The elderly population, which has grown recently, requires adequate and sufficient nutrition to maintain functional capacity, which in turn enables them to live an independent life within their own family and community( 1 , Reference Solomon 2 ). Many elderly people today have vitamin and mineral deficiencies caused by low absorption due to the fact that stomach acid secretion slows down in people as they age. Moreover, after the age of 60 years, people tend to eat less( 1 ) because they need less energy. As a result, their vitamin intake through food drops. Many other factors also affect blood nutrient levels in older people. For example, poor dentition prevents them from eating fresh fruits and vegetables plus meats that are rich in vitamins. Instead, many eat vitamin-deficient foods. Loss of a spouse, loneliness, limited mobility and social isolation also affect nutrient intake in elderly people( 1 ).
To maintain a high quality of life in people of advancing age, it is essential to regularly assess several biochemical variables because of their importance in healthy ageing( Reference Johnson and Park 3 – Reference Drewnowski and Shultz 7 ). Poor nutritional status has been associated with age-related declines in renal function, fluid imbalances, poor hydration and long-term chronic illnesses( Reference Ismail 4 , Reference Charlton, Kruger and Labadarios 6 , Reference Drewnowski and Shultz 7 ).
In developing countries, elderly people have been reported to have an inadequate intake of micronutrients such as vitamins A, B1, B2 and C( Reference Johnson and Park 3 , Reference Drewnowski and Shultz 7 – Reference Morley 9 ). Nutritional assessments performed by Ismail in Africa revealed that the diet of the elderly is generally inadequate in terms of quality and quantity( Reference Ismail 4 ). In Nigeria, like other countries in Africa with a similar socio-economic status, the elderly often suffer from poor health. While little is understood about the nutritional status of elderly people in Nigeria, it is likely that food intake is inadequate( Reference Olayiwola and Ketiku 10 ). As mentioned above, low food intake increases the risk of micronutrient deficiencies, especially when the micronutrient density and/or bioavailability in food are low, which is often the case for diets in developing countries. A factor complicating the nutritional status of elderly people in developing countries is that many experienced inadequate food intake during much of their childhood and adult life.
Since population growth projections indicate that the elderly population in Nigeria will double by the year 2015( Reference Olayiwola and Ketiku 10 ), it is important to understand the current nutritional status of this population. To address this, the present study measured serum micronutrient levels and dietary nutrient intake in a cohort of elderly Yoruba people living in an urban area of Oyo State, which is in the south-western part of Nigeria.
Materials and methods
Study design
The present cross-sectional study was carried out in an urban area of Oyo State, in Idikan community of Ibadan, North-West local government area. It is the most populated Yoruba-speaking state in the south-western region of Nigeria and is inhabited largely by the Yoruba tribe( Reference Olayiwola and Ketiku 10 ), whose occupations range from large-scale business to petty trading. Some are involved in agricultural practices, such as crop vegetable farming (especially yams, cassava, maize and plantains) and raising poultry and livestock, most of which are small ruminant animals.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Review Committee of the University of Ibadan and the University College Hospital, conforming to the international guidelines of the ethical review of epidemiological studies( 11 ). Informed verbal consent was obtained from all participants with witness and formally recorded.
Determination of sample size
The sample size was determined using the formula( Reference Gibson 12 ):
where N is the sample size, Z is the standard normal variable for a 95 % confidence level, p is the prevalence of the attribute (using a value of p for underweight of 15 % according to Olayiwola and Ketiku( Reference Olayiwola and Ketiku 10 )), q is 1–p, and d is precision (= 0·05). The sample size for the present study was calculated to be:
Thus, to estimate the prevalence of poorly nourished elderly with 95 % confidence and a precision of 5 %, a total of 196 elderly people were needed. Another 20 % was added to account for non-responses( Reference Asika 13 ), yielding a value of 234. This was rounded up to 240.
Target population
The participants lived in an urban area of Ibadan which is characterized by poor socio-economic status and low levels of education. The main occupation was petty trading. The participants were selected randomly from Idikan, the study area. All were at least 60 years of age. Persons who had a serious illness, or who were immobile or institutionalized, were excluded.
Sampling procedure
The 240 participants were selected using a random sampling procedure. Ten wards (where elderly resided) in Idikan community area of Ibadan were used in the study. In each ward, households were randomly selected until twenty-four households where elderly gave consent for the study were obtained. To be eligible for inclusion in the study, each prospective participant must have resided at the study location for at least 5 years. To contact the 240 participants, the authors made home visits with the assistance of local aides. In each household, the oldest person (male or female) was selected. If there was more than one elderly resident and they had the same age, a ballot was used to select one participant. Verbal and written consent was obtained from each participant prior to starting the study. All participants took part after the health benefits (such as more information about what foods they should eat to be in good health) of participating in the study were explained. Despite all efforts, only half of the cohort (50 %, i.e. 120 participants) agreed to donate blood for further analysis.
Data collection
Three data collection instruments were used: (i) a questionnaire about sociodemographic characteristics and health status; (ii) a questionnaire about dietary intake and direct weighing of food intake; and (iii) a biochemical assessment of blood samples.
Questionnaire about sociodemographic characteristics and health status
A questionnaire asking about the sociodemographic characteristics and health status of the participants was designed for use in the present study. Cronbach's α coefficient was 0·80.
Questionnaire about dietary intake and direct weighing of food intake
The food that was consumed by the participants was determined during visits made by the authors and other field assistants at meal times on three consecutive days. The direct weighing of food technique was used to estimate food intake( Reference Gibson 12 ). Thus, on each visit, the food that the participant was about to eat was weighed using a Salter scale and the local name of the food item was entered into the coded record form. On each visit, the participant was also asked what other foods had been eaten since the last visit( Reference Gibson 12 ). These foods were usually consumed between meals and outside the home in the form of snacks or food purchased from food vendors. The equivalent portions of these foods were purchased, weighed and the foods were entered into the coded record form of the respondent. Food composition tables for Africa of the FAO and the Total Dietary Assessment software (www.nutridata.com) were used to determine the nutrient levels in these foods. A major limitation was that extrapolated nutrient requirements of adults over 50 years of age from the FAO/WHO were used to estimate the participants’ nutrient adequacy in the present study since a specific reference for the elderly is yet to be carried out.
Biochemical assessment of blood samples
All biochemical analyses followed the official analysis methods of the Association of Official Analytical Chemists, Washington, DC, USA. Thus, in the morning, after an overnight fast, venous blood (10 ml) was collected from the participants using a sterile needle and syringe( 14 ). The blood specimens were then immediately placed in a dark, cool box (ice pack) and driven to the chemical pathology laboratory of the University College of Medicine in Ibadan (a journey time of under 3 h). Serum and plasma specimens were obtained by centrifugation of the blood, after which the samples were stored at −80°C until analysis( Reference Gibson 12 ).
Retinol and vitamin D levels were analysed by HPLC( Reference Gibson 12 ) while vitamin B6 levels were determined by HPLC with fluorescence detection( Reference Asika 13 ). Serum Ca and albumin levels were analysed using an automated analyser (Roche/Hitachi automated analyser 902, Germany). The automated technique was based on the colorimetric method( 14 ). Zn levels were determined by atomic absorption spectrophotometry( 14 ). The vitamin C levels were analysed by HPLC( Reference Gibson 12 ) and ferritin was determined using a colorimetric method( 14 ).
The following cut-off points indicated deficiency: vitamin A (retinol) <30 μg/dl; vitamin B6 <20 nmol/l; vitamin C <0·55 g/dl; vitamin D (25-hydroxyvitamin D) <10 mg/ml; Ca <8·6 mg/dl; Zn <30 μg/dl; serum ferritin <0·8 g/dl; serum albumin <3·9 g/dl( Reference Gibson 12 ).
Statistical analyses
All data were analysed using Microsoft® Excel and SPSS version 16 for simple and inferential statistics( Reference Asika 13 ). The Pearson product moment correlation was used to find out significant relationships among variables such as nutrient intakes, serum status and sociodemographic characteristics.
Results
Sociodemographic characteristics of the study population
The sociodemographic characteristics of the sampled population revealed that 52·5 % of the participants were females while 47·5 % were males. The minimum age was 60 years and the maximum age was 96 years. Family structures divulged that 100 households had monogamous couples and 140 households had polygamous couples. Of the participants, 62·5 % did not have any formal education and only 13·3 % had primary education. In this population, 86·7 % were of low socio-economic status. Only 11·7 % of the participants categorized themselves as having a poor health status. All 240 participants completed the questionnaire fully.
Mean intakes of energy, vitamins and minerals
The usual dietary intake of the 240 participants was assessed for the selected nutrients in Table 1. The energy intake of males and females ranged from 6136 to 8368 kJ/d; this was 79 % and 80 % of the Estimated Average Requirement (EAR), respectively. Mean intakes of the selected vitamins were also inadequate. The vitamin A intake of males and females was in the range of 147–535 μg/d, which is only 42 % and 49 % of the EAR, respectively. Vitamin B6 intake of males and females varied from 0·10 to 0·92 mg/d. Intakes of vitamins B1, B2, B12, C and folic acid were also deficient, being less than 55 % of the requirement for both sexes.
Table 1 Dietary adequacy of the study population: elderly Yoruba people (n 240) living in a slum of Ibadan, Nigeria
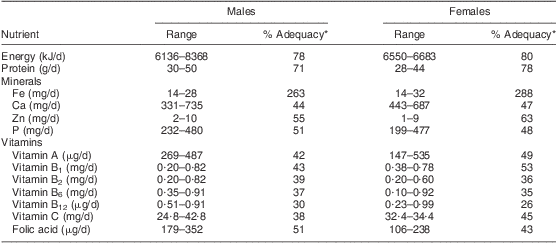
*Relative to the Estimated Average Requirement( 37 ).
As shown in Table 1, the mineral intake was poor in general. For males and females, the Zn intake varied from 1 to 10 mg/d, which was 55 % and 63 % of the EAR, respectively. The participants’ Ca intake was in the range of 331–735 mg/d (44 % and 47 % of the EAR for males and females, respectively), while their P intake ranged from 199 to 480 mg/d. Only dietary Fe intake was adequate.
Frequency of vitamin and mineral deficiencies
In total 120 participants agreed to give blood. Their blood was analysed biochemically and the levels of various nutrients and micronutrients are presented in Table 2. When 8·6 mg/dl served as the cut-off point, Ca deficiency was detected in 13 % of males and 60 % of females (40 % of the whole cohort). Thus, females were more likely to be Ca-deficient than the males. Zn deficiency occurred in 71 % of the whole cohort, with males and females showing similar rates of deficiency (73 % and 70 %). With regard to serum vitamin A (retinol) levels, when 30 μg/dl served as the cut-off point, 23 % of the elderly participants were within the marginal range; the remaining 77 % were within the normal range. Vitamin B6 deficiency was observed in 69 % of the whole cohort. Vitamin D (25-hydroxyvitamin D) deficiency occurred in 51 %. Half of the participants were deficient in vitamin C. Also, serum ferritin fell below 0·8 g/dl in 78 % of the elderly people.
Table 2 Percentage of the study population with deficient serum levels of micronutrients: elderly Yoruba people (n 120) living in a slum of Ibadan, Nigeria

VAD, vitamin A deficiency; 25(OH)D, 25-hydroxyvitamin D.
*Frequencies are presented as percentages of male, female or total population.
†The reference values of micronutrients are shown in parentheses.
‡Significant difference between males and females (P < 0·05).
§No significant difference between males and females (P > 0·05).
Relationships among variables
The results of Pearson product moment correlations of sociodemographic, dietary and micronutrient status variables are shown in Table 3. There was a significant relationship between Ca intake and Fe intake (r = 0·65; P < 0·05), serum vitamin C and serum Fe (r = 0·76; P < 0·05) and socio-economic status and micronutrient intake (r = 0·40; P < 0·05). There were no significant relationships between serum vitamin B1, B2 and B12 status and the dietary intakes of these vitamins.
Table 3 Significant (P < 0·05) Pearson moment correlation coefficients among the study population: elderly Yoruba people (n 240) living in a slum of Ibadan, Nigeria
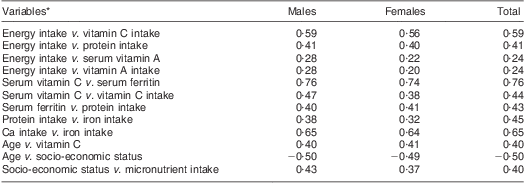
*The serum levels of vitamins B1, B6 and B12 did not show a significant relationship with the actual dietary intake of these vitamins (P > 0·05).
Discussion
The present study examined the nutritional status of apparently healthy elderly people belonging to the Yoruba tribe who lived in a slum in south-western Nigeria. That these people were healthy is indicated by the fact that all had normal serum albumin levels( Reference Gibson 12 ). Serum albumin is the most common predictor of nutrition and health status in elderly people( Reference Carl, Edward and David 15 , Reference Baumgartner, Koehler and Romero 16 ) as it can indicate the presence of nutritional anaemia and inadequate protein nourishment( Reference Johnson and Park 3 , Reference Baumgartner, Koehler and Romero 16 , Reference Sandstead, Henriksen and Greger 17 ). It is rare to find low serum albumin levels in elderly persons if they are healthy and free from diseases such as cancer, renal or hepatic diseases, or medication that may interfere with hepatic functions( Reference Baumgartner, Koehler and Romero 16 ). However, despite normal serum albumin levels, the sera of the participants were deficient in almost all of the minerals and vitamins measured.
For example, about three-quarters of the participants showed low serum Zn levels. Zn participates in many physiological functions in the elderly, including protein metabolism, immune functions, wound healing, neurosensory functions such as taste, and membrane stability( 1 , Reference Sandstead, Henriksen and Greger 17 ). Nutrition that yields adequate Zn levels significantly improves the health of elderly people( Reference Sandstead, Henriksen and Greger 17 , Reference King 18 ). Factors that contribute to Zn deficiency are mainly the amount and bioavailability of dietary Zn( Reference Sandstead, Henriksen and Greger 17 , Reference King 18 ). This is supported by the present study which showed that the food eaten by the participants was low in Zn.
The elderly participants were also deficient in serum vitamin C. This is troubling because this vitamin cannot be stored in the body. Vitamin C is associated with many metabolic functions in elderly people: it reduces cholesterol levels, lowers the risk of risk of CHD and hypertension, and has a protective role in the body( Reference Johnson and Park 3 , Reference King 18 – Reference Bowman and Russell 20 ).
About 40 % and 51 % respectively of the elderly participants had low serum Ca and vitamin D. Normal serum vitamin D levels are needed for bone health, good Ca absorption and the deposition of Ca and P into the bones( Reference Chandra 21 – Reference Brown, Finch and Slatopolsky 23 ). Moreover, epidemiological studies suggest that high circulating levels of 1,25-dihydroxyvitamin D may decrease the risk of developing prostate cancer( Reference Burckhardt 24 , Reference Brock, Graubard and Fraser 25 ).
The low serum levels of vitamins and minerals observed in the majority of the elderly participants in the present study were largely owing to their poor dietary intake of these micronutrients. The low vitamin consumption is likely due to the participants’ type of diet and their methods of cooking (as observed during the direct weighing of food intake). The cooking methods involve soaking, blanching, use of cooking soda and long cooking in a large volume of water which is later discarded; thus there is a loss of water-soluble vitamins, leading to low intake. Low consumption of vitamins B6, D and C has also been reported in some other studies( Reference Ismail 4 , Reference Bowman and Russell 20 , Reference Grabow and Linkswiler 26 ).
Analysis of the foods consumed by the elderly participants revealed that the intakes of energy, protein, vitamins and all minerals (apart from Fe) were lower than the EAR in both males and females (Table 1). The reason for the low energy intake may relate to the quantity and form in which the foods were consumed. Some of the participants consumed only soft foods in their diet, such as pap (fermented cereal), and then in only small quantities. Some of the participants also reported taking daily medications (75·5 %) that affected their appetite and thereby reduced food intake. Such food–medication interactions that affect food intake were reported previously by Morley( Reference Morley 9 ). An additional reason for the observed low energy intake was the low fat intake, which ranged from 21 to 39 g/d. This has also been observed in several studies on food consumption in African countries, including Nigeria( 27 – Reference de Groot, van den Broek and van Staveren 31 ). Moreover, Olayiwola and Ketiku documented low energy and protein intakes by elderly people in some parts of Nigeria( Reference Olayiwola and Ketiku 10 ). Similarly, lower energy intake by the elderly was recorded in Switzerland and Canada( Reference Aghdassi, McArthur and Liu 32 – Reference Davis, Murphy and Neuhaus 35 ).
The poor intake of vitamin B by the elderly Yoruba people may be due primarily to the prominent consumption of roots and tubers; little consumption of legumes, such as cooked beans, was recorded. Some elderly people have food preferences that cause them to avoid certain foods, thus potentially reducing variety and diversity in the diet. The low intake of vitamins is not limited to elderly Yoruba people, but is also found in elderly in other countries. The FAO/WHO( 27 ) and the US National Research Council( 28 ) both recommend that adults and elderly people maintain adequate intakes of thiamin, pyridoxine, biotin and vitamin A. Contrary to the commonly held belief that vitamin B6 is important only for infants, past studies demonstrate the importance of vitamin B6 in elderly people, as it plays a role in various metabolic reactions most especially in neurotransmitter release( 1 , Reference Johnson and Park 3 , Reference Grabow and Linkswiler 26 ). The nutritional implications of low intakes and low serum levels of vitamins in the elderly are significant. Many nutrition researchers have found that vitamins (retinol, cholecalciferol and ascorbic acid) are protective against oxidative stress and some chronic diseases( Reference de Groot, van den Broek and van Staveren 31 – Reference Dumartheray, Krieg and Cornuz 34 ).
Statistical analysis identified a positive correlation between energy intake and serum vitamin A for both males (r = 0·28, P < 0·05) and females (r = 0·22, P < 0·05; Table 3).
With regard to mineral intake, the intake of Fe by the elderly Yoruba participants was higher than the required estimate( 27 ). However, the elderly Yorubas had lower Ca, Zn and P intakes than their counterparts in developed countries( Reference Dumartheray, Krieg and Cornuz 34 ). Indeed, a study in Toronto found that 50 % of the surveyed elderly persons had suboptimal intakes of Ca, P, Zn and Mg, and a mean daily energy intake of 6330 (sd 3611) kJ (1513 (sd 863) kcal)( Reference Aghdassi, McArthur and Liu 32 ). In the present study the majority of foods consumed by the elderly were from plants whose micronutrients can be hindered by the presence of phytates, oxalates and the method of food preparation( Reference Bowman and Russell 20 ); in addition to decreased energy and protein intake( Reference Johnson and Park 3 , Reference Bowman and Russell 20 , Reference Aghdassi, McArthur and Liu 32 , Reference Campbell 33 ).
Other factors that influence the biochemical and nutritional status of elderly people include food intake, economic situation and health status. Food intake reflects variables that directly affect food preparation, such as regularity of cooking and the availability of fuel and cooking equipment. In the present study, 20 % of the participants lacked effective cooking facilities.
Inadequate food intake has an enormous effect on nutritional status as it can reduce the supply of energy, protein, vitamins, minerals, water and other health-beneficial substances such as Zn, Ca, Fe, vitamin A and the B vitamins in the body( Reference Ismail 4 , Reference Campbell 33 , Reference Wright, Southon and Bailey 36 ). Therefore, the poor micronutrient status of the present study is likely due to multiple factors( 37 ), with poor dietary intake (as indicated by the food intake measurements) possibly playing a major role.
In addition, high dietary oxalate and phytate levels and nutrient–nutrient interactions can also affect the availability of minerals( Reference Charlton, Kruger and Labadarios 6 , Reference Asika 13 , Reference Brock, Graubard and Fraser 25 , Reference de Groot, van den Broek and van Staveren 31 , Reference Aghdassi, McArthur and Liu 32 , Reference Dumartheray, Krieg and Cornuz 34 , Reference Davis, Murphy and Neuhaus 35 ).
To overcome the poor availability of micronutrients, elderly people may have to diversify their diet and take supplements. This may be true even for vitamin D. Although vitamin D is generally obtained directly from sunlight( Reference Johnson and Park 3 , Reference Asika 13 , Reference Aghdassi, McArthur and Liu 32 ), the fact that 50 % of the elderly Yorubas were deficient suggests that their exposure to sunlight is also not adequate.
Conclusions and recommendations
The results of the present study show that elderly Yoruba people appeared quite malnourished, particularly with regard to their serum retinol, vitamin C and vitamin B6 levels. Their serum Ca and Zn status was also poor. These elderly Yoruba people had a less favourable micronutrient status than their counterparts in developed countries, and their dietary intake was lower( Reference Fabian and Elmadfa 38 ). The eventual consequences of these deficiencies should be investigated further. In the meantime, there is clearly a need for specific intervention programmes for the elderly in Nigeria to improve their nutritional status through dietary diversification and good food preparation practices.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector. Conflict of interest: The authors have no conflict of interest to declare on any part of this study. Authors’ contributions: I.O.O. contributed to the research design, data collection, data analysis and write up. G.T.F. contributed to the data collection, data analysis and write up. S.O.A. contributed to the data collection, data analysis and write up. D.O.S. contributed to the data collection and analysis.





