Introduction
There is an emerging consensus that the transfer of knowledge from proven biomedical discoveries into patient and public benefit should be accelerated. At the same time there is little conceptual clarity, either about the precise nature of the phases of this ‘translational continuum’ or about the proper place within it of ‘implementation science’ (Eccles et al. Reference Eccles, Armstrong, Baker, Cleary, Davies, Davies, Glasziou, Ilott, Kinmonth, Leng, Logan, Marteau, Michie, Rogers, Rycroft-Malone and Sibbald2009). In this paper, we aim to: (i) develop an integrated schema to understand the whole translational medicine continuum, consisting of five sequential phases that link basic biomedical research with clinical science and practice; (ii) discuss the nature of three important blocks between these phases; (iii) consider the place of implementation science within this continuum.
A schema for the translational medicine continuum
The best developed framework describing the development of new therapeutic interventions is that which refers to pharmacological drug discoveries. Such studies are divided into the five phases shown in the first row in Table 1. Subsequently, other schemes, which refer to non-pharmacological discoveries, have been elaborated (see Table 1). Within the UK, for example, a Framework for the Evaluation of Complex (largely psycho-social) Interventions has been described by the Medical Research Council using a similar sequence (Campbell et al. Reference Campbell, Murray, Darbyshire, Emery, Farmer, Griffiths, Guthrie, Lester, Wilson and Kinmonth2007; Craig et al. Reference Craig, Dieppe, Macintyre, Michie, Nazareth and Petticrew2008). The National Institute for Health Research in England has established Biomedical Research Centres to support the conduct of translational medicine, which it sees as those investigations that begin with first-in-man studies and which continue up to, and including, early clinical trials (National Institute for Health Research, 2006). In parallel, within the USA a concerted scientific action programme has led to the National Institutes of Health Roadmap (Zerhouni, Reference Zerhouni2003), in which two ‘translational roadblocks’ have been described that delay knowledge transfer along the whole of the translational pathway (Zerhouni, Reference Zerhouni2005). Within the field of cancer research in the USA, for example, the President's Cancer Panel has distinguished ‘early’ from ‘late’ translational studies (The President's Cancer Panel, 2005). By combining these various formulations we propose a single overall schema, which consists of five phases (0–4) and three translational blocks (T1–T3), as shown in Fig. 1. This perspective integrates those elements cited in Table 1 and is proposed as a more coherent and comprehensive framework than other formulations to date.
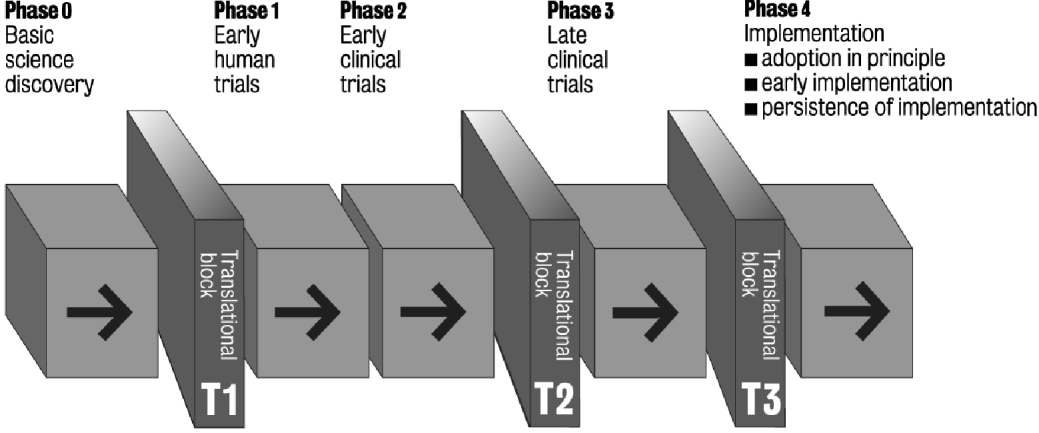
Fig. 1. Five phases and three blocks in the translational medicine continuum.
Table 1. Five phases of the translational medicine continuum and three translational blocks
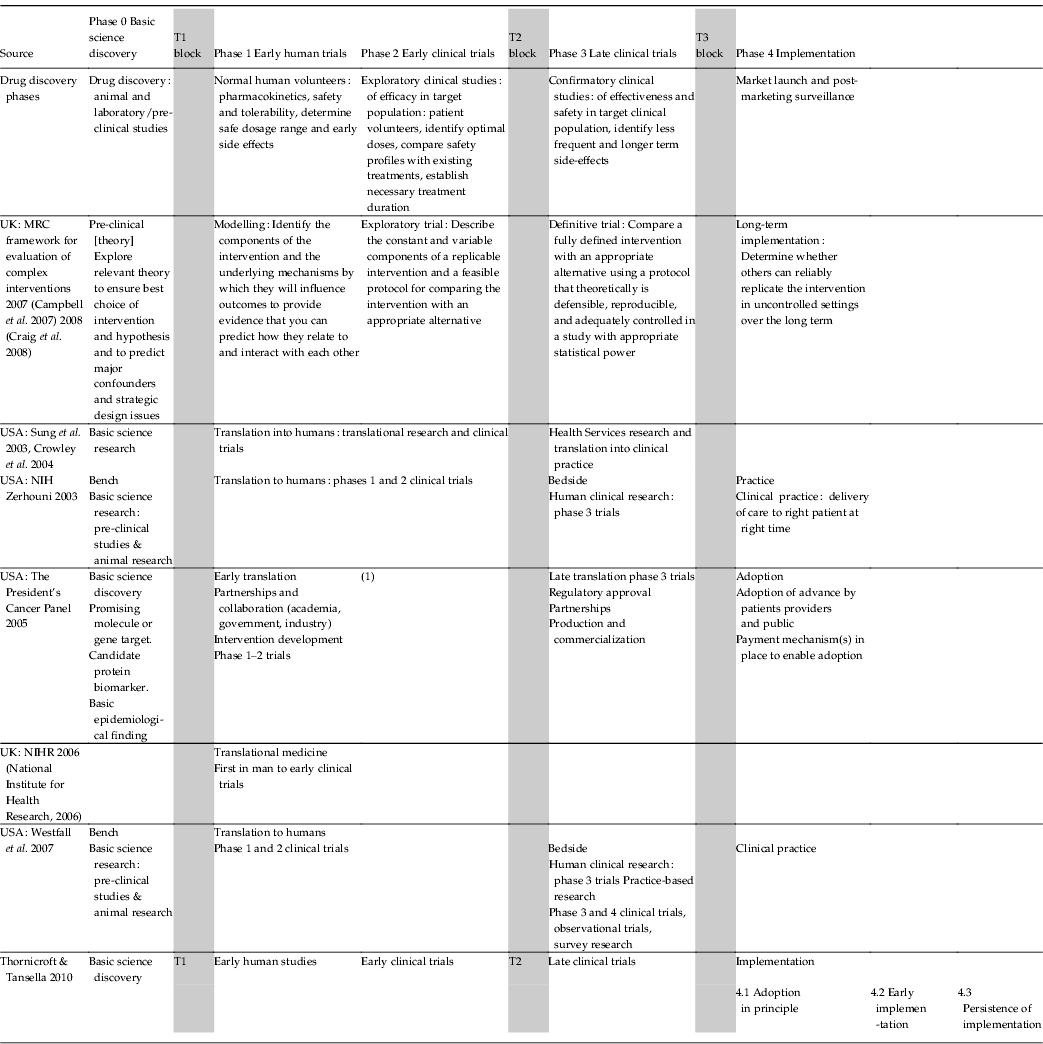
MRC, Medical Research Council; NIH, National Institutes of Health.
Westfall translational step 2 refers to clinical guidelines, meta-analyses, systematic reviews.
Translational step 3 refers to dissemination research, implementation research.
Zerhouni translational step 2 refers to moving new medical discoveries into clinical practice.
(1) Dissemination: to community health providers and to patients and public.
Phase 0: Basic science discovery
Phase 0 refers to both basic laboratory and theoretical studies. The so-called ‘bench’ phase of basic laboratory research includes understanding therapeutic mechanisms of action, identifying promising molecule or gene targets and protein biomarkers, selecting candidate drugs and animal and laboratory (pre-clinical) studies. In terms of theoretical studies, this phase includes appraising relevant theories to ensure best choice of the candidate interventions, generating relevant hypotheses and anticipating the most important confounders, making judgements on the most critical research design issues, along with fundamental (aetiological) epidemiological research.
Translational block T1
The first translational block (T1) mediates the transfer of new understandings of disease mechanisms and drug actions gained in the laboratory into the development of new methods for diagnosis, therapy and prevention, alongside their initial testing in humans. In effect, the T1 block operates at the interface between animal and first-in-man studies.
Phase 1: Early human studies
In the drug development cycle, phase 1 studies are those that include healthy human volunteers and that aim to determine safety, tolerability, dose–effect relationships and early adverse effects. For psycho-social interventions, phase 1 refers to the period during which the key components of the intervention are identified, along with the manualization of the intervention.
Phase 2: Early clinical trials
For pharmacological interventions, phase 2 consists of early exploratory clinical studies to test efficacy in the target population; namely, individuals with the disorder to be treated. Studies of such patient volunteers can identify optimal doses, compare safety profiles with existing treatments and establish treatment duration. For psycho-social treatments, phase 2 investigations include exploratory studies (including randomized clinical trials), which describe the constant and variable components of a replicable intervention and which finalize a feasible protocol for comparing the intervention with an appropriate alternative (Campbell et al. Reference Campbell, Murray, Darbyshire, Emery, Farmer, Griffiths, Guthrie, Lester, Wilson and Kinmonth2007).
Translational block T2
In describing the US national clinical research enterprise, Sung et al. have distinguished the T1 and T2 translational blocks, where T2 refers to ‘the difficulty implementing therapeutic advances proven effective in large well conducted trials into the daily practice of medicine’ (Sung et al. Reference Sung, Crowley, Genel, Salber, Sandy, Sherwood, Johnson, Catanese, Tilson, Getz, Larson, Scheinberg, Reece, Slavkin, Dobs, Grebb, Martinez, Korn and Rimoin2003). In other words, T2 can be seen as the interface between efficacy and effectiveness trials, where the former are clinical studies carried out in ideal, experimental conditions, while the latter are those investigations conducted under routine clinical conditions (Tansella et al. Reference Tansella, Thornicroft, Barbui, Cipriani and Saraceno2006).
Phase 3: Late clinical trials
The next phase of clinical discovery refers to clinical studies of effectiveness and safety in target clinical populations (those with the condition to be treated), which are conducted over a longer time-scale and which can identify less frequent and longer term side-effects (Tansella et al. Reference Tansella, Thornicroft, Barbui, Cipriani and Saraceno2006). In relation to ‘complex interventions’, phase 3 includes well-controlled investigations to compare a reproducible and fully defined intervention with an appropriate alternative under everyday clinical conditions, where the sample size is large enough to give a clear-cut answer to the primary question (Campbell et al. Reference Campbell, Murray, Darbyshire, Emery, Farmer, Griffiths, Guthrie, Lester, Wilson and Kinmonth2007).
Translational block T3
Westfall et al. have suggested a third gap (T3), at which evidence can fail to progress into clinical practice (Westfall et al. Reference Westfall, Mold and Fagnan2007). This is characterized as the distance between therapeutic interventions that are scientifically proven and applicable, for example, as formulated in clinical guidelines (Michie et al. Reference Michie, Berentson-Shaw, Pilling, Feder, Dieppe, Raine, Cluzeau, Alderson and Ellis2007a), (phase 3), and the actual content of everyday clinical encounters (phase 4).
Phase 4: Implementation
The rapidly developing sector of ‘Implementation Science’ (Madon et al. Reference Madon, Hofman, Kupfer and Glass2007; Eccles et al. Reference Eccles, Armstrong, Baker, Cleary, Davies, Davies, Glasziou, Ilott, Kinmonth, Leng, Logan, Marteau, Michie, Rogers, Rycroft-Malone and Sibbald2009) is beginning to identify the complex range of factors that interrupt the uptake of evidence-based practice at T3 in terms of: (i) the intention to implement; (ii) early implementation; (iii) persistence of implementation (Tansella & Thornicroft, Reference Tansella and Thornicroft2009). Although this field is still at an early developmental stage, the journal Implementation Science is devoted to this field and has a growing scientific reputation. Nevertheless, although there are now thousands of published papers on the development of clinical guidelines across the range of healthcare, there are relatively few on how to put these guidelines into cost-effective, routine practice in any specialty (Institute of Medicine, 2001).
Locating implementation science within translational medicine
The overall purpose of translational medicine is ‘to test, in humans, novel therapeutic strategies developed through experimentation’ (Marincola, Reference Marincola2003). More specifically, translational medicine has been defined as ‘a discipline that increases the efficiency of determining the relevance of novel discoveries in the biological sciences to human disease and helps clinical researchers identify, through direct human observation, alternative hypotheses relevant to human disease. A further goal is to accelerate the rational transfer of new insights and knowledge into clinical practice for improving patients’ outcomes and public health' (Littman et al. Reference Littman, Di, Plebani and Marincola2007).
The idea of translational medicine has been rapidly adopted in recent years and includes those studies that are related to: (i) defining the biology of disease; (ii) understanding the biological effects of therapeutics in humans; (iii) developing principles for the application of therapeutics to human disease; (iv) any clinical trial related to (i) – (iii) with an endpoint of toxicity and/or efficacy (Mankoff et al. Reference Mankoff, Brander, Ferrone and Marincola2004; Soderquest & Lord, Reference Soderquest and Lord2010). From an historical point of view, the term translational medicine was until recently used in a somewhat broader sense, largely co-terminous with the whole range of the translational continuum described in this paper. It is only within the last decade that its use has been redefined more narrowly to refer to phase 1 (Marincola, Reference Marincola2003) or phase 1 and phase 2 (National Institute for Health Research, 2006) studies within the translational continuum.
To date, one common shortcoming of the conceptions of this whole translational pathway is that they are professionally driven, from left to right in Fig. 1. In other words, this vision is a simplified supply-side schema, in which scientists deliver inventions to clinicians (Perkins et al. Reference Perkins, Jensen, Jaccard, Gollwitzer, Oettingen, Pappadopulos and Hoagwood2007), who, in turn, deliver treatments to patients. Intriguingly, such thinking is not yet integrated with the conception of patient and public participation in healthcare. Specifically, at translational block T3, to date, there are few investigations about patient-related factors that accelerate or impede knowledge transfer. For example, well-informed patients are not only ‘stakeholders’ (for example, in developing clinical interventions or guidelines), but they also exert a powerful demand-side expectation for new treatments that are publicly understood to be beneficial, as has been clear in the HIV/AIDS field.
A related issue is the need to appreciate the distinction between dissemination and implementation (Rabin et al. Reference Rabin, Brownson, Haire-Joshu, Kreuter and Weaver2008). The supply-side professional incentives that motivate scientists are primarily intended to disseminate their research findings via peer-reviewed journals, which are most often read by their scientific peers. There is a lack of clarity about who should have the responsibility and the resources to put such findings into clinical practice. In particular, there are few clear incentives for scientists to provide direct-to-patient information.
A further limitation of this field of study is that there is not as yet a clear overall theoretical paradigm for implementation science studies. Recently, however, there has been increasing attention to this theoretical deficit (Gardner et al. Reference Gardner, Whittington, McAteer, Eccles and Michie2010; Michie et al. Reference Michie, Webb and Sniehotta2010; Webb et al. Reference Webb, Sniehotta and Michie2010), including a theoretically driven approach to understanding the formulation of clinical guidelines (Michie et al. Reference Michie, Pilling, Garety, Whitty, Eccles, Johnston and Simmons2007b). One integrative framework that has been recently proposed is the ‘Knowledge to Action model’, which considers three states of knowledge (discovery, invention and innovation) and which is based upon the conceptual approach that stakeholders adopt and use knowledge that has perceived utility (Lane & Flagg, Reference Lane and Flagg2010). We anticipate a greater degree of integration in future implementation science studies between the theoretical approach used and the research designs employed (Craig et al. Reference Craig, Dieppe, Macintyre, Michie, Nazareth and Petticrew2008).
In this paper, we have proposed a simple schema, consisting of five phases, to achieve a consistent terminology to understand the discovery, development, dissemination and implementation of new interventions. This schema also sets out three points at which communication blocks can occur. By better understanding the factors that promote or delay knowledge to flow across these blocks, we can accelerate progression along these translational medicine pathways and so realize earlier patient benefit.
Acknowledgements
G.T. is supported in relation to a National Institute for Health Research (NIHR) Applied Programme grant awarded to the South London and Maudsley NHS Foundation Trust and in relation to the NIHR Specialist Mental Health Biomedical Research Centre at the Institute of Psychiatry, King's College London and the South London and Maudsley NHS Foundation Trust. H.L. is supported by the NIHR Comprehensive Biomedical Research Centre at Guy's and St Thomas' Foundation Trust. This paper expresses the personal views of the authors rather than those of their host organizations. The authors thank Professor Shitij Kapur and Professor Simon Lovestone of the Institute of Psychiatry, King's College London for their comments on an earlier draft of this paper.
Declaration of Interest
None.




