Introduction
Diarrhoeal disease is the leading infectious disease cause of morbidity and the second leading infectious cause of death globally in children under 5 years of age [Reference Lozano1]. Morbidity resulting from enteric infection can have significant consequences, including acquired malnutrition and linear growth deficits from repeated enteric infections before the age of two [Reference Kotloff2, Reference Guerrant3]. Malnutrition in early childhood may predispose children to more severe and prolonged infections, and can result in impaired cognitive development that yield negative societal outcomes and long-term health effects including cardiovascular and metabolic diseases [Reference Guerrant3–Reference Kosek5].
One of the leading causes of bacterial diarrhoea and enteric infection worldwide is Campylobacter jejuni [Reference Coker6, Reference Lee7]. In developed regions of the world, C. jejuni is a common foodborne pathogen associated with poultry and contaminated dairy products. Infection can cause an acute dysentery and/or febrile illness in children and adults [Reference Coker6]. In developing countries, C. jejuni is endemic. Poor hygiene, lack of sanitation and living in close proximity to animals contribute to recurrent infection. C. jejuni is frequently isolated in the stools of healthy children and the rates of infection between symptomatic and asymptomatic cases are often similar. Clinical disease from C. jejuni in developing countries primarily affects the paediatric population and typically results in acute watery diarrhoea with concomitant signs and symptoms such as fever, abdominal pain and vomiting [Reference Coker6, Reference Lee7]. Additionally, recent studies have also found an association of Campylobacter infection with malnutrition and growth stunting in paediatric populations in the developing world [Reference Lee7].
To date only a few virulence factors have been characterised in C. jejuni. Nevertheless, the polysaccharide capsule (CPS) present on the surface of the bacterium has been demonstrated to be one of the most important virulence determinants [Reference Guerry8]. The capsule is composed of repeating saccharide units attached to the outer membrane via to a phospholipid anchor [Reference Corcoran, Annuk and Moran9]. Many of C. jejuni CPSs are characterised by the presence of heptoses in unusual configurations (e.g. altro, ido, gulo) and non-stoichiometric modifications to the sugars, including ethanolamine and methyl phosphoramidate (MeOPN). The levels of MeOPN are non-stoichiometric due to phase variation of the genes encoding MeOPN transferases. Recent studies have shown that MeOPN contributes to complement resistance [Reference Guerry8, Reference Maue10, Reference Pequegnat11].
Capsule is the major serodeterminant of the Penner serotyping scheme, of which there are 47 serotypes of C. jejuni [Reference Karlyshev12]. However, genomic analyses of the variable regions of the CPS loci of the 47 Penner serotypes indicated that there are 35 distinct CPS types [Reference Poly13]. Strains belonging to the same serotype group are predicted to express the identical CPS structures. Nevertheless, difference in the Penner serotype can be also due to differences in lipooligosaccharide (LOS) structures; the LOSs have been suggested to be a minor serodeterminant in the Penner typing scheme [Reference Preston and Penner14]. The LOSs are additional saccharide structure present at the surface of the bacterium. Compared with the CPS, LOSs are composed only of a few repeating saccharide units and are anchored in the membrane via a different family of lipid. In this study, we used a multiplex polymerase chain reaction (PCR) method for determination of CPS types. This method can discriminate the 35 CPS types, shown in Table 1. Unlike the Penner typing scheme, the capsule multiplex PCR method is not subjected to LOS interference for the attribution of CPS type. Nonetheless, the attribution is based on the presence of gene sequences and does not provide information on the modulation of structure and expression level of the capsule. C. jejuni present homopolymeric GC track in the nucleic sequence of genes involved the capsule biosynthesis. During replication, insertion/deletion of G or C nucleotide(s) induces a frame shift that can cause the enzyme to be non-functional. This mechanism adds an additional layer of complexity to the modulation of the capsule structure [Reference Parkhill15]. It is believed that these modulations confer an advantage in escaping the host immune response.
Table 1. Summary of CPS types identified by multiplex PCR
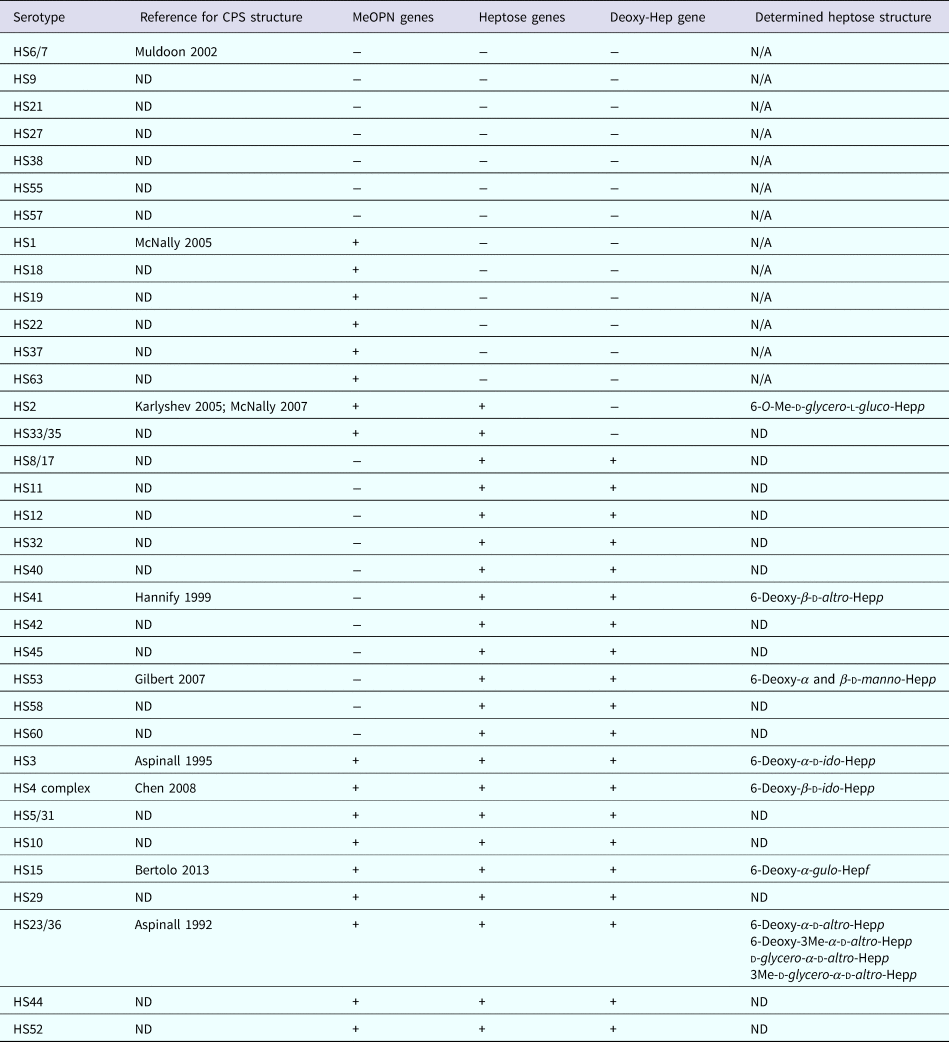
ND, not determined; N/A, not applicable; Hep, heptose; p, pyranose configuration; f, furanose configuration.
Currently, there are no vaccines against C. jejuni; however, efforts are ongoing to develop a CPS conjugate vaccine [Reference Monteiro16, Reference Riddle and Guerry17]. Capsule-based vaccines have been successfully developed for other encapsulated mucosal pathogens including type B Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumoniae. Given these successes, and the fact that the CPS is a major virulence determinant, a capsule conjugate C. jejuni vaccine is a rationale strategy that could significantly reduce the global burden of disease.
The Interactions of Malnutrition & Enteric Infections: Consequences for Child Health and Development (MAL-ED) project was established in 2009 as a worldwide collaboration to further investigate the impact of enteric infection, including C. jejuni, on child health outcomes, growth and development [Reference Kosek5]. The MAL-ED network collected data on diarrhoeal illness in poverty stricken communities in eight developing countries: Peru, Brazil, Bangladesh, India, Pakistan, Tanzania, South Africa and Nepal. Initial results from the global MAL-ED project revealed Campylobacter as the most frequently isolated pathogen, and highlight Campylobacter as a cause of significant morbidity in children in Loreto, Peru [Reference Lee7, Reference Platts-Mills and Kosek18]. This study also demonstrated an association between Campylobacter infection and linear growth shortfalls and increased intestinal permeability and inflammation in children [Reference Amour19]. Here we apply the multiplex method of determination of CPS types to C. jejuni strains isolated as part of the MAL-ED project in Peru.
Materials and methods
Clinical data were collected from three rural communities in the Department of Loreto in Peru: Santa Clara de Nanay, Santo Tomás and La Unión as previously described [Reference Richard20]. Briefly, 198 Peruvian children were enrolled within 17 days after birth and followed until 2 years of age. Field researchers visited the participant's home twice a week and collected information from the mother or caregiver regarding the child's dietary intake, general health information and surveillance for infectious diseases since the previous visit [Reference Richard20]. Stool samples were collected during diarrhoeal episodes, classified as three or more loose stools in a 24-h period if onset was after two or more diarrhoea-free days. Staff members obtained data related to diarrhoeal illness, including associated symptoms and whether any treatment or hospitalisations were needed. Associated symptoms included the presence and duration of dehydration, fever >37.5 °C, anorexia, vomiting, dysentery, the need for oral rehydration therapy or hospitalisation and any episode with four or more semi-liquid/liquid stools, among others. Routine, non-diarrhoeal stool samples were also obtained at monthly surveillance visits to assess for asymptomatic shedding during the first year of life and quarterly during the second year of life.
Definitions
Diarrhoea was defined as greater than or equal to three loose stools in a 24-h period or the presence of blood in at least one stool sample [Reference Richard20]. Two diarrhoeal episodes were considered to be distinct if separated by at least 2 days of normal stool. C. jejuni diarrhoeal illness was defined as any diarrhoeal episode in which C. jejuni was isolated. Asymptomatic infection was defined as a non-diarrhoeal stool sample that was positive for C. jejuni. The first C. jejuni positive sample, obtained from either a symptomatic or asymptomatic sample, was considered to be the first infection. Dysentery was defined as mother's observation of blood in a stool during an episode of diarrhoea.
Microbiology
Campylobacter strains were isolated as previously described and underwent phenotypic testing utilising oxidase and catalase phenotypical testing for differentiation between C. jejuni and C. coli [Reference Lee7]. Isolates were stored at −80 °C in Mueller–Hinton (MH) broth containing 15% glycerol. Strains were revived by plating onto MH agar plates and incubation under a microaerobic atmosphere (85% N2, 10% CO2 and 5% O2) at 37 °C for 24–72 h.
Capsule typing
DNA from the revived strains was extracted using the DNeasy extraction kit (Qiagen, USA). Capsule typing was performed using a multiplex PCR assay following the Poly et al. protocol. The typing system is able to discriminate all of the 35 CPS types described above for C. jejuni. It also includes a C. jejuni-specific positive primer set to confirm speciation [Reference Poly13]. Samples that tested negative with this species-specific primer set were excluded from analysis. Non-typeable C. jejuni isolates were those confirmed by multiplex PCR as C. jejuni, but with an unidentifiable capsule type. Samples that contained multiple C. jejuni CPS types were counted within the total number of isolates for each CPS type. These individuals were assumed to be co-infected.
Analyses
Analyses were performed based on capsule phenotype across the total number of infections and the number of first infections. Descriptive analyses were performed to assess the prevalence of each capsule type for all, first and subsequent total infections and diarrhoea-associated infections. Diarrhoeal illness characteristics were assessed across capsule types for all and first symptomatic infections. To determine if specific C. jejuni capsule structures were associated with diarrhoeal disease severity, we compared the prevalence of multiple clinical parameters by infection with specific capsule structures. Statistical comparisons were made using Pearson's χ 2 tests with a two-sided α = 0.05. All statistical analyses were performed using SAS version 9.3 (Cary, NC).
This study was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. The Institutional Review Board of Johns Hopkins Bloomberg School of Public Health and the ethics committee of Asociacion Benefica PRISMA in Lima, Peru approved the paediatric study.
Results
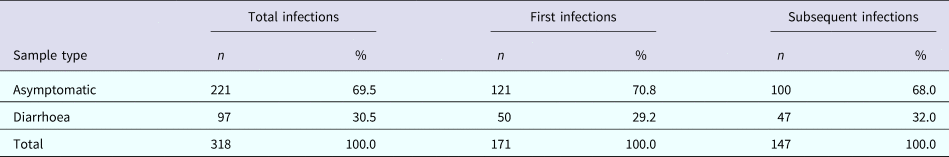
A total of 318 C. jejuni infections were identified, of which 171 (53.8%) were characterised as ‘first infections’ (Table 2). Approximately 70% of all infections were asymptomatic with no significant differences in the proportion of diarrhoeal cases between the first and subsequent infections (29.2% vs. 32.0%, respectively; p = 0.6). From the 318 infections, 352 C. jejuni isolates were identified due to co-infection with two C. jejuni CPS types in 30 subjects and three CPS types in two subjects. Co-infection with multiple Campylobacter isolates was equally prevalent among first and subsequent infections (11.4% and 7.5%, respectively; p = 0.2).
Table 2. The number of total, first and subsequent infections by the sample type
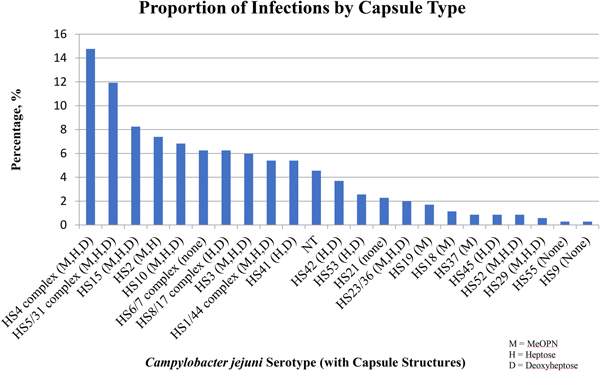
The top five most common serotypes among all infections included the HS4 complex (14.8%), the HS5/31 complex (11.9%), HS15 (8.2%), HS2 (7.4%) and HS10 (6.8%) with no significant differences in the frequency of isolation by the presence or absence of clinical illness (Figs 1 and 2). The HS4 complex, which encompasses eight related CPS types, was the most common capsule type identified among all (14.8%), first (16.1%) and subsequent (13.2%) infections. The HS4 complex was also the most common among total diarrhoea-associated infections (18.2%). However, the HS5/31 complex was the most common capsule type identified in diarrhoea-associated first infections (15.0%).
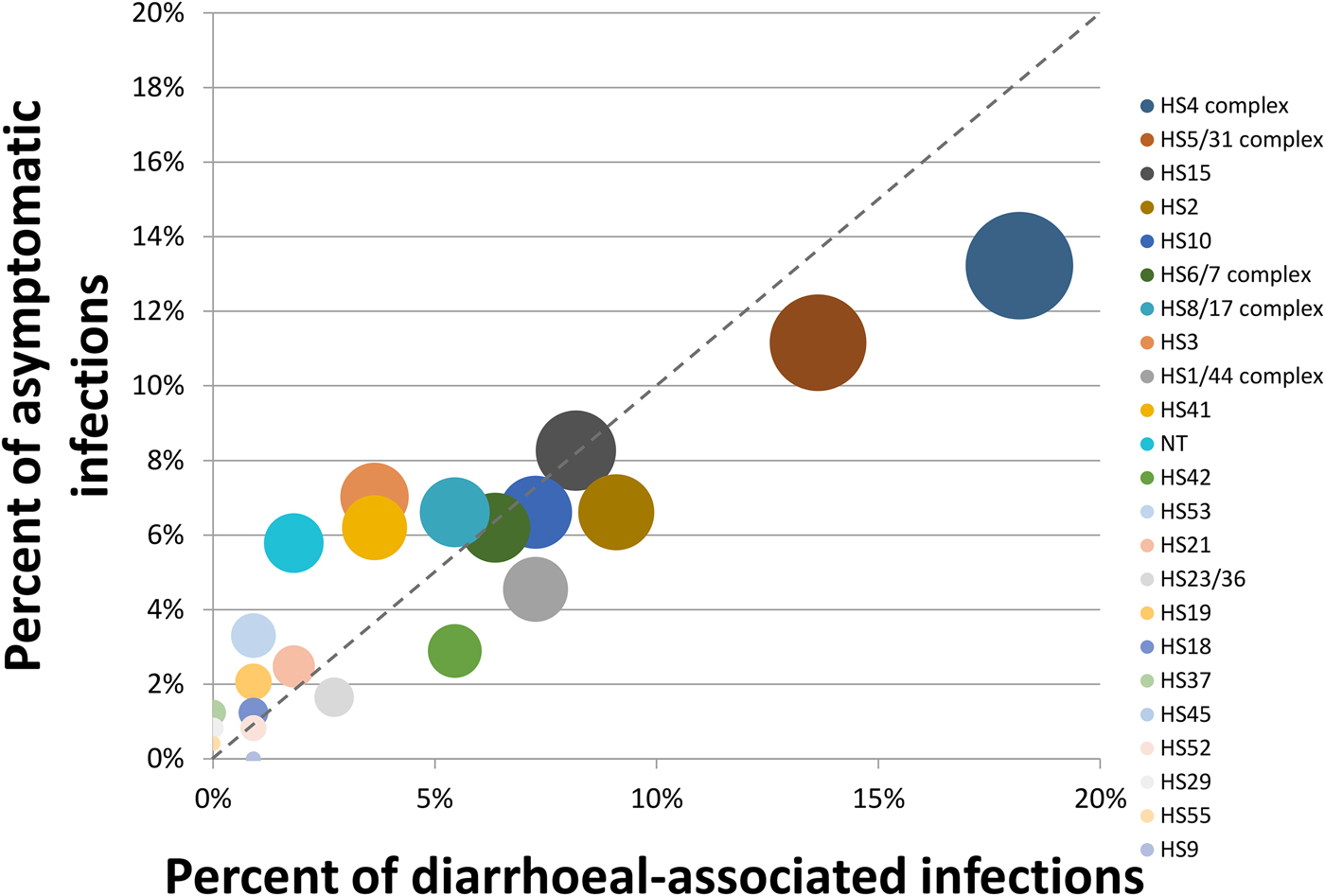
Fig. 1. Proportion of asymptomatic vs. symptomatic diarrhoeal-associated infections. The size of the circle is proportional to the overall prevalence of the capsule type. The legend is sorted from most to least prevalent capsule types.
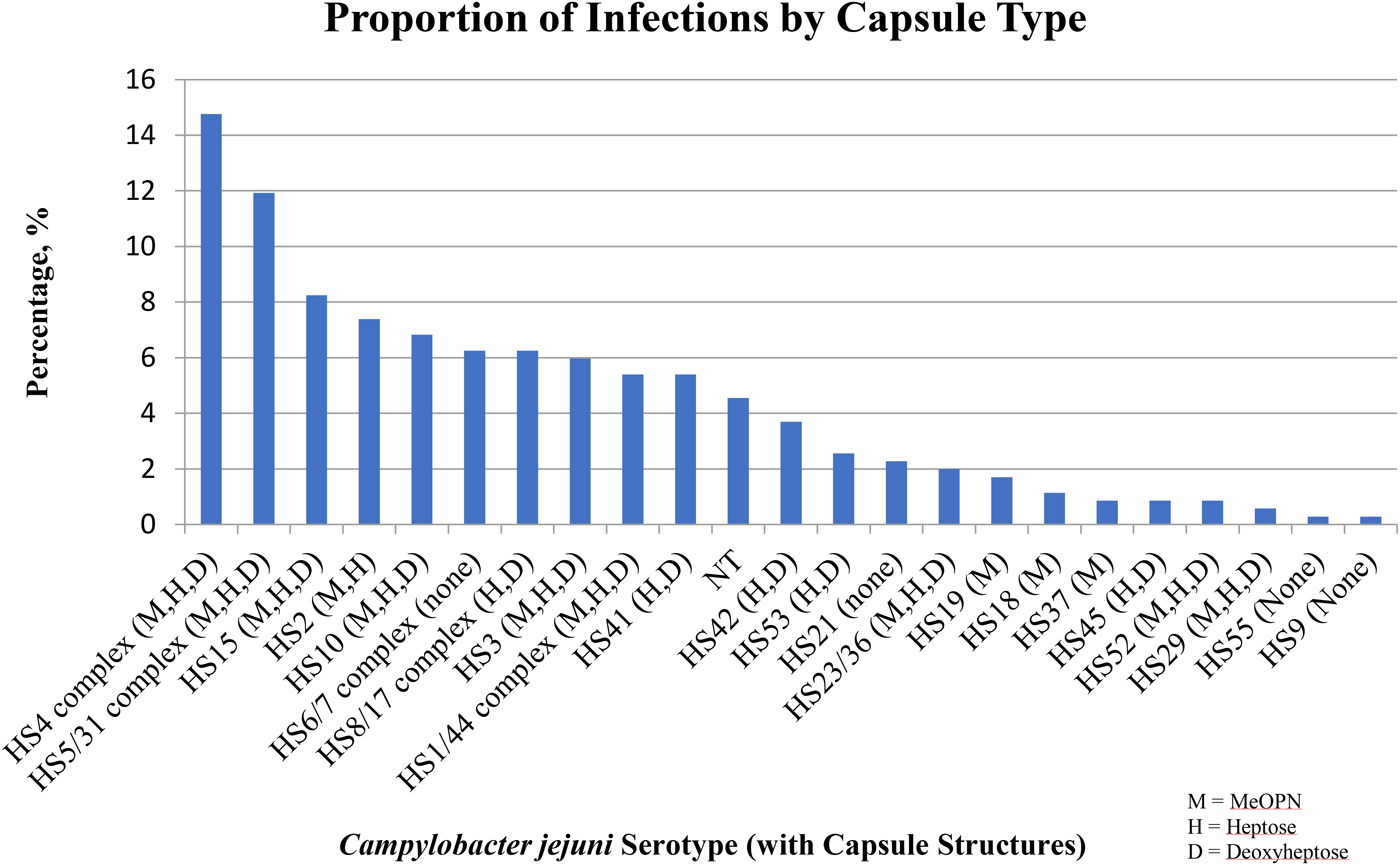
Fig. 2. The percentage of serotypes (with corresponding capsule structures) among all infections.
The majority of children (95/172; 55.2%) had between two and up to seven C. jejuni infections during the 2 years of the study. The remaining children (77/172; 44.8%) had a single C. jejuni infection. It is interesting to note that in the group with more than one infection, 97.9% of the subsequent infections were of a distinct CPS type. In the few cases in which a child shed a strain of the same CPS type seen in a previous infection, this may have been due to re-infection with a new strain or recrudescent infection with the original strain [Reference Baqar21].
The data were stratified by individual features of the different CPS types as shown in Table 1. Then, we examined whether the presence (or predicted presence based on gene content) of MeOPN, heptose or deoxyheptose residues was associated with disease outcome. Specifically, across all symptomatic infections, 52.0% of children infected with a C. jejuni strain with a capsule containing MeOPN, heptose and deoxyheptose suffered from fever, 57.5% developed anorexia, 50.0% had vomiting, 56.2% needed oral rehydration therapy, 54.8% had at least 1 day with greater than or equal to four semi-liquid/liquid stools and 46.2% suffered from dysentery (Table 3). When comparing CPS types that contained MeOPN, heptose and deoxyheptose vs. those that did not contain one of those three structures, there were no significant differences across all signs, symptoms and management for all or first symptomatic infections (all p-values >0.1). Furthermore, there were no significant differences in the duration of any of the diarrhoeal signs and symptoms across capsule types (data not shown).
Table 3. Presence [n (%)] of diarrhoeal disease signs, symptoms and management by capsule type for ALL symptomatic infections
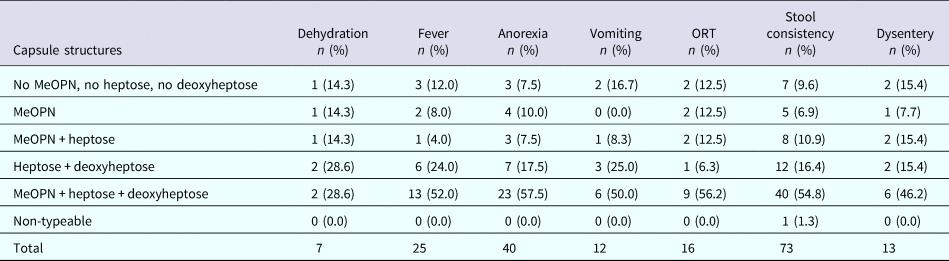
ORT, oral rehydration therapy.
The top five most common serotypes responsible for re-infection were as follows: HS4 complex (n = 21, 13.2%), the HS5/31 complex (n = 18, 11.3%), the HS8/17 complex (n = 15, 9.4%), the HS6/7 complex (n = 13, 8.2%) and HS2 (n = 12, 7.6%). In comparison with all infections, while the HS4 complex and HS5/31 complex remained the most prevalent, the HS6/7 complex and the HS8/17 complex were responsible for a larger proportion of subsequent infections than initial infections. When limited to subsequent infections that were symptomatic, 57.1% (n = 12) of infections caused by the HS4 complex resulted in diarrhoea, whereas only 33.3% (n = 6) of the infections caused by the HS5/31 complex were symptomatic.
Discussion
The most common serotypes identified in this cohort of subjects were the HS4 complex, HS5/31 complex, HS15, HS2 and HS10 which collectively accounted for ~50% of all isolates. To our knowledge, these data represent the first report of CPS types of C. jejuni strains from South America. We assessed the association between capsule type and signs and symptoms of clinical disease among all, first and subsequent infections and found only minor, non-statistically significant variations, suggesting little to no variability in the proportion of isolates responsible for infections across a range of epidemiologically important strata. In a recent systemic review on global C. jejuni Penner serotype distribution, which covered >21 000 sporadic cases of C. jejuni diarrhoea, eight C. jejuni serotypes accounted for >50% of all isolates globally [Reference Pike, Guerry and Poly22]. Interestingly, the major CPS/serotype found worldwide and in the current study was the HS4 complex, and HS2, HS5/31, HS15 and HS10 were also among the most frequent. These data also support the current understanding that the majority of C. jejuni infections are attributed to a limited number of CPS types [Reference Monteiro16, Reference Pike, Guerry and Poly22].
The overwhelming majority of isolates in our sample population (n = 291, 82.7%) are predicted to contain a heptose-containing CPS. We observed a trend towards more disease signs, symptoms and need for clinical management in CPS types with heptose; however, this association is likely due to the high proportion of C. jejuni CPS types with the potential to express heptose in our sample. Our findings do not further explain the contribution of heptose in the pathogenesis of diarrhoeal disease, which to date remains elusive [Reference Wong23].
While we were unable to determine if specific CPS types were more virulent than others, we observed trends in increased disease severity when subjects were infected with serotypes that contained MeOPN, heptose or deoxyheptose. Overall, our results do not appear to reveal any capsule-specific variability in disease signs, symptoms or need for treatment.
Approximately 70% of all infections in this study were asymptomatic with no significant difference in the proportion of cases or capsule type distribution between the first and subsequent infections. This supports previous studies that have also found asymptomatic C. jejuni infection to be common in children living in developing countries around the world [Reference Amour19, Reference Levine and Robins-Browne24]. Infection with or without diarrhoeal illness is an important factor in the development of short- and long-term sequelae [Reference Amour19]. Evidence suggests acquired immunity and resistance to C. jejuni colonisation is possible after several exposures to various capsule types and increasing age [Reference Riddle and Guerry17, Reference Tribble25]. However, the low incidence of re-infection with the same CPS type described in this study is consistent with a role for CPS in natural immunity.
Efforts to develop a C. jejuni vaccine are underway and a capsule-conjugate vaccine based on the polysaccharide CPS has yielded promising results [Reference Monteiro16]. Early studies with a recently developed monovalent capsule conjugate vaccine were shown to be 100% effective against Campylobacter-associated diarrhoeal disease in primates. This form of the vaccine eliminates the risk of several chronic sequelae, including Guillain–Barré syndrome, seen with other forms of vaccination such as oral whole cell vaccines [Reference Riddle and Guerry17]. However, a licensable capsule-based conjugate vaccine will have to be multivalent, targeting the most common and pathogenic capsule types [Reference Riddle and Guerry17]. These data contribute to the global understanding of CPS diversity and facilitate the prioritisation of CPS targets. If developed, a vaccine could be marketable to populations living in endemic regions and travellers from the developed countries, with the goal of significantly reducing the overwhelming burden of disease.
The results presented here are based on the genotypic characterisation of C. jejuni without confirmation of phenotypic expression of specific epitopes. In particular, capsule structure data are based on the genes present in specific capsules. Based on this testing, we assumed that certain phenotypic characteristics were present on the C. jejuni capsule when specific serotypes were detected by the multiplex PCR assay. However, biological variability may modify if and how these structures are expressed during infection and during clinical illness.
This study adds to the increasing understanding of the distribution of the C. jejuni capsule types associated with acute infection. These data, along with the increased recognition of Campylobacter as a global cause of morbidity in paediatric populations in low-middle income countries and as a causative agent of travellers’ diarrhoea, highlight the potential utility of a capsule-based C. jejuni vaccine as one measure in reducing the burden of enteric infections.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. This is a U.S. Government work. There are no restrictions on its use. There were no financial conflicts of interests among any of the authors. The study protocol was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. This work was supported by work unit number 6000.RAD1.DA3.A0308.
Copyright statement
FP, PG and CKP are employees of the U.S. Government. This work was prepared as part of official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Author ORCIDs
Chad K. Porter 0000-0001-7258-1690