Xylan, the major component of hemicellulose, is a heterogeneous polysaccharide with a backbone consisting of a β-d-(1 → 4)-linked xylopyranoside backbone substituted with many side chains. Complete breakdown of xylans requires the synergistic action of several enzymes of which endo-β-1,4-xylanases (EC 3.2.1.8) are the crucial enzymes for depolymerisation(Reference Bailey1). In recent years, xylanases have attracted considerable research interest because of their potential benefits in the animal nutrition and feed industry(Reference Esteve-Garcia, Brufau and Perez-Vendrell2, Reference Diebold, Mosenthin and Piepho3). The cell walls of cereals contain up to 15 % NSP; exogenous enzymes can hydrolyse these carbohydrates into smaller units that can be utilised by animals.
The fungus Trichoderma reesei is a filamentous mesophilic fungus which has been shown to secrete large amounts of efficient xylan-degrading enzymes(Reference Wong and Saddler4). The T. reesei xylanase 2 (Xyn2) has been in industrial use for many years, since it represents more than 50 % of the total xylanolytic activity of this fungus(Reference Törrönen, Harkki and Rouvinen5). However, the industrial enzymes are often used in the unpurified form. There are many side activities that can be a problem with the use of these enzymes(Reference Wong and Saddler4). For instance, the relatively crude xylanase preparation with residual cellulolytic activity requires the careful control of process parameters to avoid damage to fibres. In addition, the residual protease may degrade xylanases of interest over time(Reference Saarelainen, Paloheimo and Fagerström6, Reference La Grange, Pretorius and Van Zyl7). The T. reesei Xyn2 produced in a recombinant host has an advantage of showing xylanase activity that is practically free of harmful side activities. Therefore, the recombinant production hosts are preferred(Reference Saarelainen, Paloheimo and Fagerström6–Reference Jiang, Deng and Zhu8). We have previously expressed the xyn2 gene in Pichia pastoris and achieved a high expression level of 500 U/ml in a 5 litre fermenter (He Jun, Yu Bing, Zhang Keying and Chen Daiwen, unpublished results). To evaluate its potential benefits for industrial applications, especially in the animal nutrition and feed industry, the enzyme has been studied to understand its physical and biochemical characteristics. Furthermore, a trial with weaned pigs has been carried out to validate the potential benefits under practical conditions.
Materials and methods
Micro-organisms and media
Recombinant P. pastoris (PX-1) was previously constructed and conserved in our laboratory. PX-1 was cultivated on either buffered glycerol–complex medium (1 % yeast extract, 2 % peptone, 1·34 % yeast nitrogen base, 1 % glycerol) or buffered methanol–complex medium (1 % yeast extract, 2 % peptone, 1·34 % yeast nitrogen base, 1 % methanol), respectively. Zeocin was added to a final concentration of 100 μg/ml.
Xylanase 2 production in a 15 litre fermenter
A large-scale production was performed (six times) in a 15 litre fermenter (Nanjing Runze Bioengineering Equipment Co. Ltd, Nanjing, China). PX-1 was cultured in 2 litre Erlenmeyer flasks containing 500 ml of buffered glycerol–complex medium at 30°C on a rotary shaker at 200 rpm. After 18–20 h incubation, cell pellets were harvested by centrifugation at 3000 g for 5 min. For xylanase induction, the cell pellets were re-suspended in 9 litres of buffered methanol–complex medium. The induction was maintained for 5 d by adding absolute methanol to a final concentration of 1 % every day. Relative percentage of dissolved oxygen was maintained above 30 % via adjusting the agitation rate. Supernatant fraction samples were collected every day and kept at − 80°C before analysis. At the end of fermentation, all culture supernatant fraction was collected by centrifugation and subsequently used as the enzyme source for the animal trial.
SDS-PAGE and enzyme activity assay
SDS-PAGE on 15 % polyacrylamide was performed by the method of Laemmli(Reference Laemmli9). The protein fractions (induction supernatant fraction) were boiled for 3 min and applied to the gel. Proteins were visualised by Coomassie brilliant blue R 250 staining. The protein concentration was determined by the Bradford assay using bovine serum albumin as a standard(Reference Bradford10). Xylanase activity was assayed by the method described by Bailey et al. (Reference Bailey, Biely and Poutanen11), with 1 % oat-spelt xylan (Sigma, St Louis, MO, USA) as the substrate at 50°C for 10 min. Appropriate dilutions of the recombinant protein (culture supernatant fraction) in 50 mm-sodium citrate buffer (pH 5·0) were used as the enzyme source. The amount of released sugar was determined by the dinitrosalicylic acid method described by Miller et al. (Reference Miller, Blum and Glennon12). One unit of xylanase activity was defined as the quantity of enzyme that liberated reducing sugar at the rate of 1 μmol/s. The temperature optimum was measured by performing the xylanase activity assay at temperatures ranging from 20 to 90°C. Thermostability was tested by heating the enzyme samples for different times at various temperatures, and the activity was assayed at 50°C for 10 min. Assays at different pH values were performed at the optimal temperature over a pH range of 3·0 to 8·0. The buffers used were 50 mm-citrate (pH 3·0), 50 mm-citrate phosphate (pH 4·0 to 7·0) and 50 mm-phosphate (pH 8·0), respectively.
Analysis of substrate specificity and hydrolysis products
The substrates (birchwood xylan, oat-spelt xylan, beechwood xylan, gellan gum and carboxymethyl cellulose) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Avicel (cellulose microcrystalline) was purchased from Merck (Darmstadt, Germany). The determining of substrate specificity was carried out in 50 mm-citrate phosphate (pH 5·0) containing 2·0 mg/ml of each substrate at 50°C for 10 min. For each assay, six different substrate concentrations were prepared in 50 mm-citrate phosphate (pH 5·0), and incubated with the enzyme at 50°C for 5 min. The K m and catalytic rate constant (k cat) values were calculated from the kinetics data as described by Jiang et al. (Reference Jiang, Deng and Zhu8).
Oat-spelt xylan (40 mg) was incubated with 100 U of the enzyme in 2 ml 50 mm-citrate phosphate (pH 5·0) and the reaction was performed at 50°C for 12 h. The hydrolysis products were analysed by TLC using silica gel plates 60F 254 (E. Merck, Darmstadt, Germany). Aliquots (100 μl) of the samples were collected at 5, 10 and 30 min, and at 1, 2, 4, 8 and 12 h of the incubation period and 1 μl of the sample was spotted on the TLC plates. The plates were subsequently developed with two runs of acetonitrile–water (85:15, v/v) followed by heating for a few minutes at 130°C in an oven after spraying the plates with a methanol–sulfuric acid mixture (95:5, v/v)(Reference Jiang, Deng and Zhu8). A xylo-oligosaccharide mixture (Suntory Ltd, Osaka, Japan) consisting of xylose, xylobiose and xylotriose was used as the standard.
Animals and housing
The experimental protocols used in the present study were approved by Sichuan Agricultural University Institutional Animal Care and Use Committee. Twenty-four weaned pigs (Duroc × Landrace × Yorkshire) with an average initial body weight of 10·2 ± 0·35 kg were selected and randomly allotted to four dietary treatments (n 6) with equal numbers of males and females in each group. Treatments consisted of: (1) control without Xyn2 supplementation; (2) Xyn2 added at a concentration of 500 U/kg diet (LD); (3) Xyn2 added at a concentration of 1000 U/kg diet (MD); (4) Xyn2 added at a concentration of 1500 U/kg diet (HD).
Pigs were housed individually in metabolism cages (0·7 × 1·5 m) with woven wire flooring in an environmentally controlled room (22–24°C) and were given ad libitum access to water through a water nipple. Pigs were hand-fed four times per d (08.00, 12.00, 16.00 and 20.00 hours) in bowl feeders to make sure fresh feed was available, and allowed a 5 d adjustment to the experimental diets. The diet adjustment period was followed by a collection period, which include a 4 d collection of faeces to determine the apparent nutrient digestibility. Pig weights and feed consumption were determined daily throughout the duration of the trial.
Experimental diets
To prepare a diet containing sufficient amounts of the target substrate (xylan), both wheat and wheat bran were utilised. The diet was formulated on the basis of nutrient requirements for 10–20 kg pigs (National Research Council(13)). Dietary amino acids were supplied by maize, wheat, soyabean meal, rapeseed meal, fish meal, and vitamin and minerals were supplied by vitamin and mineral supplements (Table 1). Synthetic dl-methionine was added to the diets to meet minimal methionine–cystine concentrations. Each ingredient source was analysed for protein and amino acid content. The analysed amino acid contents of the ingredients were used to formulate the experimental diets. The unprocessed enzyme (obtained from fermentation) was added to the diet by using a portion of the basal diet (5·0 kg/100 kg of entire diet) as the carrier.
Table 1 Composition of experimental diets (as-fed basis)
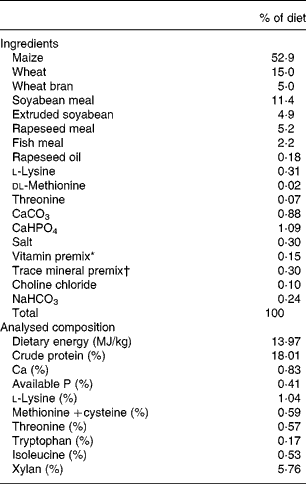
* To provide (per kg): 5·7 μg vitamin A; 24·25 μg vitamin E; 9·65 μg vitamin D; 1·1 mg vitamin K (menadione dimethylpyrimidinol bisulfate); 5 mg vitamin B1; 15 mg riboflavin; 25 mg niacin; 30 mg d-pantothenic acid; 0·05 mg vitamin B12.
† To provide the following (mg/kg entire diet): Fe as FeSO4·7H2O, 100 mg; Mn as MnSO4·7H2O, 40 mg; Zn as ZnO, 80 mg; Cu as CuSO4·5H2O, 10 mg; Se as NaSeO3, 0·3 mg; iodine as KI, 0·3 mg.
Sample collection and analysis
Faeces were collected and weighed every 24 h, with 5 % samples being saved and blended for each pig within the collection period. Then the total quantity of faeces collected was pooled and freeze-dried to a constant weight. The dried faecal matter was ground and stored at 4°C until it was analysed for nutrients.
Quantitative composition was determined on each of the samples using the following analytical methods: DM content according to AOAC method 964·22; crude protein according to method 955·04; ash according to method 923·03; Ca according to method 927·02; P according to method 965·05(14). The acid-detergent fibre content was determined following the procedures of Van Soest et al. (Reference Van Soest, Robertson and Lewis15). Gross energy was determined using an adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, IL, USA).
Calculation and statistical analysis
The experimental model used was a completely randomised design with four treatments of added enzyme. Data from the animal trial were analysed with an ANOVA procedure of SPSS 13.0 (SPSS Inc., Chicago, IL, USA) followed by Duncan's test for multi-group comparisons. To determine whether there was a significant linear response to the enzyme, the orthogonal polynomial contrasts were carried out using the general linear model (GLM) procedure. Data were expressed as mean values with their standard errors. Differences with P < 0·05 were considered to be significant.
Results
Xylanase 2 production in a 15 litre fermenter
The recombinant P. pastoris PX-1 was cultured in a 15 litre fermenter. To determine the highest xylanase yield, the experiment was repeated three times. The size of recombinant xylanase (Xyn2) determined by SDS-PAGE was 21 kDa, similar to that of the native xylanase secreted by T. reesei (Fig. 1). Xyn2 was the major protein (over 90 % of total protein as detected by densitometry) secreted by PX-1 into the culture medium. Therefore, the procedure for protein purification was not necessary. The highest xylanase activity (560 U/ml) in the culture supernatant fraction was recorded after 78 h induction (Fig. 2). However, the highest cell wet weight (78 g/l) was obtained at 96 h. Both xylanase activity and cell wet weight decreased slightly in the late induction period (after 96 h).

Fig. 1 SDS-PAGE analysis for products in the culture supernatant fraction. M, protein marker; 1–6, culture supernatant fraction after 60, 66, 72, 78, 84 and 90 h fermentation.

Fig. 2 Enzyme production of Pichia pastoris (PX-1) and cell wet weight after induction at different times. (–●–), Xylanase activity; (–△–), cell wet weight. Values are means, with standard errors represented by vertical bars.
Effect of pH and temperature on β-xylanase activity
The recombinant β-xylanase activity peaked between pH 4·5 and 7·5, with the highest activity measured at pH 6·0 in 50 mm-citrate buffer (Fig. 3(A)). The optimal temperature for this enzyme was at 60°C (Fig. 3(B)). Although the highest activity was measured at 60°C, the enzyme was not stable at this temperature (only 30 % activity retained after 30 min incubation at 60°C). However, the enzyme was stable at 50°C, and the total activity remained more than 94 % after 30 min incubating at this temperature (Fig. 3(C) and (D)).

Fig. 3 Characterisation of the recombinant xylanase 2 (Xyn2). (A) Effect of pH on the activity of Xyn2. (B) Effect of temperature on the activity of Xyn2. (C) Determination of temperature stability after preincubating the enzyme in the absence of the substrate for 30 min at 50, 60 and 70°C. (D) Determination of thermostability at different temperatures (50°C (–♦–), 60°C (–□–) and 70°C (–▲–)) by preincubating the enzyme at these temperatures in the absence of substrate for 5, 10, 15, 20, 25 and 30 min before measuring its activity. The xylanase activity before the preincubations at different temperatures was taken as 100 %. Values are means, with standard errors represented by vertical bars.
Analysis of substrate specificity and hydrolysis products
The hydrolytic activity of the recombinant enzyme on various substrates was determined (Table 2). The highest activity (100 %) was observed with the oat-spelt xylan followed by the birchwood xylan (91 %). The enzyme exhibited relatively low activities towards cellulosic substrates, such as gellan gum (12 %), Avicel (1·7 %) and carboxymethyl cellulose (1·5 %). The Michaelis–Menten constants were determined for the substrates. The K m and catalytic rate constant (k cat) were 1·8 mg/ml and 435·7/s for birchwood xylan, and 1·1 mg/ml and 512·4/s for oat-spelt xylan, respectively. The predominant endproducts of hydrolysis of oat-spelt xylan were xylobiose and xylotriose (Fig. 4). The results confirmed that the recombinant Xyn2 was an endoxylanase.
Table 2 Substrate specificity and kinetic constants for the recombinant xylanase
(Mean values with their standard errors of six replications)
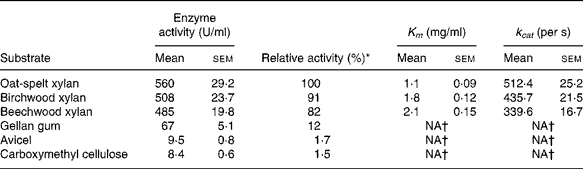
k cat, Catalytic rate constant; NA, not available.
* The activity for oat-spelt xylan was defined as 100 %.
† Difficult to measure, so not presented.

Fig. 4 Analysis of the hydrolysed products by recombinant xylanase 2. Oalt-spelt xylan (40 mg) was incubated with 100 U of the enzyme in 2 ml 50 mm-citrate phosphate (pH 5·0) and the reaction was performed at 50°C for 12 h and then the hydrolysates were analysed by TLC. M, protein marker.
Effect of xylanase 2 on performance of weaned pigs
As shown in Fig. 5, there was a positive effect of enzyme treatment (500 U/kg) on average daily body-weight gain (increased 16·9 %; P < 0·05). But the MD and HD groups (a higher concentration of Xyn2 supplementation) failed to present a positive effect on average body-weight gain (P>0·10). There was no effect of enzyme treatment on feed intake in the LD group (P>0·10). However, the feed intake for the MD and HD groups decreased by 6·7 % (P < 0·05) and 5·1 % (P = 0·07), respectively (Fig. 6(A)). The ratio of feed intake:body-weight gain significantly decreased in the LD group (P < 0·05). Like with the feed intake, a higher concentration of Xyn2 supplementation did not improve the feed efficiency (Fig. 6(B)).

Fig. 5 Effect of recombinant xylanase 2 (Xyn2) supplementation on average daily body-weight gain (ADG) in weaned pigs (n 6). Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).

Fig. 6 Effect of recombinant xylanase 2 (Xyn2) supplementation on feed intake (A) and feed efficiency (B) in weaned pigs (n 6). Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Effect of xylanase 2 on nutrient digestibility of weaned pigs
There was a positive effect (0·05 < P < 0·10) on the apparent digestibility values of crude protein, ash, Ca and acid-detergent fibre upon Xyn2 supplementation in the LD group (Table 3). However, there was no positive effect of enzyme treatment on energy utilisation (P>0·10). Compared with the control group, most of the digestibility values in the MD and HD groups were not improved by enzyme supplementation. On the contrary, there was a negative effect on the digestibility values of Ca in the MD and HD groups.
Table 3 Influence of recombinant xylanase 2 (Xyn2) supplementation on nutrient digestibilities of weaned pigs (n 6)
(Mean values with their standard errors)

Control, without Xyn2 supplementation; LD, Xyn2 added at a concentration of 500 U/kg diet; MD, Xyn2 added at a concentration of 1000 U/kg diet; HD, Xyn2 added at a concentration of 1500 U/kg diet.
a,b,c Mean values within a row with unlike superscript letters were marginally significantly different (P < 0·10).
Discussion
In recent years, several commercial enzymes have been produced to act on carbohydrates not readily digested by single-stomached animals. These are known as NSP and have anti-nutritional properties, depressing animal performance(Reference Jaroni, Scheideler and Beck16). NSP of particular concern include β-glucans, xylans, pentosans and arabinogalactans. Today, the two extensively used enzymes are xylanase in wheat-based diets and β-glucanase in barley-based diets. Enzyme supplementation to diets for poultry has usually shown more beneficial effects than supplementation to diets for pigs, particularly if highly viscous diets are used(Reference Diebold, Mosenthin and Piepho3). The mechanisms of xylanase action can be attributed to the reduced water-holding capacity and subsequent gut fill, and increased digestibility of the cell wall fraction, and a decrease in gut viscosity, resulting in a greater exposure of the gut contents to absorptive surfaces(Reference Cadogan, Choct, Campbell and Granwell17).
Currently, heterologous expression has become one of the main tools for the production of industrial enzymes(Reference Kirk, Borchert and Fuglsang18). Many xylanase genes have been successfully expressed in different hosts(Reference Saarelainen, Paloheimo and Fagerström6–Reference Jiang, Deng and Zhu8). In our laboratory, the xyn2 gene of T. reesei, coding Xyn2, was previously expressed in P. pastoris. The complete characterisation of the recombinant enzyme (Xyn2) is a key-point condition to evaluate the possibility of its use in industrial applications. As shown in the present study, the recombinant Xyn2 is the predominant protein in the extracellular medium (Fig. 1). The molecular mass (21 kDa) is close to previously purified T. reesei Xyn2(Reference Törrönen, Mach and Messner19). The highest β-xylanase activity (560 U/ml) for PX-1 was obtained in a 15 litre fermenter after 78 h fermentation (Fig. 2). The expression level is higher than that of levels obtained from a recombinant Saccharomyces cerevisiae strain (90 U/ml)(Reference La Grange, Pretorius and Van Zyl7) or any Escherichia coli expression system(Reference Junli, Guixue and Li20, Reference Chenyan, Jiangyu and Shanshan21). The expression level compares well with that of hyperproducing mutant T. reesei VTT-D-86 271 (324 U/ml) if one takes into account that the T. reesei culture supernatant fraction contains the complete battery of enzymes involved in xylan degradation(Reference Bailey, Buchert and Viikari22). The pH and temperature optima (pH 6·0; 60°C) of the recombinant Xyn2 compares well with Xyn2 secreted by recombinant S. cerevisiae (Reference La Grange, Pretorius and Van Zyl7). Both of them have a moderate thermostability and inactivated rapidly above 60°C. The hydrolytic activity of the recombinant Xyn2 on various substrates was determined in the present study (Table 2). The highest activity (100 %) was observed with the oat-spelt xylan as the substrate followed by the birchwood xylan (91 %). The K m for oat-spelt xylan and birchwood xylan was 1·1 and 1·8 mg/ml, respectively. The higher affinity towards oat-spelt xylan indicated that the recombinant Xyn2 is more suitable for use in the feed industry other than the paper industry. The products of hydrolysis of oat-spelt xylan were predominantly xylotriose and xylobiose and a smaller amount of xylose (Fig. 4), which confirmed the endo-acting nature of the recombinant Xyn2. The xylo-oligosaccharides (i.e. xylobiose) have been found to have a stimulatory effect on the selective growth of intestinal bifidobacteria, which are important for the maintenance of a healthy intestinal microflora(Reference Jeong, Park and Kim23).
In the present study, the potential benefits of the recombinant enzyme in the nutrition of weaned pigs have been validated. Previous study has shown that pigs receiving enzyme-supplemented diets usually fail to show the same consistent improvements in nutrient digestibilities and growth performance as observed in poultry(Reference Partridge, Bedford, Partridge and Bedford24). However, in young pigs, poor nutrient utilisation has been attributed, in part, to the immaturity of the digestive system, including hydrolysis of NSP. Significant improvements in growth performance and nutrient digestibilities were observed when xylanase was supplemented to wheat-based diets(Reference Diebold, Mosenthin and Piepho3). In the present study, our statistics results showed a significant linear response to the recombinant xylanase. The supplementation of recombinant Xyn2 (500 U/kg diet) increased the apparent digestibilities of crude protein, ash, Ca and acid-detergent fibre, which is consistent with an improved performance (average body-weight gain) and feed efficiency (Fig. 5). Yin et al. (Reference Yin, McEvoy and Schulze25) also reported increases in the digestibilities of some nutrients when xylanase was supplemented to a wheat-based diet in studies with grower pigs. It has to be mentioned that xylanase supplementation may not be effective if sufficient amounts of the target substrate are unavailable. In the present study, the entire diet contained xylans at a level of 5·76 %.
Despite the positive effect of enzyme supplementation on performance and nutrient digestibilities in the LD group, there was no improvement of such parameters in the MD and HD groups. This might be explained, in part, by the liquid form of the enzyme. The enzyme prepared through fermentation usually has a bad odour. The bad odour together with a greater enzyme supplementation in the diet may decrease the feed intake. This was supported by the present results, as the feed intake decreased 6·7 % in the MD group and 5·1 % in the HD group, respectively (Fig. 6(A)). Therefore, a proper process step, such as ultrafiltration, dilution and spray drying is indispensable to the fermentative products(Reference Susanto, Arafat and Janssen26, Reference Langrish and Fletcher27).
In conclusion, the results obtained from the present study indicated that the recombinant Xyn2 is more suitable for use in the feed industry. A diet supplemented with the unprocessed enzyme at a lower level improves both the performance and nutrient digestibilities in weaned pigs. However, a proper process is needed to improve its potential benefits in the nutrition of young pigs.
Acknowledgements
The present study was funded by the Feed Biotechnology Project of Sichuan Province of China with grant no. 2007Z06-050, the Youthfund Project of Sichuan Ministry of Education with grant no. 00924200 and the Program for Changjiang Scholars and Innovative Research Team in University with grant no. IRTO555-5, China Ministry of Education.
J. H., J. Y. and L. W. participated in the experimental design, carried out the molecular genetic and biochemical experiments, participated in data interpretation and helped draft the manuscript. B. Y. conceived of the study. D. C. directly supervised the project, participated in its experimental design and data interpretation and was responsible for writing the manuscript.
The authors state that there are no conflicts of interest in this field.











