Lung cancer is the most common cause of death in men and second only to breast cancer in women. It is responsible for 1·38 million deaths annually(Reference Ferlay, Shin and Bray1). Non-small cell lung cancer (NSCLC) accounts for approximately 80–85 % of all cases of lung cancer. About 40 % of patients with NSCLC present at an advanced stage, with metastatic or locally advanced disease, which underscores the importance of identifying therapeutic schemes that may benefit this large patient population. Combination chemotherapy, usually platinum-based, is currently the first-line therapy of choice; however, the prognosis for patients with advanced NSCLC remains poor with a median survival time of 8–11 months, a 1-year survival rate of 30–45 %, and a 5-year survival rate < 5 %(Reference Rudd, Gower and Spiro2, Reference Martoni, Marino and Sperandi3). The treatment of NSCLC is therefore a major unmet need, and development of novel anti-cancer drugs is urgently needed.
Cruciferous vegetables have been widely accepted as potential diet components that may decrease the risk of cancer(Reference Lam, Gallicchio and Lindsley4). Isothiocyanates are abundant in cruciferous vegetables such as broccoli, watercress and Brussels sprouts. They have recently been of intense interest for their anti-carcinogenic activities and potential use in the chemoprevention of cancer. Chemopreventive activity is thought to be associated with the inhibition of the metabolic activation of carcinogens by phase I enzymes, cytochrome P450 isozymes and increased excretion of carcinogens by inducing increased activities of phase II enzymes, quinone reductase and GSH S-transferases(Reference Wu, Zhou and Xu5). A further feature of the pharmacological activity of isothiocyanates such as phenethyl isothiocyanate (PEITC), benzyl isothiocyanate (BITC) and sulforaphane is their anti-cancer activity. They inhibit several types of cancer cell growth, such as leukaemia(Reference Xu and Thornalley6, Reference Xu and Thornalley7), prostate cancer(Reference Gong, He and Krishna Vanaja8), breast cancer(Reference Kang, Ding and Wang9), lung cancer(Reference Mi, Gan and Cheema10), cervical cancer(Reference Mukherjee, Dey and Bhattacharya11), colorectal cancer(Reference Prawan, Saw and Khor12), etc.
Recent studies have revealed that isothiocyanates have anti-angiogenic and anti-metastatic effects. Isothiocyanates inhibited tumour-specific angiogenesis by down-regulating nitric oxide, TNF-α and proinflammatory cytokine production, and by the inactivation of Akt(Reference Xiao and Singh13–Reference Thejass and Kuttan15). Isothiocyanates also suppressed the metastasis potential of human hepatoma cells(Reference Hwang and Lee16), colon cancer cells(Reference Lai, Huang and Hsu17) and breast cancer cells(Reference Hunakova, Sedlakova and Cholujova18). These effects are mediated by decreasing the expression of matrix metalloproteinases, proinflammatory cytokines, growth factors such as vascular endothelial growth factor, transcription factor twist, and by increasing the expression of tissue inhibitors of matrix metalloproteinase. In our previous study, by using a highly metastatic human large cell lung cancer cell line L9981, we showed that BITC and PEITC suppressed the metastasis potential of NSCLC by the modulation of metastasis-related gene expression, inhibition of Akt/NF-κB pathway and induction of oxidative stress(Reference Wu, Zhu and Yan19).
In the present study, we further investigate the effect of BITC and PEITC (Fig. 1) on highly metastatic L9981 cell growth, including the induction of apoptosis and cell cycle arrest and regulation of the mitogen-activated protein kinase (MAPK) signalling pathway. Moreover, to explore potential molecular targets, we examined their effects on global gene expression profile.
Fig. 1 Structures of (a) benzyl isothiocyanate and (b) phenethyl isothiocyanate.
Materials and methods
Materials
PEITC, BITC, N-acetyl cysteine (NAC), RNase A and propidium iodide were purchased from Sigma-Aldrich Inc. (St Louis, MO, USA). Mouse monoclonal antibodies against cyclin D1, cyclin B1 and rabbit polyclonal antibodies against p-c-jun N-terminal kinase (JNK), p-p38, p-extracellular signal-regulated protein kinase (ERK1/2) were purchased from Cell Signaling (Beverly, MA, USA); mouse monoclonal antibody against β-actin was purchased from Sigma; secondary antibodies coupled to HRP were purchased from ZSGB-BIO (Beijing, China). TRIzol was purchased from Invitrogen (Carlsbad, CA, USA); reverse transcription and real-time PCR kits were purchased from TaKaRa Biotechnology Company (Dalian, China). An RNeasy kit was purchased from Qiagen (Valencia, CA, USA). Microarray GeneChips were purchased from Affymetrix, Inc. (Santa Clara, CA, USA). pAP-1-luc was purchased from Clontech (Mountain View, CA, USA); pRL-SV40 was purchased from Promega (Madison, WI, USA).
Cell culture
The highly metastatic human lung cancer cell line L9981 was established from a human lung large cell carcinoma cell line (WCQH29801)(Reference Zhou, Wang and Che20). Cells were grown and maintained in the RPMI-1640 medium supplemented with 10 % fetal bovine serum, 2 mm-glutamine (GIBCO BRL, Grand Island, NY, USA) at 37°C and 5 % CO2. Penicillin and streptomycin were not added into the culture medium to avoid any cross-effect with isothiocyanates.
Apoptosis assay
The cells were incubated with PEITC or BITC for the indicated times. The percentage of cells undergoing apoptosis was determined by an Annexin V-FITC Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA), following the manufacturer's instructions. Briefly, cells were washed with PBS and re-suspended in a 1 × binding buffer at a concentration of 1 × 106 cell/ml. Annexin V-FITC and propidium iodide were added to cells and incubated for 15 min at room temperature in the dark. Then 1 × binding buffer was added and analysed on a FACSAria flow cytometer (Becton Dickenson, San Jose, CA, USA).
Cell cycle analysis
Cell cycle analysis was performed as described previously(Reference Xu and Thornalley7). Briefly, L9981 cells were incubated with PEITC or BITC for 24 h, sedimented by centrifugation (300 g, 5 min), washed with PBS, and the cell pellet was fixed by the addition of 70 % ethanol. The fixed cells were stored in the dark at 4°C overnight. The cell pellets were re-suspended in PBS, with 1 mg/ml of RNase A for 30 min at 37°C. The cells were then collected by centrifugation and re-suspended in 100 μm-propidium iodide in 0·1 % sodium citrate with 0·1 % Triton X-100 and transferred to a flow cytometric analysis tube. Flow cytometric analysis was performed on a FACSAria flow cytometer (Becton Dickenson). Data were analysed by ModFit LT software (Verity Software House, Topsham, ME, USA).
Western blotting analysis
Western blotting was performed as described previously(Reference Wu, Zhu and Yan19). Briefly, L9981 cells were incubated with PEITC or BITC for 24 h, washed with PBS, and the cell pellets were prepared in lysis buffer (20 mm-Tris (pH 7·5), 150 mm-NaCl, 1 % Triton X-100, sodium pyrophosphate, β-glycerophosphate, EDTA, Na3VO4, leupeptin). Lysates were electrophoresed on SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked for 1 h at room temperature with 5 % milk protein, 0·1 % Tween 20 in PBS, and then were probed with mouse anti-cyclin B1, cyclin D1 or rabbit anti-p-JNK, p-p38, p-ERK1/2 antibodies at 1:1000 dilution in 0·1 % Tween 20 in PBS with 5 % BSA overnight at 4°C. After washing, the membranes were probed with HRP-conjugated secondary antibodies at 1:5000 dilution in 0·1 % Tween 20 in PBS with 3 % milk protein for 1 h. The blots were developed with the Phototope HRP Western Blot Detection System (Cell Signaling).
DNA transfection
Transfection of L9981 cells was carried out using Lipofectamine 2000 (Invitrogen), following the manufacturer's instructions. Briefly, L9981 cells were plated in a twenty-four-well plate at 1 × 105 cells/well. The cells were co-transfected with 400 ng of pAP-1-luc, and 4 ng of pRL-SV40 served as an internal control. The cells were rested for 8 h after transfection and were then incubated with BITC or PEITC for 18 h. Luciferase assays were performed using the Dual-luciferase Reporter Assay System (Promega) following the manufacturer's instructions, on a BERTHOLD TriStar LB 941 microplate reader (Berthold Technologies, Bad Wildbad, Germany).
Reverse transcription
Total RNA was extracted from the cells using TRIzol (Invitrogen). Reverse transcription was performed as described previously(Reference Xu, Guidez and Glasow21) using the TaKaRa reverse transcription kit following the manufacturer's instructions, on a DNAEngine Peltier Thermal Cycler (Bio-Rad, Richmond, CA, USA). Briefly, RNA and random primers were denatured for 10 min at 70°C; then M-MLV reverse transcriptase, deoxynucleotide triphosphates, RNase inhibitor and reverse transcription buffer were added and incubated for 10 min at 30°C, 60 min at 42°C and 15 min at 70°C.
Real-time quantitative PCR
The primers were synthesised by SBS Genetech Company (Beijing, China). mRNA levels were detected by SYBR Green-based real-time PCR. PCR were performed as described previously(Reference Xu, Guidez and Glasow21). Briefly, PCR were performed in the following conditions: 10 s at 95°C, then forty cycles at 95°C for 5 s and 65°C for 34 s on an ABI Prism 7500 Sequence Detector System (Applied Biosystems, Foster City, CA, USA). The primers for the detected genes are listed in Table 1.
Table 1 Real-time PCR primer sequences
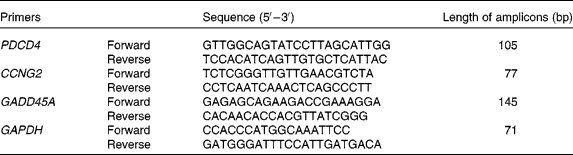
PDCD4, programmed cell death 4; CCNG2, cyclin G2; GADD45A, growth arrest and DNA damage-inducible α; GAPDH, glyceraldehyde-3-phoshate dehydrogenase.
Microarray analysis
L9981 cells were incubated with BITC for 24 h. Total RNA was extracted from the cells using an RNeasy kit (Qiagen), following the manufacturer's instructions. The quality of each RNA sample was determined on an Experion Automated Electrophoresis Station (Bio-Rad). Gene expression profiles were analysed by microarray experiments, using Affymetrix GeneChip Human Genome U133 Plus 2.0 (Affymetrix, Inc.), which contains more than 47 000 transcripts and variants. The targets were prepared according to Affymetrix Gene Chip 3′ IVT Express Kit protocol. Briefly, First-Strand cDNA was synthesised by reverse transcription and then Second-Strand cDNA was synthesised. The biotin-modified aRNA was synthesised by in vitro transcription and fragmented. Then the targets were hybridised to the GeneChip Human Genome U133 plus 2.0 Array.
Microarray data analysis
Raw data were acquired and later analysed using Affymetrix GeneChip Operating Software (GCOS) 1.4, scaled to a default target signal value of 500. Absolute and comparative analysis was performed using the Affymetrix MAS 5.0 algorithm in GCOS 1.4. Probe sets with increase/decrease calls, signal log ratio higher than 1 or lower than − 1 and change in P value lower than 0·05 were defined as significant probe sets and selected for further analysis. Additionally, calls of probe sets with higher signal values and with a detection P value lower than 0·05 must be present for single chip analysis. Annotations were analysed using a combination of interactive query at NetAffx (www.affymetrix.com) and R suite (annaffy package from the Bioconductor suite). Clustering analysis was done using MultiExperiment Viewer (MeV) 4.5.1 (J. Craig Venter Institute, Rockville, MD, USA; www.tigr.org). Hierarchical clustering analysis was done using Euclidean distance and average linkage clustering.
Statistical analysis
Data are presented as means and standard deviations. Variance analysis between groups was performed by one-way ANOVA, and significance of difference between control and treatment groups was analysed using Dunnett's multiple comparison test. The differences with P values < 0·05 were considered statistically significant.
Results
Benzyl isothiocyanate and phenethyl isothiocyanate induced apoptosis
Our previous study showed that both BITC and PEITC inhibited L9981 cell growth in a dose-dependent manner, and IC50 values were 5·0 and 9·7 μm, respectively(Reference Wu, Zhu and Yan19). In the present study, we found that BITC and PEITC induced L9981 cell apoptosis in a dose-dependent manner (Fig. 2(a)). After 24 h treatment, 53·1 and 70·0 % cells were apoptotic when treated with 7·5 and 10 μm of BITC (P < 0·001), respectively; and 17·7 % and 45·1 % cells were apoptotic when treated with 12·5 and 20 μm of PEITC (P < 0·001), respectively (Fig. 2(b) and (c)).

Fig. 2 Induction of apoptosis by benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC). L9981 cells were treated with 7·5–20 μm of BITC or PEITC for 24 h. For N-acetyl cysteine (NAC) protection, cells were pretreated with NAC (1 mm) for 1 h. (a) Dose–response of induction of apoptosis was determined by flow cytometry after Annexin V-FITC/propidium iodide staining. (b) The percentage of apoptotic cells induced by BITC. (c) The percentage of apoptotic cells induced by PEITC. (d) Caspase-3 activities determined by Western blotting. The samples were harvested, then lysed and subjected to SDS-PAGE, transferred to the membrane and blotted with the antibody against cleaved caspase-3. Values are means, with standard deviations of three independent experiments represented by vertical bars. * Mean values were significantly different (P < 0·001). Similar results were obtained in three independent experiments.
Numerous studies showed that isothiocyanates could induce reactive oxygen species (ROS) generation and caused oxidative stress. Our previous study also showed that BITC and PEITC elevated ROS level in L9981 cells(Reference Wu, Zhu and Yan19). Therefore, we investigated whether antioxidant NAC could protect cells from apoptosis. The cells were pretreated with 1 mm of NAC for 1 h before incubation with isothiocyanates. Flow cytometric analysis showed that apoptosis induced by both BITC and PEITC was effectively blocked (P < 0·001; Fig. 2(a)). Caspase-3 is an effector caspase, triggering the apoptotic process. We evaluated the role of caspase-3 in the apoptosis induction by isothiocyanates. The cells were treated with 10 μm of BITC or 20 μm of PEITC for 24 h, and the activation of caspase-3 was detected by Western blotting. Fig. 2(d) shows that caspase-3 was activated by both BITC and PEITC. Furthermore, pretreatment of cells with antioxidant NAC (1 mm) for 1 h abolished caspase-3 activation. This was consistent with our apoptosis analysis.
Benzyl isothiocyanate and phenethyl isothiocyanate-induced cell cycle arrest
Since the induction of apoptosis could not fully explain the reduction of cell growth, we determined whether BITC and PEITC lead to cell cycle arrest. The cells were treated with 7·5 μm of BITC or 12·5 μm of PEITC for 24 h, and the cell cycles were analysed by flow cytometry. Both BITC and PEITC significantly arrested cell cycle progression (Fig. 3(a)). Flow cytometric analysis showed that BITC reduced the G0/G1 phase from 68·0 to 45·1 % and increased G2/M phase from 11·9 to 31·2 % (P < 0·01); however, S phase was not affected, whereas PEITC reduced G0/G1 phase from 68·0 to 35·6 % and increased both S and G2/M phases, from 20·1 to 36·5 % and 11·9 % to 27·9 % (P < 0·01), respectively (Fig. 3(b)). These results demonstrated that BITC and PEITC arrested L9981 cell cycle progression; the cell cycles were arrested at the G2/M phase.

Fig. 3 Effect of benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC) on cell cycle. L9981 cells were treated with 7·5 μm of BITC or 12·5 μm of PEITC for 24 h. (a) DNA content was determined by flow cytometry after fixation with ethanol and staining the cells with propidium iodide ![]() , G0/G1;
, G0/G1; ![]() , S;
, S; ![]() , G2/M. (b) The percentage of cell cycle phases (
, G2/M. (b) The percentage of cell cycle phases (![]() , G2/M;
, G2/M; ![]() , S;
, S; ![]() , G0/G1) was analysed by ModFit LT software. (c) Cyclin B1 expression level was determined by Western blotting. The samples were harvested, then lysed and subjected to SDS-PAGE, transferred to a membrane and blotted with the antibody against cyclin B1. Values are means, with standard deviations of three independent experiments represented by vertical bars. * Mean values were significantly different (P < 0·01). Similar results were obtained in three independent experiments.
, G0/G1) was analysed by ModFit LT software. (c) Cyclin B1 expression level was determined by Western blotting. The samples were harvested, then lysed and subjected to SDS-PAGE, transferred to a membrane and blotted with the antibody against cyclin B1. Values are means, with standard deviations of three independent experiments represented by vertical bars. * Mean values were significantly different (P < 0·01). Similar results were obtained in three independent experiments.
To investigate whether cell cycle arrest at the G2/M phase was mediated by cyclins, we examined the effects of isothiocyanates on cyclin expression. The cdc2-cyclin B1 kinase activity is pivotal in regulating the G2/M transition; therefore, we detected cyclin B1 protein expression level by Western blotting. Our data showed that cyclin B1 expression was reduced by BITC and PEITC in a dose-dependent manner (Fig. 3(c)). This suggested that cyclin B1 down-regulation may play a role in isothiocyanate induced G2/M phase arrest.
Benzyl isothiocyanate and phenethyl isothiocyanate induced phosphorylation of mitogen-activated protein kinases
There are a few signalling pathways involved in the induction of apoptosis; one of them is the MAPK pathway. MAPK respond to extracellular stimuli and regulate various cellular activities, such as gene expression, mitosis, differentiation, proliferation and cell survival/apoptosis. To understand the role of the MAPK pathway in apoptosis induced by BITC and PEITC in L9981 cells, the activation/phosphorylation of MAPK was investigated by Western blotting. As shown in Fig. 4, 24 h treatment of L9981 cells by PEITC resulted in a strong and dose-dependent activation of three MAPK (JNK, ERK1/2 and p38). It was found that 20 μm of PEITC were more potent than 12·5 μm of PEITC. BITC also activated JNK, ERK1/2 and p38; however, it was not in a linear dose-dependent manner. It was found that 7·5 μm of BITC were more potent than 10 μm of BITC. Pretreatment with 1 mm of NAC attenuated the activation of MAPK by both BITC and PEITC.

Fig. 4 Effect of isothiocyanates on mitogen-activated protein kinase (MAPK) phosphorylation. L9981 cells were treated with 7·5–20 μm of benzyl isothiocyanate (BITC) or phenethyl isothiocyanate (PEITC) for 24 h. For N-acetyl cysteine (NAC) protection, cells were pretreated with NAC (1 mm) for 1 h. Samples were harvested, then lysed and subjected to SDS-PAGE, transferred to membrane and blotted with the antibodies against phospho-c-Jun N-terminal kinase (p-JNK), phospho-extracellular signal-regulated protein kinase (p-ERK1/2) and phospho-p38. Similar results were obtained in three independent experiments.
Benzyl isothiocyanate and phenethyl isothiocyanate repressed activator protein 1 transcriptional activation
Following the findings that isothiocyanates activated MAPK, we further examined their effects on the transcriptional factor AP-1, a downstream target of MAPK. AP-1 controls a number of cellular processes including differentiation, proliferation and apoptosis. In the present study, we investigated the effects of BITC and PEITC on AP-1 transcriptional activation, by luciferase reporter assay. As shown in Fig. 5(a), both BITC and PEITC inhibited the transcriptional activation of AP-1 in a dose-dependent manner. After treatments for 18 h, 12·5 and 20 μm of PEITC significantly inhibited the transcriptional activity of AP-1 to 75·9 % (P < 0·05) and 18·2 % (P < 0·001) of control, respectively. Similar to PEITC, 7·5 and 10 μm of BITC significantly inhibited the transcriptional activity of AP-1 to 18·8 % (P < 0·001) and 7·4 % (P < 0·001) of control, respectively. We further investigated the protective effect of antioxidant NAC. Pretreatment with antioxidant NAC (1 mm) for 1 h attenuated the inhibitory effect of BITC and PEITC on AP-1 transcriptional activation (P < 0·05).

Fig. 5 Effect of isothiocyanates on activator protein 1 (AP-1) transcriptional activation. (a) L9981 cells were transfected with pAP-1, and pRL-SV40 served as an internal control. Then the cells were treated with 7·5–20 μm of benzyl isothiocyanate (BITC) or phenethyl isothiocyanate (PEITC) for 18 h. For N-acetyl cysteine (NAC) protection, cells were pretreated with NAC (1 mm) for 1 h. AP-1 transcriptional activation was detected by luciferase reporter assay. ![]() , − NAC;
, − NAC; ![]() ,+NAC. (b) L9981 cells were treated with 7·5–20 μm of BITC or PEITC for 24 h. Samples were harvested, then lysed and subjected to SDS-PAGE, transferred to membrane and blotted with the antibody against cyclin D1. Values are means, with standard deviations of three independent experiments represented by vertical bars. Mean values were significantly different: *P < 0·05, **P < 0·01 and ***P < 0·001. Similar results were obtained in three independent experiments.
,+NAC. (b) L9981 cells were treated with 7·5–20 μm of BITC or PEITC for 24 h. Samples were harvested, then lysed and subjected to SDS-PAGE, transferred to membrane and blotted with the antibody against cyclin D1. Values are means, with standard deviations of three independent experiments represented by vertical bars. Mean values were significantly different: *P < 0·05, **P < 0·01 and ***P < 0·001. Similar results were obtained in three independent experiments.
To examine the effects of isothiocyanates on AP-1-regulated genes, we next detected the expression level of cyclin D1 in L9981 cells after treatments with BITC and PEITC. Cyclin D1 has been known to be an endogenous gene that is under the control of AP-1 and plays an important role in cell proliferation. Western blotting results showed that cyclin D1 levels were reduced by both BITC and PEITC, in a dose-dependent manner (Fig. 5(b)). This was well correlated with AP-1 transactivation result.
Effect of benzyl isothiocyanate on gene expression profile
To further detail the global gene transcriptional changes induced by isothiocyanate and explore the potential targeted genes, high-density microarrays were performed. As BITC was more effective than PEITC, we examined L9981 cells treated with 10 μm of BITC for 24 h using Affymetrix GeneChip. After a data normalisation procedure, a total of 1305 genes were found to be differentially expressed. Among these genes, 1166 genes were up-regulated and 139 genes were down-regulated.
Based on the present findings that iosthiocyanates induced L9981 cell apoptosis and cell cycle arrest, we further evaluated the potential targeted genes related to apoptosis and cell cycle in array data. For apoptosis-related genes, a total of eighty genes were regulated by BITC; seventy-seven of them were up-regulated and three of them were down-regulated. For genes involved in the cell cycle, fifty-seven genes were regulated by BITC; fifty-two of them were up-regulated and five of them were down-regulated. Fig. 6 illustrates the hierarchical clustering analysis of differentially expressed genes. Regulated genes are listed in Tables 2 and 3. Expression profiles of selected genes PDCD4, CCNG2 and GADD45A were then confirmed using real-time PCR. Using RNA isolated from a different experiment, real-time PCR analysis generated data highly similar to that derived from the microarray (Figs. 6 and 7, Table 3).

Fig. 6 Effect of benzyl isothiocyanate (BITC) on the gene expression profile. L9981 cells were treated with 20 μm of BITC for 24 h. Sample preparation and microarray analysis were described in Materials and Methods. (a) Hierarchical clustering of total genes differentially expressed. (b) Hierarchical clustering of apoptosis-related genes differentially expressed. (c) Hierarchical clustering of cell cycle-related genes differentially expressed.
Table 2 Apoptosis-related genes modulated by benzyl isothiocyanate

Table 3 Cell cycle-related genes modulated by benzyl isothiocyanate


Fig. 7 Effect of benzyl isothiocyanate (BITC) on gene expression. The expression patterns observed using microarray analysis were confirmed with quantitative RT-PCR using programmed cell death 4 (PDCD4), cyclin G2 (CCNG2) and growth arrest and DNA damage-inducible α (GADD45A). L9981 cells were treated with BITC as described previously, and total RNA was harvested 24 h post-treatment. Relative expression levels for PDCD4, CCNG2 and GADD45A derived from array (![]() ) and RT-PCR (
) and RT-PCR (![]() ) were indicated.
) were indicated.
Discussion
Metastasis is the most common cause of death in cancer patients. It was found that 90 % of lung cancer patients die of metastasis(Reference Gupta and Massague22). New strategies to prevent and treat cancer metastasis are urgently needed. Therefore, research and development of novel anti-metastatic drugs is one of the most active fields in cancer research. Recent studies(Reference Hwang and Lee16–Reference Hunakova, Sedlakova and Cholujova18) have shown that isothiocyanates effectively suppressed metastasis potential of tumour cells; however, the mechanism is not fully understood. There are several possible ways to suppress tumour cell metastasis: for example, to reverse the metastasis potential by regulating metastasis-related genes and pathways, to inhibit metastatic cell growth by inducing cell cycle arrest or to eliminate the metastatic cells by inducing apoptosis. Our previous study demonstrated that BITC and PEITC suppressed metastasis potential of highly metastatic human lung cancer the L9981 cells by the modulation of metastasis-related matrix metalloproteinases-2, Twist and β-catenin expression, inhibition of the Akt/NF-κB pathway and induction of oxidative stress(Reference Wu, Zhu and Yan19). In the present study, we explored other possible ways to suppress NSCLC cell metastasis.
We first examined the effect of BITC (7·5 and 10 μm) and PEITC (12·5 and 20 μm) on cell migration and invasion by Wound Healing Assay and Cell Invasion Assay. Cell migration and invasion were more significantly reduced than BITC (5 μm) and PEITC (10 μm) in our previous study(Reference Wu, Zhu and Yan19) (data not shown).
The induction of apoptosis by isothiocyanates was first documented by Yu et al. (Reference Yu, Mandlekar and Harvey23). Their study showed that PEITC and other structurally related isothiocyanates, phenylmethyl isothiocyanate, phenylbutyl isothiocyanate and phenylhexyl isothiocyanate, induced HeLa cell apoptosis in a time and dose-dependent manner. Since then, the apoptosis induction effect of isothiocyanates has been studied in several types of cancer cells. The mechanism is not fully understood, although there is evidence showing that apoptosis is mediated by caspase-3 and -8 activation, poly-(ADP-ribose) polymerase and Bid cleavage, JNK and ERK1/2 activation(Reference Xu and Thornalley6, Reference Xu and Thornalley7, Reference Hu, Kim and Chen24). However, there is no report on their apoptosis induction effect in metastatic lung cancer cells. The present study demonstrated, for the first time, that BITC and PEITC induced highly metastatic human lung cancer cell apoptosis in a dose-dependent manner. Caspase-3 plays a central role in the execution phase of cell apoptosis. It responds to caspase-8, -9, -10, and cleaves and activates caspase-6, -7, -9. In the present study, caspase-3 was activated in L9981 cells. This is consistent with our previous findings in human leukaemia cells, in which HL60 and ML-1 cells induced apoptosis by PEITC and allyl isothiocyanate via caspase-3 and -8 activation(Reference Xu and Thornalley7).
Isothiocyanates also inhibit cell cycle progress. The cell cycle may be arrested at the G1 phase or G2/M phase, depending on the cell types(Reference Wu, Zhou and Xu5). Our data showed that BITC and PEITC delayed highly metastastic human lung cancer cell cycle progress and caused G2/M phase arrest. Although the exact molecular mechanisms responsible for cell cycle arrest are still mostly unknown, some potential targets of isothiocyanates have been postulated. Cyclins are a family of proteins that control the progression of cells through the cell cycle by activating cyclin-dependent kinase enzymes, such as cdc2 and cdk2. Activation of cdc2 is controlled at several steps including cyclin B1 binding. The cdc2-cyclin B1 kinase activity is pivotal in regulating G2/M transition. In human prostate cancer cells, AITC-induced G2/M phase arrest was accompanied by a significant decrease in the cyclin B1 level(Reference Xiao, Srivastava and Lew25). The down-regulation of cdk1 and cdc25B was also observed in the AITC-induced G2/M-arrested cells. Miyoshi et al. (Reference Miyoshi, Uchida and Osawa26, Reference Miyoshi, Uchida and Osawa27) reported that BITC inhibited human leukaemia cell G2/M progression by up-regulating the expression of the G2/M cell cycle arrest-regulating genes including p21 and inhibiting cyclin-dependent kinase activity by directly binding to CDK/cyclin complexes including Cdc2/cyclin B1 kinase. We found that both BITC and PEITC reduced cyclin B1 expression in a dose-dependent manner in L9981 cells. Altogether, these findings suggested that cyclin B1, cdk1 and cdc25B may be targeted by isothiocyanates.
The present study also aimed at elucidating the underlying network of signalling events in the BITC- and PEITC-induced apoptosis of L9981 cells. MAPK are important mediators involved in the intracellular network of interacting proteins that transduce extracellular signals to intracellular responses. When the MAPK cascade is activated, signals lead to the activation of diverse molecules that regulate cell growth, survival and differentiation(Reference Inamdar, Madhunapantula and Robertson28). The present study showed that activations of JNK, ERK1/2 and p38 are involved in the induction of apoptosis by BITC and PEITC. Hu et al. (Reference Hu, Kim and Chen24) reported that three MAPK (JNK, ERK1/2 and p38) were activated after PEITC treatment in colon adenocarcinoma HT-29 cells. However, in ovarian cancer cells OVCAR-3, PEITC suppressed the activation of ERK1/2 while simultaneously activating pro-apoptotic p38 and JNK1/2(Reference Satyan, Swamy and Dizon29). This indicated that the role of ERK in isothiocyanate-induced apoptosis is controversial.
AP-1 is one of the transcription factors regulated by MAPK. AP-1 regulates a wide range of cellular processes, including cell proliferation, death, survival and differentiation. AP-1 has been proposed to play important roles in carcinogenesis and cancer development(Reference Shaulian and Karin30, Reference Oya, Takayanagi and Horiguchi31). Isothiocyanates activate AP-1 activity in prostate cancer cells(Reference Xu, Shen and Yuan32) and bladder cancer cells(Reference Yao, Zhang and Li33). Interestingly, Xu et al. reported that in colon cancer cells, a low concentration of PEITC and sulforaphane activates AP-1 activity, but at higher concentrations, they suppress AP-1 activity(Reference Jeong, Kim and Hu34). Our data showed that BITC and PEITC suppressed AP-1 activity in highly metastatic lung cancer L9981 cells. These suggested that the effect of isothiocyanates on AP-1 activity may depend on the types and concentrations of isothiocyanates, as well as on the cell types.
Cyclin D1 is one of the genes under the control of AP-1. Cyclin D1 forms a complex with and functions as a regulatory subunit of CDK4 or CDK6, whose activity is required for cell cycle G1/S transition. Cell proliferation is regulated by cyclin D/CDK4/6. Numerous studies show that isothiocyanates inhibit several types of cancer, including prostate cancer(Reference Xu, Shen and Yuan32), colon carcinoma(Reference Shen, Xu and Chen35), pancreatic carcinoma(Reference Batra, Sahu and Kandala36), which is associated with the inhibition of cyclin D1 expression. Further studies show that these effects are mediated by MAPK, NF-κB and STAT3 pathways. The present study demonstrated that BITC and PEITC inhibited cyclin D1 expression via the MAPK/AP-1 pathway.
ROS are essential for biological functions. ROS are involved in signalling cell growth and differentiation, regulating the activity of enzymes, mediating inflammation and eliminating pathogens and foreign particles. The generation of ROS is part of the mechanism by which most chemotherapeutic agents or ionising radiation kill tumour cells(Reference Sun and Rigas37). Isothiocyanates elevate ROS level in different types of cancer, such as leukaemia(Reference Trachootham, Zhang and Zhang38), breast cancer(Reference Xiao, Powolny and Singh39) and pancreatic cancer(Reference Sahu, Zhang and Batra40). Recent work from our laboratory has shown that BITC and PEITC induce the generation of ROS in L9981 cells. To explore whether the induction of oxidative stress plays a role in the induction of apoptosis, we pre-treated L9981 cells with antioxidant NAC. Our data showed that NAC attenuated apoptosis induction, MAPK activation and AP-1 suppression by BITC and PEITC, suggesting that cell death signalling was triggered by oxidative stress.
The inhibitory effect of BITC and PEITC on highly metastatic lung cancer cells prompted us to perform further work aimed at identifying isothiocyanate-targeted genes related to apoptosis or cell cycle. For this, we performed microarray studies to define the gene expression profiles observed in L9981 cells in response to BITC treatment. A number of genes that seem to be regulated by BITC and have relevance to apoptosis and the cell cycle were identified by using such an approach. Among the apoptosis-related genes, TNF is well known in the regulation of a wide spectrum of biological processes including cell proliferation, differentiation, apoptosis, lipid metabolism and coagulation(Reference Faustman and Davis41). Programmed cell death 4 is a tumour suppressor and plays an important role in cell apoptosis(Reference Sheedy, Palsson-McDermott and Hennessy42). Caspase-4 is a member of the caspase family. This caspase is able to cleave and activate its own precursor protein as well as a caspase-1 precursor. It promotes apoptosis when activated(Reference King, Fong and Griffin43). Our microarray data analysis also showed that a number of cell cycle-related genes were regulated by BITC. Growth arrest and DNA damage-inducible-α is up-regulated following stressful growth arrest conditions and treatment with DNA-damaging agents. It is closely related with cell cycle arrest in human liver carcinoma cells treated with fucoxanthin(Reference Satomi and Nishino44). Another cell cycle-related gene is cyclin G2. Ectopic expression of cyclin G2 inhibits cyclin-dependent kinase 2 activity, Rb phosphorylation, cell cycle progression and cellular proliferation. A study showed that elevated cyclin G2 expression is associated with anti-human epidermal growth factor receptor 2 antibody-mediated inhibition of breast caner cell growth(Reference Le, Arachchige-Don and Mao45).
In summary, the present results showed that BITC and PEITC suppressed the metastasis potential of highly metastatic human lung cancer L9981 cells by the induction of apoptosis and cell cycle arrest. The MAPK pathway was involved in suppression and was the potential targeted pathway of isothiocyanates. Apoptosis and MAPK activation were blocked by antioxidant NAC, suggesting that cell death signalling was triggered by oxidative stress. Targeted genes related to apoptosis and the cell cycle were identified, and further work on the definition of new targeted signalling pathways and genes is warranted.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (30873035), Key Project of Tianjin Municipal Education Commission (ZD200714), Key Project of Tianjin Municipal Science and Technology Commission (10JCZDJC20800), and the Start-Up Fund of the Ministry of Education of China. K. X., X. W. and Q. Z. designed the study; H. Y., Y. Z., B. L., H. W. and Y. L. performed the experiments; K. X., X. W. and Q. Z. analysed the data and wrote the paper. The authors declare that there is no conflict of interest.












