Contact precautions, a category of transmission-based precautions, require healthcare personnel to don a gown and gloves prior to entering a patient’s room. Reference Siegel, Rhinehart, Jackson and Chiarello1 Based on the 2007 Healthcare Infection Control Practice Advisory Committee (HICPAC) Guidelines, the Centers for Disease Control and Prevention (CDC) recommends routine use of contact precautions when caring for patients with multidrug-resistant organisms (MDROs). Reference Siegel, Rhinehart, Jackson and Chiarello1,Reference Siegel, Rhinehart, Cic and Jackson2 In this document, MDROs are defined as microorganisms resistant to 1 or more classes of antimicrobial agents, but they are often resistant to most available antimicrobial agents. The widely referenced CDC “Appendix A: Type and Duration of Precautions Recommended for Selected Infections and Conditions” comments that MDROs should be “of clinical and epidemiologic significance” and includes methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), extended-spectrum β-lactamase (ESBL)–producing organisms, and resistant Streptococcus pneumoniae as examples. 3
Which MDROs should require contact precautions is unclear and frequently debated. Reference Young, Doernberg, Snedecor and Mallin4–Reference Bearman, Harris and Tacconelli7 Much of the data referenced in the 2007 HICPAC guidelines evaluated the impact of contact precautions on MDRO transmission as part of larger infection-prevention bundles, making it difficult to assess the relative contribution of contact precautions. Reference Siegel, Rhinehart, Jackson and Chiarello1,Reference Siegel, Rhinehart, Cic and Jackson2 Prior to the coronavirus disease 2019 (COVID-19) pandemic, some healthcare facilities stopped routinely recommending contact precautions for MRSA and VRE and did not see an increase in healthcare associated infections (HAIs) due to these organisms. Reference Haessler, Martin and Scales8–Reference Martin, Russell and Rubin10 However, many of these studies were conducted at a single center and were limited by quasi-experimental designs. Others have argued that contact precaution policies specifically for MRSA have played a large role in the significant decline in prepandemic MRSA rates both in the Veterans’ Affairs (VA) system and nationally. Reference Rubin, Samore and Harris11–Reference Popovich, Aureden and Ham13 In the Benefits of Universal Glove and Gown (BUGG) cluster-randomized controlled trial, universal gown-and-glove use was associated with decreased MRSA acquisition and no increase in adverse events. Reference Harris, Pineles and Belton14 Even less is known about the impact and utility of contact precautions on the transmission of multidrug-resistant gram-negative organisms. A cluster-randomized crossover trial in 4 European acute-care hospitals, did not demonstrate a reduction in ESBL-producing Enterobacterales carriage with the addition of contact precautions. At least 1 healthcare facility has discontinued contact precautions for ESBL-producing organisms and has shown similar findings. Reference Maechler, Schwab and Hansen15,Reference Gottlieb, Walits, Patel and Schaefer16
During the coronavirus disease 2019 (COVID-19) pandemic, shortages in personal protective equipment (PPE), and the limited availability of single-occupant patient rooms in some facilities forced many hospitals to reconsider the use of contact precautions. We hypothesized that COVID-19 may have been the initial impetus to discontinue contact precautions for MRSA or other MDROs but that many facilities did not return to prepandemic contact precautions polices, even after the supply shortage ended. Using the Emerging Infections Network (EIN), we surveyed clinicians involved in infection prevention or hospital epidemiology about which MDROs require contact precautions in their facility and what adjunctive measures are employed to minimize MDRO transmission. We compared our results to a similar survey that was administered through the EIN in 2014. Reference Russell, Beekmann, Polgreen, Rubin and Uslan17
Methods
The EIN is a CDC-funded cooperative program that serves as a “sentinel network” of infectious disease clinicians to help detect, identify, and gather information on emerging infectious diseases. Reference Pillai, Beekmann, Santibanez and Polgreen18 All authors reviewed the original 2014 EIN survey on contact precautions, Reference Russell, Beekmann, Polgreen, Rubin and Uslan17 and through iterative feedback, agreed on the revised 2022 version. The updated 8-question survey asked about the respondent’s primary inpatient healthcare facility’s (self-defined) recommendations on transmission-based precautions and adjunctive measures employed to reduce MDRO transmission. Compared to the 2014 survey, we added questions related to multidrug-resistant gram-negative organisms, Candida auris, and the impact of the COVID-19 pandemic. The questions included both discrete answer choices as well as free-text responses. The full survey is included in Supplementary Materials (online).
On September 8, 2022, we distributed the survey via email to the EIN physician members who had reported infection prevention or hospital epidemiology responsibilities. According to EIN protocol, we excluded physician members who had never answered any EIN surveys from the denominator when reporting results. We sent 2 reminder emails over the following month. We used descriptive statistics to summarize our results and, where applicable, we compared our results to the 2014 survey.
Results
Of the 708 EIN members with reported infection prevention or hospital epidemiology responsibilities, 283 (40%) responded to the survey. Most respondents were adult infectious diseases physicians (80%) with at least 15 years of experience (63%). Nearly all the respondents worked in the United States (99%), with relatively equal geographic distribution. Similar proportions of respondents reported working in community (25%), university (36%) and nonuniversity teaching facilities (29%) (Table 1). Of the initial 283 respondents, 201 (71%) reported being involved in infection prevention, and they completed at least 1 of the remaining survey questions. Another 82 (29%) were not involved in infection prevention and were excluded from the remaining survey.
Table 1. Characteristics of All Survey Respondents

Note. DOD, Department of Defense; VA, Veterans’ Affairs.
a This respondent answered the survey questions based on the policies of the inpatient facility that was connected to ambulatory clinic.
Most respondents reported that their facility routinely used contact precautions for MRSA (66%) and VRE (69%), which decreased from 93% and 92%, respectively, in 2014. Reference Russell, Beekmann, Polgreen, Rubin and Uslan17 Nearly all (>90%) reported requiring contact precautions for C. auris, carbapenem-resistant Enterobacterales (CRE), and carbapenem-resistant Acinetobacter baumannii. More variability was reported in the use of contact precautions for carbapenem-resistant Pseudomonas aeruginosa and ESBL-producing organisms (Fig. 1). Recommendations for contact precautions appeared similar between community and academic healthcare settings. Nearly 100% of respondents working in VA hospitals reported using contact precautions for MRSA, VRE, CRE and C. auris (Supplementary Table 1 online).
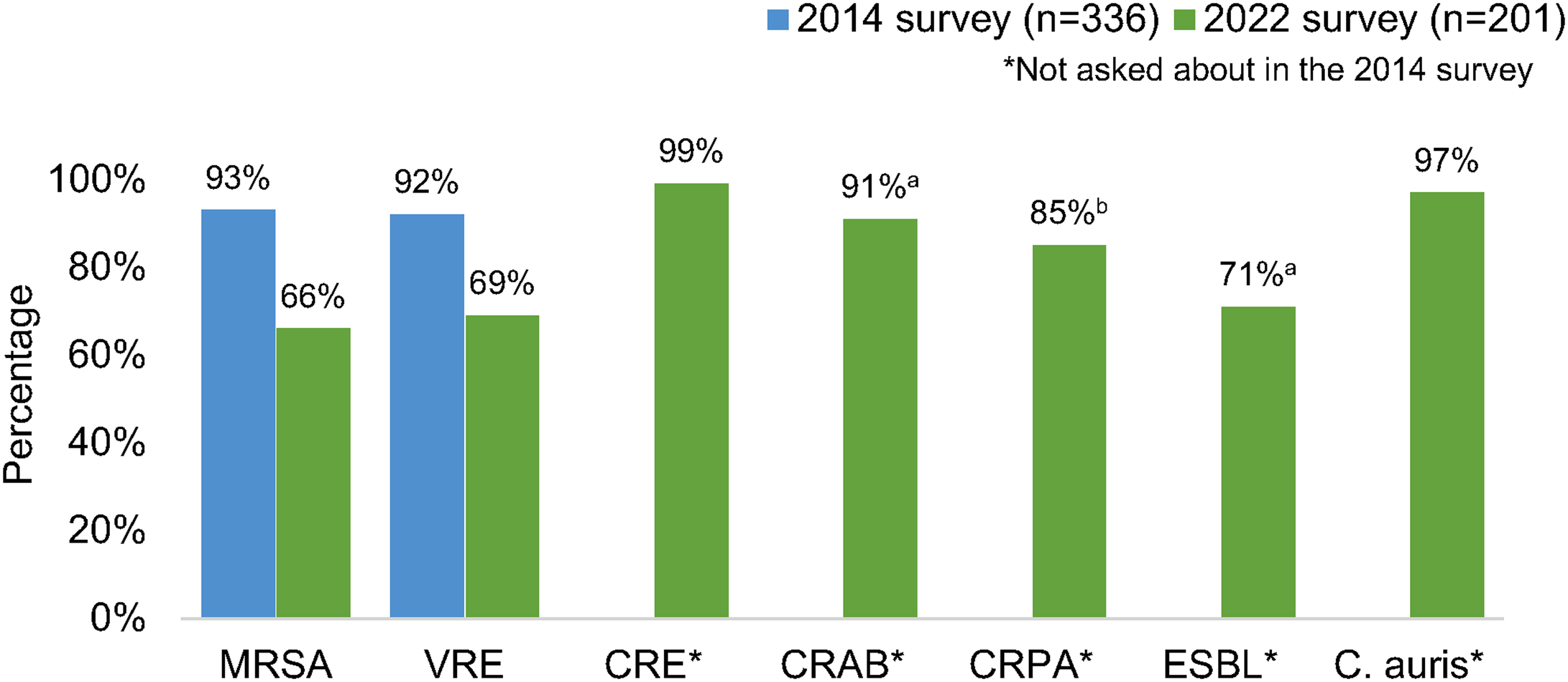
Figure 1. Percentage of respondents whose primary facility uses contact precautions for selected multidrug-resistant organisms. (a) Answered by 196 respondents. (b) Answered by 192 respondents. Note. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CRE, carbapenem-resistant Enterobacterales; CRAB, Carbapenem-resistant Acinetobacter baumannii; CRPA, Carbapenem-resistant Pseudomonas aeruginosa; ESBL, extended-spectrum β-lactamase–producing organisms.
Active surveillance for MRSA (54%) was still performed more frequently than for other MDROs (including VRE, CRE or C. auris) but was lower than reported in 2014 (81%). Active surveillance for VRE also decreased from 2014 to 2022 (Fig. 2). The duration of contact precautions employed varied by organism (Table 2). Compared to 2014, facilities in this survey were less likely to use contact precautions indefinitely for MRSA (18% vs 6%) and VRE (31% vs 11%). Reference Russell, Beekmann, Polgreen, Rubin and Uslan17 For CRE and C. auris, >75% of respondents reported that their facility either used contact precautions indefinitely or only removed contact precautions if the patient was “cleared or decolonized” (Table 2).

Figure 2. Percentage of respondents whose primary facility performs active surveillance for selected multidrug-resistant organism. (a) Answered by 197 respondents. (b) Answered by 196 respondents. Note. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CRE, carbapenem-resistant Enterobacterales.
Table 2. Duration of Contact Precautions Once a Patient Is Identified to Have a Multidrug-Resistant Organism

Note. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CRE, carbapenem-resistant Enterobacterales.
a Answered by 190 participants.
b Answered by 183 participants.
c Answered by 191 participants.
Most facilities (90%) performed chlorhexidine gluconate (CHG) bathing either in all inpatients or a subset of inpatients (Table 3). Compared to the 2014 survey, more respondents in the 2022 survey reported using CHG bathing on all inpatients (7% in 2014 vs 19% in 2022). Reference Russell, Beekmann, Polgreen, Rubin and Uslan17 Similarly, for environmental cleaning, more respondents reported using ultraviolet light or hydrogen peroxide vapor disinfection at time of any patient discharge (23% in 2014 vs 53% in 2022). Adenosine triphosphate bioluminescence assays were the most common technique for monitoring inpatient environmental cleaning (50%) (Table 4).
Table 3. Number of Respondents Whose Facility Performs CHG Bathing on the Following Inpatient Populations

a Respondents could select all subsets that applied.
b The 2014 survey included different options than the 2022 survey, so data on the 2014 subsets were not include here.
c Denominator for the percentage is 142.
d Respondents could select only a subset of surgical patients to whom this applied.
Table 4. Number of Respondents whose Facility Routinely Uses the Following Practices for Monitoring Environmental Cleaning

Note. ATP, adenosine triphosphate. Respondents were instructed to select all practices that applied.
Lastly, many (44%) reported institutional changes to contact precautions policies after the start of the COVID-19 pandemic that remained in place at the time of the survey. Furthermore, 60% did not anticipate their contact precautions practices changing in the next year.
Discussion
In this nationwide survey of >200 experienced physicians with expertise in infection prevention, contemporary use of contact precautions was heterogeneous and varied by the MDRO. Although routine contact precautions for MRSA or VRE were used in hospitals for >66% of the survey respondents, this rate notably decreased from 2014 when >90% reported using contact precautions for these pathogens. We also observed a similar decline in active surveillance for these gram-positive organisms, and active surveillance for any pathogen was rare. Contact precautions were nearly universally recommended for CRE and C. auris; however, variability existed in the recommendations for other multidrug-resistant gram-negative pathogens.
Recently, the Society for Healthcare Epidemiology of America (SHEA), in partnership with the Infectious Diseases Society of America (IDSA) and the Association for Professionals in Infection Control and Epidemiology (APIC), published updated guidance on strategies to prevent MRSA transmission in acute-care hospitals, recommending use of contact precautions for all patients infected or colonized with MRSA. Reference Popovich, Aureden and Ham13 Based on our survey, a significant proportion of facilities will now be nonadherent to this guidance, but it remains to be seen whether this SHEA update will prompt more facilities to return to prepandemic policies. The SHEA guidance acknowledges that not all hospitals are routinely using contact precautions for MRSA and includes guidance about when deviation from this approach could be considered, in the setting of performing a risk assessment, monitoring MRSA rates, and ensuring other horizontal infection prevention practices are in place to prevent MDRO transmission. Our survey did not exhaustively assess every horizontal or adjunctive infection prevention measure for MRSA, and notably, we did not ask about hand-hygiene-monitoring programs. The survey results indicated that many facilities are employing CHG bathing for a large subset of hospitalized patients. There may be room for improvement in using environmental cleaning and auditing to minimize MRSA transmission, as >20% of our respondents, who have expertise in infection prevention, were unsure if their facility routinely monitored environmental cleaning.
Although facilities appear to use contact precautions more consistently for multidrug-resistant gram-negative organisms compared to gram-positive organisms, even fewer data are available on the benefit of contact precautions for gram-negative organisms. Reference Harris, Morgan, Pineles, Magder, O’Hara and Johnson19,Reference Derde, Cooper and Goossens20 Policy decisions for gram-negative organisms are particularly challenging as they often consider both molecular and phenotypic definitions of resistance. The CDC toolkit on CRE recommends contact precautions for CRE, although it acknowledges that some institutions will only do this for carbapenemase-producing isolates. 21 Whether or not contact precautions should be used for other carbapenem-resistant organisms like P. aeruginosa, which is unlikely to be carbapenemase-producing in the United States, is unknown. Reference Reyes, Komarow and Chen22 The lack of unified policy on this and heterogeneity in practice patterns throughout the United States may confuse patients, staff, and clinicians without specific infection-control expertise when they move between multiple facilities. One potential solution may be to transform the SHEA pathogen-specific MRSA guidance into more universal MDRO guidance given the number of horizontal interventions in the MRSA guidance that may be broadly applicable to other MDROs. This guidance document could recommend shared infection prevention strategies that would be effective across different MDROs, while also highlighting any unique considerations of each MDRO included.
The COVID-19 pandemic fundamentally changed the landscape of infection prevention and hospital epidemiology. Although many initial changes to contact precautions policies in early 2020 were due to an urgent need to conserve gowns and gloves, in late 2022, >40% of respondents said changes to contact precautions remained in place. During the pandemic, HAIs increased, including hospital-onset MRSA infections. Reference Witt, Howard-Anderson, Jacob and Gottlieb23–Reference Weiner-Lastinger, Pattabiraman and Konnor25 Although this rise in HAIs is multifactorial, it brings into question whether the tail end of a pandemic is the ideal time to relax contact precautions policies. Additionally, our survey did not ask about COVID-19, but a few respondents added in free-text comments calling for the removal of contact precautions for COVID-19, which is still recommended by the CDC even though evidence of transmission of SARS-CoV-2 via contaminated surfaces or fomites is minimal. 26,27 It's possible that the continued recommendation for use of contact precautions for COVID-19 without perceived benefit by some healthcare personnel has contributed to negative attitudes toward the use of contact precautions for MDROs.
This study was strengthened by surveying experienced clinicians with expertise in infection prevention throughout the country. Because the survey respondents work in diverse settings including academic and community hospitals, our results are likely representative of most facilities across the country. However, we did not capture facilities that do not have physicians engaged in hospital epidemiology or infection prevention.
The study also had several limitations. First, respondents who work at facilities where contact precautions policies had recently changed may have been more likely to respond to the survey. However, the use of contact precautions is frequently debated among infection-prevention professionals, so major differences between respondents and nonrespondents are unlikely. Second, all our data were self-reported and, thus, were subject to bias. We did not ask respondents to include the name of their primary facility, and duplicate data may have been submitted from the same facility. Third, the survey was cross-sectional, and we could not directly compare individual or facility responses between 2014 and 2022. Fourth, we know some states and health systems require contact precautions for MRSA, so practice may not necessarily reflect attitudes or beliefs of the institution’s infection preventionists or hospital epidemiologist. Lastly, we were unable to determine whether the decline in contact precautions was specifically due to the COVID-19 pandemic; use of contact precautions may have already been declining after 2014 but prior to the pandemic.
In conclusion, we found large variation in institutional infection prevention policies aimed at preventing MDRO transmission in healthcare facilities. We believe that updated and more specific public health guidance defining which organisms require contact precautions and for what duration could help reduce this heterogeneity. Guidance documents may also benefit from including alternative recommendations or considerations for when facilities are not able to implement all recommended infection prevention measures. To inform and iteratively refine such guidance, well-designed trials are needed to investigate the benefits and any potential harms of contact precautions for prevention of MDRO transmission. As we look to the future and emerge from the COVID-19 pandemic, many healthcare organizations are struggling with burnout, workplace violence, and staffing shortages. Reference Murthy28 Our findings suggest a need to critically re-evaluate how contact precautions are used, which measures are the most effective to prevent the transmission of infectious organisms, and how we can ensure the safety of patients and healthcare personnel.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2024.11
Acknowledgments
Financial support
This research was supported by the the Centers for Disease Control and Prevention (Cooperative Agreement No. 5, grant no. NU50CK000574 to P.M.P. and S.E.B.). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Competing interests
L.B.G., J.H.A., and J.T.J. report grant funding from the Centers for Disease Control and Prevention unrelated to this work. P.M.P. reports grant funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, the CDC, and Pfizer Inc, as well as consulting fees from Eli Lily Inc, unrelated to this work.









