yeshurun, Magid, Algali and Mechoulam6
Cannabidiol (CBD) is a major nonintoxicating compound found in C. sativa and C. indica, along with hemp and other plants,Reference Atakan7,Reference Hilderbrand8 and is thought to possess therapeutic potential due to its major neurological and anti-inflammatory properties.Reference Maroon and Bost9,Reference Li, Kong and Chambers10 It is thought that when CBD is administered alongside other phytocannabinoids, such as tetrahydrocannabinol (THC or Δ9-THC), there is an entourage effect that elevates the therapeutic properties of both CBD and THC.Reference Russo11–Reference Russo13 Human research in this realm in relation to concussion is very limited, presumably due to ethical issues surrounding administration of intoxicating and often illegal compounds such as THC, even if CBD does have the potential to counteract THC psychosis.Reference Zuardi, Crippa and Hallak14,Reference Niesink and Van Laar15 Numerous animal studies have supported the idea that CBD and THC administered simultaneously can lead to changes in behavioral effectsReference Klein, Karanges and Spiro16 and altered THC metabolism.Reference Hlozek, Uttl and Kaderabek17 It is also important to note that human studies observing pharmacokinetic and pharmacodynamic effects of CBD are also very limited,Reference Liu and Martin18–Reference MacCallum and Russo20 although it has been suggested that the maximum measured plasma concentration of CBD increases in a dose-dependent manner, occurring between 0 and 4 h, depending on administration route.Reference Millar, Stone, Yates and O’Sullivan19
This review will focus on the neuroprotective effects of cannabinoids, specifically the phytocannabinoid CBD following TBI. Considering the prevalence of the entourage effect, it is also important to discuss what the research suggests regarding the combined effects of endocannabinoids and phytocannabinoids, specifically related to concussion. While an animal model has suggested a role for CBD to regulate glutamate and gamma-aminobutyric acid (GABA)Reference Belardo, Iannotta and Boccella21 responses following mTBI, the fundamental mechanisms underlying these effects are still not clear, especially when considering human studies.
The Endocannabinoid System
After the identification and synthesis of THC,Reference Gaoni and Mechoulam22 the scientific world expanded much of their knowledge of the cannabis plant constituents. This increase in interest of the exogenous cannabinoids also led to a greater understanding of the endocannabinoid system, which is known to help regulate pain processing and perception,Reference Woodhams, Sagar, Burston and Chapman23 maintain immune system homeostasis,Reference Acharya, Penukonda, Shcheglova, Hagymasi, Basu and Srivastava24 and influence cardiovascular functioning.Reference Sierra, Luquin and Navarro-Otano25 The endocannabinoid system consists of at least 2 receptors coupled through G-protein inhibition: cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2).Reference Munro, Thomas and Abu-Shaar26–Reference Mackie30 Other receptors sensitive to cannabinoids include vanilloid receptor 1 (TRPV1),Reference Corcoran, Roche and Finn31,Reference Ryskamp, Redmon, Jo and Križaj32 adenosine receptors,Reference Olah, Toth and Borbiro33,Reference Oláh and Bíró34 5-hydroxytryptamine (5-HT1A),Reference Russo, Burnett, Hall and Parker35,Reference Resstel, Tavares, Lisboa, Joca, Corrêa and Guimarães36 and G-protein coupled receptors.Reference Morales and Reggio37
The CB1 receptors are most abundant within the central nervous system, located primarily on the axon and synaptic terminals on the neurons,Reference Mackie38 while the CB2 receptors are much more concentrated within the peripheral nervous system, such as the immune system.Reference Mackie38,Reference Turcotte, Blanchet, Laviolette and Flamand39 Interestingly, CB2 receptors are also present at the brain microglia.Reference Atwood and Mackie40–Reference Cabral, Raborn, Griffin, Dennis and Marciano-Cabral42 CB2 receptors are therefore present at the central nervous system as well, suggesting that the CB2 receptor is not only a peripheral receptor, as implied by earlier studies.Reference Munro, Thomas and Abu-Shaar26,Reference Schatz, Lee, Condie, Pulaski and Kaminski43,Reference Galiegue, Mary and Marchand44 Specifically, CB2 mRNA expression was found on B Cells in the immune system,Reference Galiegue, Mary and Marchand44 spleen macrophages,Reference Munro, Thomas and Abu-Shaar26 and the thymus.Reference Schatz, Lee, Condie, Pulaski and Kaminski43 With CB1 receptors densely concentrated in the central nervous system,Reference Kendall and Yudowski45 this led to the belief that the CB1 and CB2 receptors can be considered as tissue-selective antigens,Reference Galiegue, Mary and Marchand44 although the presence of CB2 at the microglia does put this statement into question. Microglia activation can result in a release of proinflammatory cytokines and reactive oxygen intermediatesReference Dheen, Kaur and Ling46 and the presence of the CB2 receptor at the microglia suggests a neuroprotective purpose. For example, CB2 receptor stimulation in CB1 receptor knockout mice produced antinociceptive effects in response to inflammatory pain.Reference Li, Carey, Mackie and Hohmann47 Discussed in more detail in the following section, activation of the CB1 receptor is thought to be responsible for the common psychosis symptoms associated with THC,Reference Mackie30 while activation of the CB2 receptor can attenuate inflammation and accelerate regeneration in many disease states,Reference Pacher and Mechoulam41 including liver regeneration in an animal model of acute hepatitisReference Teixeira-Clerc, Belot and Manin48 and attenuation of inflammation and tissue injury in an animal model of spinal cord injury.Reference Pacher and Mechoulam41,Reference Baty, Zhang and Li49 Therefore, the CB1 and CB2 receptors are present in neural and peripheral tissue, suggesting involvement in many physiological processes.
The entourage effect suggests a synergistic effect when different phytocannabinoids are administered together. For assistance across the blood brain barrier, adenosine triphosphate-binding cassette transporters have been shown to aid in THC efflux,Reference Spiro, Wong, Boucher and Arnold50 and with CBD’s ability to inhibit these transporters,Reference Holland, Panetta and Hoskins51 it is possible that THC may exert its therapeutic influence at the brain for longer periods.Reference Britch, Wiley, Yu, Clowers and Craft52 This evidence suggests that the location of CB2 receptors on the microglia can be due to a protective mechanism as THC has been shown to increase CB2 receptor protein expression, thereby attenuating inflammatory responses, an effect which was abolished when CB2 receptor antagonists were introduced.Reference Yang, Li, Han, Jia and Ding53 Furthermore, sustaining high level of THC at the brain can increase its influence on the brain’s dopamine system.Reference Bloomfield, Ashok, Volkow and Howes54 Considering that TBIs can impair the nigrostriatal and mesocorticolimbic (mesolimbic and mesocortical) pathways,Reference Chen, Huang, Kuo, Miller, Chiang and Hoffer55 THC’s ability to potentially increase dopamine firing rates on dopaminergic neurons can aid to alleviate dopamine synthesis, reuptake, and metabolism following TBI.Reference Chen, Huang, Kuo, Miller, Chiang and Hoffer55
Concussion Implication and Endocannabinoid Neurophysiology
Introduction to Concussion
Concussion or mTBI-related injuries have been a growing issue, both in adults and youths.Reference Daneshvar, Nowinski, McKee and Cantu56–Reference Halstead and Walter59 While return to baseline after 3 weeks is common in individuals aged 5–14 years,Reference Chrisman, Lowry and Herring58 post-concussion syndrome (concussion-like symptoms lasting over 3 months following mTBI) has been shown to persist for over 6 months in 40% of mTBI patients.Reference Voormolen, Polinder, von Steinbuechel, Vos, Cnossen and Haagsma60 Post-concussion syndrome can have negative effects on the individual’s day-to-day activities, as shown through its negative associations with health-related quality of life, assessed by the 36-item Short-Form Health Survey and Perceived Quality of Life Scale.Reference Voormolen, Polinder, von Steinbuechel, Vos, Cnossen and Haagsma60 Persistence of one symptom was much more common than multiple symptoms (with headache, fatigue, forgetfulness, and poor concentration among the most common),Reference Polinder, Cnossen and Real61,Reference Theadom, Parag and Dowell62 and being female was a significant predictor of symptoms lasting 12 months post injury.Reference Theadom, Parag and Dowell62
Concussion can initiate a neurometabolic cascade,Reference Giza and Hovda63 which can lead to an energy crisis, thereby impairing cerebral blood flow,Reference Len, Neary, Asmundson, Goodman, Bjornson and Bhambhani64 increasing intracellular calcium (Ca2+) levels,Reference Giza and Hovda63,Reference Buki and Povlishock65 and disrupting autonomic functioning,Reference Bishop, Dech, Baker, Butz, Aravinthan and Neary66,Reference Neary, Singh, Bishop, Dech, Butz and Len67 among other physiological consequences (Figure 1).Reference Giza and Hovda63,Reference Bishop, Dech, Baker, Butz, Aravinthan and Neary66,Reference Study, Hocke, Duszynski, Debert, Dleikan and Dunn68–Reference Jang, Huang and Hammer76 mTBIs generally occur due to rotational or twisting forces (accelerations or decelerations)Reference Barth, Freeman, Broshek and Varney77,Reference Ivancevic78 of the brain within the skull, thereby resulting in a disruption in homeostasis. In comparison, the endocannabinoid system is thought to have neuroprotective properties,Reference Xu and Chen79,Reference Mechoulam, Spatz and Shohami80 which could therefore ameliorate these disturbances in physiological homeostasis. This may partly be due to the fact that the endocannabinoids are retrograde messengers with the ability to cause depolarization-induced suppression of inhibition.Reference Diana and Marty81 Once neurotransmitters are released from the presynaptic neuron, they bind to the postsynaptic receptors to induce either excitatory or inhibitory postsynaptic potentials. This binding to the postsynaptic receptors allows for entry of Ca2+ into the cell, leading to the activation of phospholipase (PL) and diacylglycerol lipase (DAGL).Reference Barrie and Manolios82 Activation of PL and DAGL in combination with arachidonic acid leads to biosynthesis of N-arachidonylethanolamine (also called anandamide; AEA) and 2-arachidonoyl glycerol (2-AG).Reference Prescott and Majerus83–Reference Di Marzo85 These endocannabinoids are thought to be produced in vivo where increased intracellular Ca2+ concentration is the major trigger for synthesis.Reference Placzek, Okamoto, Ueda and Barker86 The endocannabinoid ligands, such as AEA and 2-AG,Reference Hillard29 are released from cells in a stimulus-dependent manner by cleavage of membrane lipid precursors.Reference Giuffrida, Beltramo and Piomelli87–Reference Grotenhermen89 These endocannabinoids travel back to the presynaptic neuron and bind to the CB1 receptors which causes a decrease in presynaptic neurotransmitter release across the synaptic cleft. This is done by inhibition of adenylate cyclase (leading to a decrease in sodium (Na+) inflow) and calcium channels, along with activation of the potassium (K+) channels,Reference Diana and Marty81 thus potentially leading to hyperpolarization as depolarization-induced suppression of inhibition decreases the frequency of miniature inhibitory postsynaptic currents. Taken together, these changes in ionic balance can increase the percentage of synaptic failures.Reference Diana and Marty81
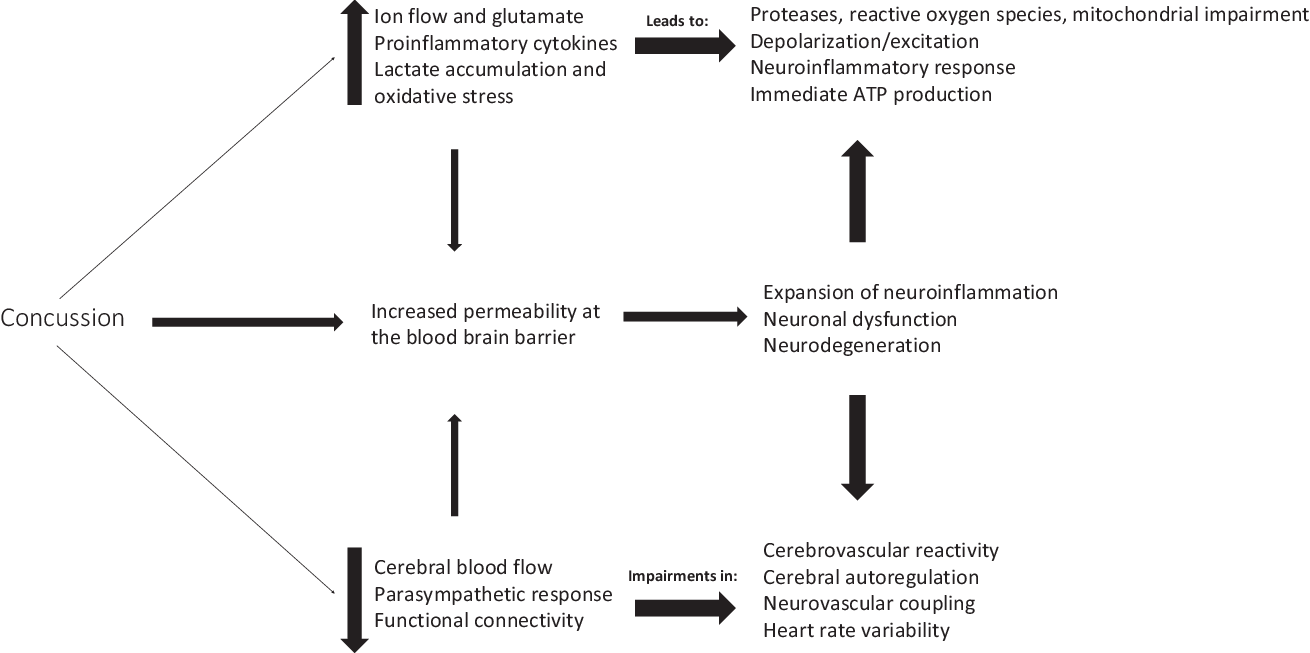
Figure 1: Overview of physiological consequences following concussion.
The endocannabinoid ligands are synthesized by the body from arachidonic acid of membrane phospholipidsReference Pope, Mechoulam and Parsons90 in response to pain, inflammation, anxiety, emotional and physical stressors, and pathological conditions. Completion of the endocannabinoid signaling is thought to require cellular reuptake through a carrier-mediated transport process followed by enzymatic degradation by enzyme fatty acid amide hydrolase primarily for AEAReference Deutsch and Chin91 and monoacylglycerol lipase for 2-AG.Reference Pope, Mechoulam and Parsons90 The Ca2+-dependent synthesis and release of endocannabinoids for its retrograde mechanism could have a significant role in protecting the body by limiting overstimulation, such as protection against the energy crisis which occurs during the neurometabolic cascade following a mTBI.Reference Giza and Hovda63
Cannabidiol Implications for Neuroprotection following Concussion
CBD is a phytocannabinoid found in the C. sativa and hemp plant that is well known for its therapeutic potential but is devoid of psychosis.Reference Mechoulam, Peters, Murillo-Rodriguez and Hanus92,Reference Iffland and Grotenhermen93 Phytocannabinoids, including CBD, are also known to act on other receptors aside from the CB1 and CB2 receptors in the body (Table 1). CBD can inhibit fatty acid amide hydrolase, thereby inhibiting the enzymatic hydrolysis and uptake of AEAReference Bisogno, Hanus and De Petrocellis101 from the synapse. This implies that CBD can therefore indirectly influence the effects on AEA in the endocannabinoid system, possibly allowing for sustained neuroprotective effects of AEA,Reference Veldhuis, van der Stelt and Wadman102,Reference Correa, Mestre, Docagne, Borrell and Guaza103 such as influencing CB2 receptors to alleviate lipopolysaccharide-induced neuroinflammation.Reference Malek, Popiolek-Barczyk, Mika, Przewlocka and Starowicz104
Table 1: Non CB1 and CB2 receptors stimulated by CBD and their relevance to concussion pathophysiology
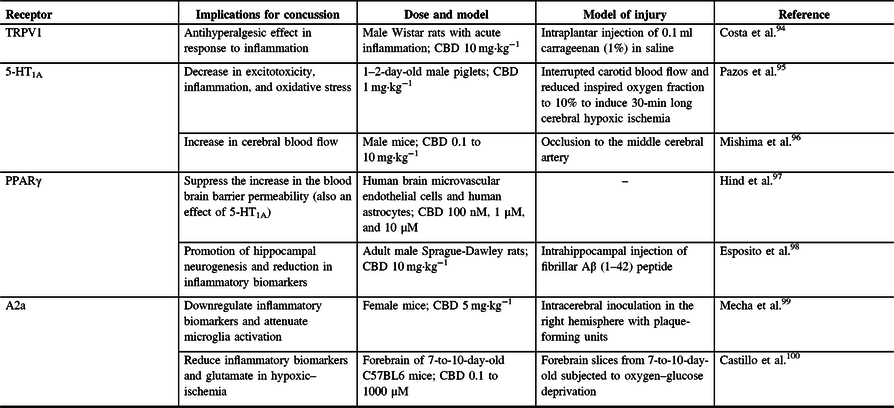
TRPV1 = vanilloid receptor 1; A2a = adenosine receptor; 5-HT1A = 5-hydroxytryptamine; PPARγ = peroxisome proliferator-activated receptor gamma; CBD = cannabidiol.
TRPV1 is a ligand-gated ion channel and acts on afferent neurons where it is expressed both presynaptically, and postsynaptically. This receptor is involved in the sensation of pain and thermal hyperalgesia.Reference Caterina, Leffler and Malmberg105 CBD can function as a weak TRPV-1 agonist, which may explain its therapeutic ability in neuropathic pain,Reference Costa, Giagnoni, Franke, Trovato and Colleoni94,Reference Bisogno, Hanus and De Petrocellis101 as 10 mg·kg−1 of CBD administered to rats with acute inflammation showed an antihyperalgesic response.Reference Costa, Giagnoni, Franke, Trovato and Colleoni94 Research by Benyó et alReference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 highlights that the activation of TRPV1 is known to control vascular responses, thus hinting at the idea of CBD having different actions within the cerebral vasculature. Furthermore, Benyó et al suggest that phytocannabinoids and endocannabinoids have the ability to directly influence cerebrovascular resistance and blood perfusion of the brainReference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 and that all cerebrovascular control pathways contain cells capable of being modulated by the different forms of cannabinoids. Considering that concussions can impair cerebrovascular reactivity,Reference Len, Neary and Asmundson73,Reference Len and Neary107,Reference Bishop and Neary108 it is possible that CBD’s influence on TRPV1 can protect against these changes.
Another group of receptors upon which CBD is known to act are adenosine receptors, such as A2a receptors. These receptors can downregulate the release of other neurotransmitters such as dopamine and glutamate, and CBD is thought to increase brain adenosine levels by reducing adenosine reuptake.Reference Maroon and Bost9 Furthermore, CBD-mediated activation of A2a receptors may also allow for increased anti-inflammatory effects, further increasing CBD’s potential for enhancing neuroprotection.Reference Castillo, Tolon, Fernandez-Ruiz, Romero and Martinez-Orgado100 Indeed, CBD was shown to downregulate inflammatory biomarkers and attenuate microglia activation in a viral mouse model of multiple sclerosis by interacting with A2a receptors.Reference Mecha, Feliu, Inigo, Mestre, Carrillo-Salinas and Guaza99 Finally, it was suggested that CBD can blunt the THC-induced cognitive and memory impairments in an A2a receptor-dependent manner,Reference Aso, Fernandez-Duenas and Lopez-Cano109 as shown by A2a receptor knockout mice performance in a two-object recognition test.Reference Aso, Fernandez-Duenas and Lopez-Cano109
CBD is also known to act directly upon the 5-HT1A receptor, which is found in the brain in large concentrations, with large densities at the prefrontal cortex, hippocampus, and amygdala.Reference Garcia-Garcia, Meng and Canetta110,Reference Pompeiano, Palacios and Mengod111 CBD has been shown to exhibit a high potency for this receptor and serves as an agonist, to further stimulate the receptor’s properties of decreasing anxiety, pain, and headaches. 5-HT1A is a G-protein-coupled serotonin receptor, which further suggests the potential of CBD to provide therapeutic benefits,Reference Russo, Burnett, Hall and Parker35 similar to those of serotonin itself. For example, it was found that CBD can enhance both serotonergic and glutamate cortical signaling in a mouse model, possibly allowing for rapid-acting antidepressant-like effects.Reference Linge, Jimenez-Sanchez and Campa112 Furthermore, human cell culture, rat, and pig research has also shown CBD to be neuroprotective by inhibiting the reuptake of 5HT,Reference Russo, Burnett, Hall and Parker35,Reference Pazos, Mohammed and Lafuente95 resulting in decreased excitotoxicity, inflammation, and oxidative stress.Reference Pazos, Mohammed and Lafuente95
Peroxisome proliferator-activated receptor (PPAR) gamma (γ) is also thought to be influenced by phytocannabinoids to produce therapeutic effects.Reference O’Sullivan113 PPARs are nuclear receptor proteins that function as transcription factors and are essential in the body’s ability to regulate energy homeostasis and metabolic function.Reference Tyagi, Gupta, Saini, Kaushal and Sharma114 As such, their involvement becomes increasingly important in stabilizing cell homeostasis. For example, it was shown that CBD suppresses the increased permeability of the blood brain barrier associated with oxygen–glucose deprivation by PPARγ and 5-HT1A activation.Reference Hind, England and O’Sullivan97,Reference O’Sullivan113 This was done using human brain microvascular endothelial cells and human astrocytes,Reference Hind, England and O’Sullivan97 providing a valuable in vitro point of view rather than an animal model. In relation to other neurodegenerative disorders, cell culture research has shown that CBD reduces β‐amyloid expressionReference O’Sullivan113,Reference Scuderi, Steardo and Esposito115 and can possibly induce apoptosis in some forms of cancer cells, such as lung tumor cells,Reference O’Sullivan113,Reference Ramer, Heinemann and Merkord116 via PPAR activation. PPARγ is thought to inhibit the expression of inflammatory cytokines,Reference Tyagi, Gupta, Saini, Kaushal and Sharma114,Reference Wang, Shi and Xin117 suggesting possible CBD and PPARγ synergy to further regulate inflammation. This was further shown by CBD’s inhibition of necrosis factor kappa b, nitric oxide, tumor necrosis factor alpha, and interleukin 1 beta, along with promoting hippocampal neurogenesis in a rat model. It is important to note that CBD’s neuroprotective effects were completely abolished after introduction of a PPARγ antagonist (GW9662).Reference Esposito, Scuderi and Valenza98
Finally, a receptor initially known as the endothelial cannabinoid receptor, now thought to be G-protein-coupled receptor 18,Reference Console-Bram, Brailoiu, Brailoiu, Sharir and Abood118,Reference Penumarti and Abdel-Rahman119 also serves as a receptor at which abnormal CBD (ABN-CBD), a synthetic CBD product, was shown to react.Reference Console-Bram, Brailoiu, Brailoiu, Sharir and Abood118–Reference Begg, Pacher and Batkai120 This receptor is believed to be in existence as administration of CBD to mice lacking the cannabinoid receptors still resulted in hypotension and endothelium-dependent mesenteric vasodilation, yet administration of a CB1 antagonist was shown to block the CBD effect.Reference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 It is therefore possible that CBD can help to regulate cerebral blood flow due to its influence on vasomotor control, meaning that the endothelial cannabinoid receptor and G-protein-coupled receptors stimulated by CBD can mediate neuroprotective effects and regulate cell migration.
Concussion can result in a cascade of pro- and anti-inflammatory cytokines,Reference Patterson and Holahan121 ionic imbalance,Reference Giza and Hovda63 increased lactate accumulation due to increased glycolysis and oxidative stress,Reference Kawamata, Katayama, Hovda, Yoshino and Becker122,Reference Kim, Han, Gallan and Hayes123 and impaired cerebral blood flow.Reference Len, Neary and Asmundson73 Through its influences on the cerebrovasculature, its anti-inflammatory properties, and its neuroprotective properties, CBD can theoretically help to reduce impairments following concussion. Furthermore, to control for the calcium sequestration and ionic inflow,Reference Choe69 it has been suggested that CBD can regulate Ca2+ homeostasis against mitochondrial dysfunction and toxins, and Ca2+ dysregulation, as shown in tissue cultures of rat pups.Reference Ryan, Drysdale, Lafourcade, Pertwee and Platt124
Due to its neuroprotective capabilities and its lack of intoxicating side effects, CBD shows promising potential in helping individuals with TBIs. Mice induced with bilateral common carotid artery occlusion were administered 10 mg·kg−1 CBD which attenuated hippocampal neurodegeneration and white matter injury, increased hippocampal brain-derived neurotrophic factor protein levels, stimulated neurogenesis, and promoted dendritic restructuring.Reference Mori, Meyer, Soares, Milani, Guimaraes and de Oliveira125 Furthermore, CBD administration to mice both before and after occlusion of the middle cerebral artery showed that CBD suppressed the impairment in cerebral blood flow by the failure of cerebral microcirculation after reperfusion, providing further evidence of CBD’s neuroprotective properties.Reference Hayakawa, Mishima and Nozako126 Adding to this evidence of neuroprotective potential, CBD administration to piglets exposed to acute hypoxia-ischemia increased the brain activity post-ischemia back to normal as shown by brain electrical activity, cerebral tissue oxygenation, and neurobehavioral responses, such as motor performance.Reference Lafuente, Alvarez and Pazos127
Blood Brain Barrier
Concussions can alter cerebrovascular actions by disrupting the blood brain barrier integrity.Reference Sahyouni, Gutierrez, Gold, Robertson and Cummings128 Cannabinoids, specifically 2-AG and dexanabinol, a synthetic cannabinoid also known as HU-211, have been shown to decrease proinflammatory cytokines such as tumor necrosis factor-alphaReference Gallily, Breuer and Mechoulam129 and necrosis factor kappa b.Reference Juttler, Potrovita and Tarabin130 Cytokines have been shown to overexpress following concussion,Reference Patterson and Holahan121 which can lead to a heightened sensitivity for disease, such as multiple sclerosis or Alzheimer’s, at the blood brain barrier.Reference Varatharaj and Galea131 Furthermore, head injuries tend to enhance the activity of water-soluble antioxidants in the brain.Reference Shohami, Beit-Yannai, Horowitz and Kohen132This occurs along with an increase in 2-AG,Reference Panikashvili, Simeonidou and Ben-Shabat133 which can further increase the levels of the water-soluble antioxidants,Reference Mechoulam and Shohami134 suggesting a regulator ability for the endocannabinoid in neuroprotection with the blood brain barrier,Reference Panikashvili, Simeonidou and Ben-Shabat133 especially as synthetic 2-AG administration in a mouse model resulted in reduced brain edema, infarct volume, and hippocampal cell death.Reference Panikashvili, Simeonidou and Ben-Shabat133 The blood brain barrier controls the amount of material transported into the brain, and in combination with the CB1 and CB2 receptors, it can limit and protect the brain against the influx of neurotoxins, immune cells, and macromolecules,Reference Vendel and de Lange135 thus allowing maintenance of an optimal extracellular environment in the brain.Reference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 This allows for prevention of apoptosis, glia cell activation, and scar tissue formation, among other symptoms caused by these substances. While CB1 and CB2 receptors are involved in the blood brain barrier, 2-AG is effective as a full CB receptor agonist,Reference Di Marzo and De Petrocellis136,Reference Sugiura, Kishimoto, Oka and Gokoh137 which may help explain its anti-inflammatory propertiesReference Turcotte, Blanchet, Laviolette and Flamand39,Reference Castillo, Tolon, Fernandez-Ruiz, Romero and Martinez-Orgado100 due to its influence on the CB2 receptor. CBD’s lipophilic properties allow it to easily pass the blood brain barrier.Reference Maroon and Bost9 While research pertaining to CBD’s influence at the blood brain barrier in humans is limited, it has been shown that CBD can preserve the barrier’s integrity (permeability), possibly by protecting against the loss of tight junction proteins by acting on PPARγ and 5‐HT1A receptors.Reference Hind, England and O’Sullivan97,Reference Brook, Mamo and Wong138
Adding to CBD’s neuroprotective properties, oxidative stress is greatly modulated by CBD as well.Reference Cassol, Comim and Silva139–Reference Saito, Rezende and Teixeira142 Following a TBI, there is an increase in free radical production, such as reactive oxygen species.Reference Kontos and Povlishock143,Reference Povlishock and Kontos144 Under oxidative stress, the protective effect of CBD is thought to be mediated by a decrease in reactive oxygen species production.Reference Mecha, Torrao, Mestre, Carrillo-Salinas, Mechoulam and Guaza140 This can be due to the electrophilic aromatic molecular region and hydroxyl groups of CBD, potentially allowing it to act as an antioxidant itself.Reference Borges, Batista and Viana145–Reference Atalay, Jarocka-Karpowicz and Skrzydlewska147 Post TBI, N-methyl-d-aspartate (NMDA) receptor binding by glutamate allows for intracellular accumulation of Ca2+, causing cellular damage via proteases, reactive oxygen species, and mitochondrial impairment.Reference Choe69,Reference Cheng, Kong, Zhang and Zhang70 NMDA-induced retinal neurotoxicity in rats was also shown to be treated by intravenous injection of CBD to the eye at 2 mg·kg−1, which further shows the antioxidative properties of CBD, as it reduced both oxidative and nitrative stress.Reference El-Remessy, Khalil and Matragoon148
Brain-Derived Neurotrophic Factors
Brain-derived neurotrophic factors (BDNF) is a protein that promotes the survival of nerve cells. It is known to increase the frequency of miniature excitatory postsynaptic currents, possibly strengthening excitatory, glutamatergic synapses and weakening inhibitory, GABAergic synapses.Reference Binder and Scharfman149,Reference Lohof, Ip and Poo150 In cases involving encephalopathy, the administration of 5 mg·kg−1 CBD was shown to help restore BDNF levels in mice through the activation of the 5-HT1A receptor.Reference Magen, Avraham, Ackerman, Vorobiev, Mechoulam and Berry151 Furthermore, withdrawal from amphetamines can diminish BDNF levels due to oxidative stress.Reference Fuller, Murray and Horner152 This decrease in BDNF has been shown to be blocked by the administration of 60 mg·kg−1 dosages of CBD in a rat model,Reference Campos, Fogaça, Sonego and Guimarães153,Reference Valvassori, Elias and de Souza154 suggesting that CBD may have a great potential for aiding in addiction recovery and attenuate reductions in BDNF levels. Finally, 30 mg·kg−1 dosages of CBD have been shown to upregulate BDNF following cerebral malaria in a rodent model through the nitric synthase pathway.Reference Campos, Brant, Miranda, Machado and Teixeira155 This ability of CBD to increase BDNF expression can suggest an important role in neuroprotection, leading to decreased neuronal damage.Reference Campos, Fogaça, Sonego and Guimarães153 BDNF is also thought to enhance neurogenesis,Reference Binder and Scharfman149 further suggesting CBD’s restorative potential. At the prefrontal cortex and hippocampus, CBD was shown to elevate BDNF levels, resulting in sustained antidepressant-like effects in mice.Reference Sales, Fogaca and Sartim156 Considering that BDNF levels can be impaired following TBI in humansReference Korley, Diaz-Arrastia and Wu157,Reference Giza and Difiori158 and there is a correlation between BDNF serum levels and cognitive impairments,Reference Siuda, Patalong-Ogiewa and Zmuda159 it is possible that CBD administration post-concussion can further aid in the recovery process.
Cognitive Capacity
Cognitive capacity is the total amount of information the brain can retain at any moment. Functional magnetic resonance imaging (fMRI) is a neuroimaging method used to measure brain activity by observing correlations relative to blood flow, thus allowing measurements of neural mechanisms of cognitive capacities.Reference Logothetis160 Using this fMRI technique, for example, it was found that THC and CBD have distinct effects on regional brain activation,Reference Winton-Brown, Allen and Bhattacharyya161 sometimes even completely opposite effectsReference Winton-Brown, Allen and Bhattacharyya161,Reference Bhattacharyya, Morrison and Fusar-Poli162 when 10 mg THC, 600 mg CBD, or placebo capsules were administered to 14 healthy human volunteers. This study found that found that THC decreased the activation of bilateral temporal cortices during auditory processing and was associated with increases in anxiety, intoxication, and positive psychotic symptoms. It also influenced visual processing. In contrast, CBD administration did not have any reductive effects on areas such as visual processing and showed no significant symptomatic effects such as psychotic symptoms and increases in anxiety,Reference Winton-Brown, Allen and Bhattacharyya161,Reference Bhattacharyya, Morrison and Fusar-Poli162 suggesting that CBD alone can provide therapeutic benefits without the intoxication of THC.
Interestingly, THC and CBD have also shown opposite effects on cognition-related brain activation. It has been suggested that the harmful effect of cannabis might be driven by high THC/low CBD compositions,Reference Colizzi and Bhattacharyya163 as shown by poor performances on verbal memory task,Reference Morgan, Schafer, Freeman and Curran164 reduced emotional processing accuracy,Reference Hindocha, Freeman and Schafer165 and poorer verbal memory performance.Reference Englund, Morrison and Nottage166 Combination of CBD and THC allows a reduction in the psychotic effects of THC, and thus CBD is able to partially prevent the detrimental effects of THC on working memory.Reference Colizzi and Bhattacharyya163,Reference Englund, Morrison and Nottage166 Finally, higher THC concentrations found in hair strands were shown to negatively impact memory and psychological well-being, whereas lower psychosis-like symptoms were found in individuals with higher CBD concentrations in their hair,Reference Morgan, Gardener and Schafer167 further showing CBD’s nonintoxicating therapeutic influence.
Cerebrovasculature
CBD administration following TBI possesses strong potential to significantly reduce inflammation via the reduction in microglia activation and proinflammatory cytokines.Reference Pope, Mechoulam and Parsons90,Reference Mechoulam and Shohami134,Reference Campos, Fogaça, Sonego and Guimarães153 As discussed earlier, during brain trauma, the endocannabinoid system is activated by the rise in intracellular Ca2+. Activation of the endocannabinoid system suggests that it is part of the compensatory repair mechanism for the brain.Reference Bahr, Karanian, Makanji and Makriyannis168 Since cerebral blood flow is tightly regulated by myogenic, endothelial, metabolic, and neural mechanisms, all major cell types involved in cerebrovascular control pathways are capable of synthesizing endocannabinoids.Reference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 Finally, CBD administration has been shown to increase cerebral blood flow following middle cerebral artery occlusion by action on 5-HT1A receptors.Reference Mishima, Hayakawa and Abe96,Reference Hayakawa, Mishima and Fujiwara169 As such, it is logical to hypothesize that the endocannabinoid system modulates the regulation of cerebral circulationReference Richter, Quenardelle and Rouyer170 under both physiological and pathophysiological conditions.Reference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106
Regulation of blood flow following a TBI can be beneficial in assuring adequate nutrient supply to areas of altered metabolic activity. This compensatory phenomenon in healthy individuals is internally regulated through a mechanism known as neurovascular coupling. While no direct studies have shown CBD’s influence on this mechanism, the role of the endocannabinoid system in cerebral circulationReference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 and the presence of TRPV1 at the sensory vagal afferent neurons and the tunica of blood vesselsReference Sierra, Luquin and Navarro-Otano25 give CBD the cardioprotective potential. Concussion may lead to changes in the neurovascular coupling response,Reference Neary, Singh, Bishop, Dech, Butz and Len67,Reference Jang, Huang and Hammer76,Reference Tan, Meehan, Iverson and Taylor171 and CBD has been shown to regulate cerebral blood flowReference Crippa, Zuardi and Garrido172 and influence pial vessel responses by regulating vascular effects in combination with protecting the blood brain barrier.Reference Ruiz-Valdepeñas, Martínez-Orgado, Benito, Millán, Tolón and Romero173 Keeping in mind that the cannabinoid receptors and TRPV1 are located at the cerebrovasculature,Reference Benyó, Ruisanchez, Leszl-Ishiguro, Sándor and Pacher106 CBD’s ability to sustain high AEA levels by inhibiting fatty acid amide hydrolaseReference Leweke, Piomelli and Pahlisch174 suggests an indirect effect on CB receptors. Further stimulation of CB2 receptors by AEA can allow for regulation of nitric oxide, thereby potentially suppressing neuroinflammatory reactions,Reference Malek, Popiolek-Barczyk, Mika, Przewlocka and Starowicz104 which suggests great potential in combination with the CB1 receptor stimulation to promote neuroprotection, as the neuroprotective effects of AEA are also mediated by the CB1 receptor.Reference Veldhuis, van der Stelt and Wadman102
Finally, functional connectivity impairments have also been shown following concussion. Specifically, patients with persistent post-concussion symptoms have reduced connectivity and reduced coherence during memory tasks,Reference Hocke, Duszynski, Debert, Dleikan and Dunn175 and reduced connectivity in the default mode network during resting states has also been shown in subacute patients and patients assessed less than 3 weeks post injury.Reference Johnson, Zhang and Gay176,Reference Mayer, Mannell, Ling, Gasparovic and Yeo177 Human research has shown that THC intake resulted in decreases in the default mode network activation (a large scale of interacting brain regions known to have activity highly correlated with each other, especially during task disengagementReference Raichle178), whereas a combination of THC and CBD attenuated the disruption in this network seen when THC is taken alone.Reference Wall, Pope and Freeman179 Patients with autism spectrum disorder were administered 600 mg of CBD and they also showed changes in functional connectivity as assessed by the fractional amplitude of low-frequency fluctuations.Reference Pretzsch, Voinescu and Mendez180 While the direct underlying mechanism by which CBD regulates functional connectivity requires more research, there is a clear therapeutic effect on functional connectivity following CBD intake.
Dopaminergic Impairments following Concussion
TBIs can result in impaired dopamine signaling at the brain. Specifically, the long axonal projections in the dopaminergic nigrostriatal and mesocorticolimbic pathways can be disrupted due to mechanical damage, which induces oxidative stress, axonal damage, and postsynaptic neuron impairments.Reference Chen, Huang, Kuo, Miller, Chiang and Hoffer55,Reference Palmer, Marion, Botscheller, Swedlow, Styren and DeKosky181 Following these acute changes, sustained dopamine impairments due to TBI results in BDNF reduction, increase in inflammatory processes, and metabolic dysregulation.Reference Chen, Huang, Kuo, Miller, Chiang and Hoffer55 In combination with epigenetic effects and mitochondrial dysregulation,Reference Chen, Huang, Kuo, Miller, Chiang and Hoffer55,Reference Sauerbeck, Hunter, Bing and Sullivan182 TBIs can therefore result in impairments in dopamine synthesis and metabolism.Reference Chen, Huang, Kuo, Miller, Chiang and Hoffer55 Rodent studies have shown that THC can result in increased dopamine neuron firing rates, while human studies have shown mixed responses.Reference Bloomfield, Ashok, Volkow and Howes54 CBD has been shown to be a partial agonist at dopamine receptors in rat striatal tissue, potentially explaining some of its antipsychotic effects.Reference Seeman183 Furthermore, in a rat model of Parkinson’s disease, CBD was shown to attenuate loss of dopaminergic neurons and microglia activation.Reference Garcia, Palomo-Garo, Garcia-Arencibia, Ramos, Pertwee and Fernandez-Ruiz184,Reference Peres, Lima, Hallak, Crippa, Silva and Abílio185 CBD and THC both seem to aid in neuroprotection of dopaminergic pathways following mTBI and TBI.
Cardiovascular Physiology in Relation to Concussion
CBD has also been shown to exert its protective influences over the cardiovascular system. For example, diabetes is known to cause dysfunction and cardiac autonomic abnormalitiesReference Vinik and Dan186 by mechanisms such as hyperglycemia-induced overproduction of reactive oxygen species and impaired antioxidant enzyme activities. Administration of ABN-CBD has been shown to attenuate these effects in rats and even promote vagal responses (such as decreased heart rate and mean arterial pressure), supported by a high frequency increase in the electrocardiogram (ECG) R-R index spectral analysis.Reference Matouk, Taye, El-moselhy, Heeba and Abdel-rahman187 It is important to note that heart rate variability impairments have been noted following concussion,Reference Bishop, Dech, Guzik and Neary74,Reference Gall, Parkhouse and Goodman188,Reference Paniccia, Verweel and Thomas189 although the specific physiological mechanism(s) is still unknown. As concussion can occur due to any transient neurologic dysfunction resulting from a biomechanical force,Reference Giza and Hovda63 an injury leading to autonomic dysfunction may further lead to changes in heart rate variability. Furthermore, decreased global heart rate variability is also thought to be related to arrhythmic mortality.Reference Counihan, Fei, Bashir, Farrell, Haywood and McKenna190–Reference Esterov and Greenwald192 CBD’s ability to reduce arrhythmic events (ventricular tachycardia and total length of arrhythmias)Reference Gonca and Darici193 suggests further potential to treat cardiovascular complications arising from concussion. It has been shown that there are some changes in heart rate variability when getting the participant to exercise,Reference Bishop, Dech, Baker, Butz, Aravinthan and Neary66,Reference Gall, Parkhouse and Goodman188 but the data are still unclear as to what the changes imply. Regardless, it is clear is that dysfunction occurs following concussion, and considering the effect of ABN-CBD to influence endothelial cannabinoid receptors, it follows that phytocannabinoids can potentially help regulate the cardiovascular system following dysfunction.
Neurogenesis
Following mTBI, neuronal cell death is usually a result of increased intracellular Ca2+ concentration, as this increases glutamate release across the synapse, activation of NMDA receptors, and thereby increasing Ca2+ concentration. This can further lead to increased enzymatic activity, specifically enzymes which can induce apoptosis. The ability of phytocannabinoids and endocannabinoids to inhibit N- and P/Q-type calcium channelsReference Twitchell, Brown and Mackie194 and the ability of CBD to induce a hyperpolarizing shift in the steady state inactivation potentials of the T-type calcium channelsReference Ross, Napier and Connor195 imply that there is potential for CBD to aid in neurogenesis and protection against neurodegenerative processes.
Endocannabinoids are known to be produced by neural progenitor cells which can stimulate proliferation at the hippocampal and subventricular zones via CB1 receptors, as documented by neurosphere generation.Reference Galve-Roperh, Aguado, Palazuelos and Guzman196 CBD has been suggested to have a restorative effect on regions of the hippocampus which may be damaged due to prolonged cannabis use.Reference Beale, Broyd and Chye197 Furthermore, mice with knocked-out fatty acid amide hydrolase enzyme express a higher concentration of 2-AG, which has been shown to induce astrogliogenesis.Reference Aguado, Palazuelos and Monory198 The mechanism behind CBD’s ability to regulate neurogenesis seems to involve the mitogen-activated protein kinase (MAPK) pathway, activation of which allows neural progenitor cell proliferation, whereas the CB1 receptor -mediated inhibition of the proliferation is due to the attenuation of sustained MAPK activity.Reference Galve-Roperh, Aguado, Palazuelos and Guzman196,Reference Rueda, Navarro, Martinez-Serrano, Guzman and Galve-Roperh199 These results suggest that CBD and the endocannabinoids themselves can be key compounds in neurogenesis.
Gaps and Limitations in the Literature
While there is strong preclinical and basic science evidence to show that CBD has the potential to be a candidate for concussion treatment, it is important to note that no double-blind, randomized controlled trials have been completed to show CBD as an efficacious medication following these mTBIs. As our review suggests, there is substantial evidence shown through rodent models which does imply that there are benefits for administration of CBD for concussion recovery. Following this note, there is a clinical trial currently in the recruitment phase (Identifier number: NCT03826368) utilizing CBD as a dietary supplement to aid in recovery from brain injury in humans. Pharmacological treatment for concussion and post-concussion syndrome has been a persistent issue because of the complex nature of the injury,Reference Giza and Hovda63 underreporting of the injury,Reference Wallace, Covassin, Nogle, Gould and Kovan200 and the absence of common physiological impairments or symptomology.Reference McGeown, Hume, Kara, Neary and Gardner3 The exact pathophysiology of concussion is still being researched, and as such there may be effects of CBD upon concussion which can potentially go unnoticed. Regardless, the presented research does provide a strong framework for future studies on the therapeutic evaluation of CBD on concussion.
Conclusion
This review presents research to suggest the potentially beneficial effects of CBD in treatment following mTBI such as concussion. CBD appears to regulate ionic balance, act as an anti-neuroinflammatory, attenuate dopaminergic pathway damage, and suppress the impairments of cerebral blood flow, heart rate variability, and the blood brain barrier. The general consensus for treatment following concussion is continuing to evolve,Reference McCrory, Meeuwisse and Dvorak201 as the exact pathophysiology is not yet understood. Because there are many physiological consequences that arise due to head trauma, a single remedy to treat all impairments seems like a monumental task without assuming a poly-prescription approach, which may result in a large amount of unwanted side effects. Although direct human research and double-blind randomized controlled trials are still lacking, there is much preclinical evidence and theoretical framework available to support the idea of using CBD following concussion due to its neuroprotective properties.
Acknowledgements
The authors would like to thank all those who contributed to the ideas presented in this review.
Disclosures
The authors report no conflicts of interest.
Statement of Authorship
JS and JPN conceived and revised this paper.




