INTRODUCTION
The incidence of leptospirosis, a bacterial zoonotic disease, is increasing worldwide [Reference Pappas1]. Although asymptomatic infections are most common, symptomatic disease ranges from mild anicteric febrile illness to severe forms (acute renal failure, pulmonary forms, etc.) and sometimes death [Reference Levett2]. Many wild or domestic animals serve as a reservoir for pathogenic Leptospira and people mostly become infected through contact of abraded skin or mucous membranes with water/soil contaminated by the urine excreted by reservoir animals. Leptospira are able to survive in soil and water for a long period depending on temperature and humidity [Reference Levett2], with warm and humid environments considered as favourable for Leptospira survival. Therefore, the incidence is highest in tropical countries with a peak during rainy seasons, while in temperate areas most human cases are recorded during summer. The emergence of this neglected disease is mainly due to the occurrence of more frequent natural disasters (floods, hurricanes, etc.), the important expansion of slums as well as the development of aquatic leisure or sport competitions both in tropical and temperate areas [Reference Wright, Goot and Rogers3–Reference Naranjo9].
Réunion Island is a French overseas department situated in the South West Indian Ocean, 700 km East of Madagascar. The climate is tropical with a rainy season from December to May and a dry season from June to November. Leptospirosis is endemic on Réunion Island with an annual average incidence of 5·6 cases/100 000 inhabitants [Reference Pagès10–Reference Mailloux12] with most cases occurring during the rainy season [Reference Pagès10]. Leptospira interrogans serogroup Icterohaemorrhagiae, the dominant species causing leptospirosis on Réunion Island is responsible of many severe forms [Reference Paganin13–Reference Law-Koune15]. The black rat, Rattus rattus is considered the primary reservoir of Leptospira spp. [Reference Mollaret, Mailloux and Debarbat16–Reference Desvars, Michault and Chiroleu18]. Since 2004, a specific surveillance system of leptospirosis has been implemented including systematic reporting together with environmental investigations around incident cases. This surveillance showed that 12% of infections were actually associated with aquatic leisure (fishing, swimming, kayaking, canyoning) between 2004 and 2012 [Reference Pagès10]). In the present study, we investigated a leptospirosis outbreak occurring after a triathlon competition with the aim of characterizing Leptospira in both patients and rodents at the serological and molecular level and highlighting variables at risk for infection.
OUTBREAK DETECTION
On 29 March 2012, a general practitioner and the medical referent of the triathlon league informed the Regional Health Agency (Agence Régionale de Santé Océan Indien) that leptospirosis cases had occurred in participants of a triathlon taking place in the eastern part of the island (Triathlon de la Rivière des Roches, 3 March). A cluster of five cases linked to the event was identified and included three confirmed cases (real-time PCR positive from blood or urine samples), one possible case (fever > 38·5 °C with arthralgia and myalgia together with positive ELISA IgM) and one suspected case (fever with arthralgia and myalgia together without diagnostic investigation). The contamination was linked to the competition as the confirmed or possible cases were from different clubs and geographical origin. Immediately, an investigation was initiated in order to assess the number of athletes who developed symptoms following the event, identify risk factors, and provide the general public with health recommendations. We present herein the results of this investigation together with recommendations for the implementation of some protective measures in order to reduce leptospirosis risk during aquatic races.
METHODS
Epidemiological investigation
Several competitions were co-organized on 3 March during this same event, including adult races (biathlon, triathlon, triathlon relay) and child/adolescent races (biathlon, triathlon). A retrospective cohort study was designed to investigate the outbreak. The contact details of all participants were obtained from the organizers. All participants or their legal representative were contacted by phone or email and any sign of illness in the month following the triathlon was recorded. For each illness episode, the clinical and biological manifestations and the final diagnosis were recorded. A standardized questionnaire was administered by phone to all adults that had participated in an aquatic race in order to collect demographic data (age, sex, district of residence), to report the existence of any skin lesions before and/or after the triathlon, the protection/disinfection measures of these wounds following the triathlon, to record if water was swallowed while swimming and the implementation of any preventive measure (swimming goggles, swim suit) by the participants as well as all medical data of possible importance (use of antibiotics, existence of symptoms within the month following the race including fever, chill, headache, arthralgia and myalgia). All activities at risk for leptospirosis during the month preceding the onset of symptoms such as fishing, swimming, gardening, farming or hunting were also recorded for confirmed, possible and suspected cases. Finally, the participants were asked whether they had any knowledge on leptospirosis before the triathlon (way of contamination, protective measures) and whether they intended to preventively use any protective measures during future races.
Outbreak case definition
All persons participating in one of the races on 3 March and reporting a fever within the following 30 days were considered as suspected leptospirosis cases. Laboratory-confirmed cases were classified according to the case definition routinely used by the regional surveillance system and based on clinical and/or biological data. Cases were considered as confirmed when testing positive through either culture, polymerase chain reaction (PCR) from blood or urine, or microscopic agglutination test (MAT) on at least a single serum with titre >1/400; cases were considered possible ones when patients tested positive for leptopsirosis ELISA IgM and showed clinical manifestations compatible with leptospirosis, i.e. fever > 38·5 °C with arthralgia and/or myalgia.
Laboratory investigation of clinical cases
Samples were obtained from suspected cases that underwent medical consultation. Blood collected on EDTA and urine samples were obtained from patients during the first 10 days of illness and were tested by real-time PCR following a previously described protocol [Reference Stoddard19, Reference Woo20]. DNA extracts obtained from patients were sent for 16S rDNA direct sequencing to the French National Reference Center (NRC) for Leptospirosis (Institut Pasteur, France) using a previously described protocol [Reference Cerqueira21]. In addition, blood samples obtained following the first week of symptoms were tested with a commercial kit (Serion GmbH ESR 125, Germany) or an in-house kit for ELISA IgM detection and MAT [Reference Levett2]. Serogroups included in the MAT screening panel were those routinely used by the regional hospital [Reference Gomard22].
Environmental investigation
Rainfall data were obtained from a Météo France station located 800 m from the estuary of the Rivière des Roches where the aquatic race took place. In the past, leptospirosis cases have been registered both in the estuary and upstream in swimmers and fishermen and also in people collecting grass for their livestock. Rats are considered to be abundant along the rivers and in the crops (sugarcane, pineapple, etc.). Four weeks preceding the triathlon, rats were trapped for an independent research programme aimed at describing leptospirosis epidemiology (LeptOI Programme) along a transect edging the coastline and located in a wetland, 600–900 m south of the rivermouth (see [Reference Guernier23]) for sampling description. Rattus spp. were identified using morphological criteria and were submitted to Leptospira detection through real-time PCR [Reference Smythe24]. Leptospira-positive samples were further sequenced at the 16S rRNA locus in order to compare the bacterial haplotypes infecting rats to those detected in human cases. MAT was performed on rats’ sera using the same screening panel as used for human tests.
Statistical analysis
Results of the investigation were entered into EpiData v. 3.1 (EpiData Association, Denmark). Data were analysed with Stata v. 11 statistical software (StataCorp., USA). Laboratory-confirmed cases were compared to people without clinical manifestations. The aim was to identify risk or protective factors associated with the occurrence of leptospirosis in competitors. χ 2 test was used to compare for categorical data (i.e. use of a neoprene suit, presence of wounds, etc.). The t test was used to compare continuous data (i.e. age) and McNemar's test was used to compare paired data (knowledge about leptospirosis before and after the outbreak, intention of using protective measures for future races). The risk ratio (RR) for dichotomous variables was calculated.
RESULTS
Epidemiological investigation
Of the 160 athletes competing in the different races, 102 were contacted and 101 (63·1%) agreed to participate in the study. The specific participation rates were 69·61% (n = 101) and 87·8% (n = 44) for all swimmers and adult swimmers, respectively. Participation rates by categories are presented in Table 1. The participation rate was significantly lower for children aged 5–8 years (47%) but due to the weather conditions, their aquatic race had been cancelled and children were only involved in a short running race. There was no significant difference between respondents and non-respondents in terms of sex or age (data not shown). The median age was 37 (range 16–60) years for adults and 10 (range 5–16) years for children and adolescents. All participants were French; 158 were actually living in Réunion while two participants had arrived from continental France.
Table 1. Description of response rates to the survey by sex and by type of race and comparison of ages of responders and non-responders using t test, Triathlon de la Rivière des Roches athletes, Réunion Island, March 2013
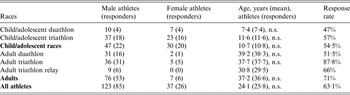
n.s., Not significant.
No illness was reported in the 50 adults or children that participated in the biathlon (without swimming race). Eleven suspected leptospirosis cases were identified in the 44 adults participating in a swimming contest: nine were laboratory confirmed (eight males, one female), one case was considered as negative (PCR, IgM and MAT negative) while no biological data could be obtained from the last suspected case (fever spontaneously resolved within 3 days). As shown in Table 2, only 7/9 suspected cases were confirmed cases [one blood positive quantitative PCR (qPCR), one urine positive qPCR, one qPCR positive from blood and urine DNA, and four positive MAT on a single serum with titre >1/400] following our case definition, while the two remaining cases were considered as possible cases (positive ELISA IgM and clinical manifestations compatible with leptospirosis). All cases occurred 10–17 days after the race (Fig. 1) with a median incubation period of 13 days. All confirmed leptospirosis cases were adult swimmers (eight triathletes and one relay swimmer) leading to an attack rate of 23% [95% confidence interval (CI) 12·6–33·8] within this category (see Table 3). Of note, two cases reported other possible exposures during the month preceding the first signs: one lived in a house surrounded by sugarcane fields and had trapped rats in his garage, the other bathed in a natural basin during a trail in the forest 1 week after the race.
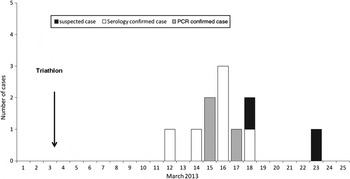
Fig. 1. Date of fever onset for suspected and laboratory-confirmed cases of leptospirosis in Triathlon de la Rivière des Roches athletes, Réunion Island, March 2013.
Table 2. Laboratory results for leptospirosis outbreak in Triathlon de la Rivière des Roches athletes, Réunion Island, March 2013
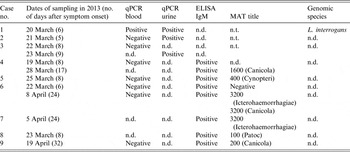
n.d., Not done/not available; n.t., not tested.
Table 3. Attack rates (AR) calculated for the whole population (respondents and non-respondents) and for respondents, Triathlon de la Rivière des Roches athletes, Réunion Island, March 2013
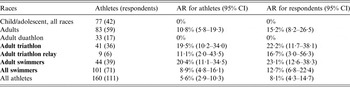
CI, Confidence interval.
As shown in Figure 2, the majority of patients had non-specific algo-febrile illness accompanied by digestive symptoms, one patient had jaundice, one patient had pulmonary manifestations and two patients had neurological manifestations (e.g. paresthesia of hands and feet). Five patients were hospitalized but all patients eventually recovered without sequelae.

Fig. 2. Clinical manifestations in nine cases of leptospirosis that occurred after the Triathlon de la Rivière des Roches, Réunion Island, March 2013.
Investigation of risk factors
The epidemiological investigation was implemented for adult swimmers only. Thirty-seven (36 triathletes and one relay swimmer; 32 men, five women) out of the 39 responders including all positive cases agreed to participate in the study. The attack rates were not statistically different between men and women (33·3% vs. 25%, respectively) or according to age (mean age 33·4 and 38·9 years for men and women, respectively). Knowledge on leptospirosis or protective behaviours did not significantly protect against infection (data not shown). Swallowing river water was not associated with leptospirosis and the use of antibiotics within days following the event did not show any significant difference in attack rates: although none of the positive cases had taken any antibiotic following the race, five negative cases had received antibiotic treatment for distinct reasons: two for ear, nose and throat (ENT) infections, one for an infected wound during the race, one for dental care and one as self-medication for leptospirosis chemoprevention. Positive cases reported significantly more wounds (either old or directly resulting from the race) (RR 4·5, 95% CI 1·6–13) than controls although there was no significant effect of wound disinfection following the race. In addition, positive cases wore complete neoprene suits less often than did negative cases (RR 4·3, 95% CI 1·3–14·5). There were significantly more cases in competitors unlicensed to the triathlon league (4/4) than in licensed competitors (5/33) (RR 6·6, 95% CI 2·9–14·8). Before the race, only 8% of triathletes had taken preventive measures against leptospirosis while following the outbreak 48·8% of the competitors claimed they would consider preventive measures in future races. This increase was statistically significant (P < 0·001, McNemar's test for paired data).
Laboratory investigation of human cases
Of the three DNA extracts sent to the NRC, 16S rDNA could be amplified only for one patient (using 70 cycles), the sequencing allowed identification of the genomospecies as L. interrogans, but it was not possible to proceed further typing (VNTR negative) due to low DNA concentration (Table 2).
Environmental investigation
According to Météo France, there was no significant rain episode during the week preceding the race except for heavy rainfall beginning on the eve of the race and continuing during the races, resulting in a brownish coloration of the river obviously loaded with organic matter while riverbanks were consistently muddy. Rising river level led organizers to cancel the swimming competition for children while adult competitors had to walk barefoot in puddles and mud before and after the swimming event.
Altogether, 25 rats (two Rattus norvegicus, 23 Rattus rattus) were trapped near the river before the race and analysed by real-time PCR for the presence of pathogenic Leptospira. Infection rate was 68% for Rattus spp. (17 positive animals) and 16S partial sequencing revealed the presence of a single haplotype identical to the haplotype found in the single sequenced human case. Out of the ten rat sera tested by MAT, two were positive and reacted against Icterohaemorrhagiae serogroup.
DISCUSSION
We report herein the first leptospirosis outbreak following a sports event on Réunion Island. The presence of cutaneous cuts was the only investigated risk factor found associated with leptospirosis whereas the use of complete neoprene suits was shown to protect against the disease. We associated a higher attack rate in unlicensed competitors (RR 6·6, 95% CI 2·9–14·8) with a possible under-equipment of this category of competitors. Both findings are in favour of an infection of adult swimmers by abraded or wounded skin. Presence of wounds, type of swimming suit and membership of a triathlon club are obviously correlated but due to the low number of cases and missing data, it was not possible to conduct a robust multivariate analysis.
The participation rate in the present study was 63%, which is in the range of rates reported in previous recreational leptospirosis outbreaks (i.e. 28–100%) [Reference Sejvar7–Reference Naranjo9, Reference Morgan25–Reference Haake29]. As there was no difference between respondents and non-respondents in terms of sex and age, our sample may be considered as representative especially for adults (87·8% of participants), who are most exposed to Leptospira infection. Due to a lack of response, the estimation of the total attack rate (8·1%), the children's attack rate (0%) and the swimmers’ attack rate (12·7%) may have been underestimated. Nevertheless, the attack rate in adult swimmers (23·1%) is reliable as is the description of their risk factors. Although none of the confirmed leptospirosis patients had used antibiotics following the race, five competitors reported antibiotic treatment, of which one was for leptospirosis prevention. This treatment may have prevented the occurrence of clinical leptospirosis in these five individuals [Reference Haake29]. For water sports, clinical leptospirosis has been found to be statistically associated with several risk factors such as swimming, swallowing water, the presence of skin cuts and the duration of swim or stay in the water [Reference Sejvar7, Reference Morgan25, Reference Perra26, Reference Brockmann30]. Swallowing water was not associated with leptospirosis in this outbreak since most swimmers (60%) reported water ingestion during the swim.
A possible limitation of the present study is that only symptomatic patients were tested for the presence of anti-Leptospira antibodies and/or Leptospira DNA. Given that Leptopsira infections can lead to subclinical or mild manifestations, a seroinvestigation of all participants would have provided more robust information on the actual exposure of competitors [Reference Ganoza31, Reference Silva32]. However, such a comprehensive investigation is obviously challenging and only a limited number of studies have thus far succeeded in including all participants of a recreational event associated with a leptospirosis outbreak. For instance, 85/566 individuals tested positive by IgM ELISA in an outbreak investigated in Nicaragua, out of which only 30% displayed symptoms [Reference Ashford33]. In the present study, the number of asymptomatic cases was not measured, which could underestimate the importance of some of the investigated risk factors such as swallowing water.
Regarding children, mild and severe leptospirosis have been previously reported on Réunion Island with infection most likely linked to contact with natural water courses [Reference Agésilas34]. In a leptospirosis outbreak associated with swimming in Illinois, six (14%) children were seropositive by MAT out of 43 asymptomatic children [Reference Jackson35]. In another outbreak in Andaman Island, 90% of the seropositive children identified during an outbreak were actually asymptomatic [Reference Vijayachari36]. In our study, in the absence of testing asymptomatic patients, we cannot state whether the absence of clinical forms in child swimmers results from a reduced exposure or alternatively from a lower risk of developing symptomatic disease in childhood.
Heavy rainfall was recorded the day before the races, resulting in flooding of riverbanks that made the aquatic race exceptionally difficult, as reported by many swimmers. This is congruent with previously published outbreaks also reporting heavy rainfall [Reference Sejvar7, Reference Morgan25, Reference Brockmann30, Reference Radl37, Reference Koay38]. These heavy rains or flooding over contaminated soil may disperse pathogenic leptospires thus increasing the risk of infection, which is substantiated by the short delay (24 h) between heavy rain and Leptospira infection.
The environmental investigation presented herein shows Leptospira infection rates of 70% in rats sampled at the vicinity of the rivermouth during the month preceding the competition. MAT revealed that 20% of the sera obtained from a subset of these same rats reacted against Icterohaemorrhagiae serogroup. In addition, the species L. interrogans was identified in one patient and in rats by sequencing a partial sequence of the rrs gene. Sequence alignments further showed a 100% match at the nucleotide level but the weak load of bacterial DNA in humans did not allow further genotyping of the infecting bacteria and thus providing more compelling evidence. Taken together, these results strongly suggest that rats are the likely reservoirs of L. interrogans at Rivière des Roches and contaminate the environment thus exposing mammals to this infection, as substantiated by positive MAT results in rats and humans. Indeed, MAT on human and rat samples shows that sera are reactive against Icterohaemorrhagiae and/or Canicola serogroups, both serological responses probably resulting from an exposure to L. interrogans. Last, our data do not rule out the possibility that other mammal species, including stray dogs, whicht are numerous on the island, may act as reservoirs as previously suggested [Reference Desvars17]. It is important to note that our investigation presents data obtained from both human and animal samples through serological and molecular methods which is hardly the case in comparable investigations where rodents or cattle have been identified as reservoirs and sources of human infection [Reference Perra26, Reference Jackson35, Reference Desai39] without genotyping of the prevalent Leptospira [Reference Katz, Manea and Sasaki40].
Leptospirosis was biologically confirmed for 9/10 suspected cases either by real-time PCR or serological tests (MAT or ELISA). The first cases were diagnosed by real-time PCR within the first week of disease onset. These results would thus have allowed a prompt investigation and the diffusion of public health recommendations in order to prevent severe forms [Reference Perra26]. Unfortunately, such a prompt response was impaired because incident cases were hospitalized in different centres and non-hospitalized cases were distributed throughout the island, which slowed down the identification of the cluster. This failure was in part due to the design of the surveillance scheme, i.e. with investigations around cases only taking place after hospitalization. In order to reduce this delay, the clinical notification form sent by physicians has been modified so that they now have to assess if there might be additional cases due the same exposure period (i.e. triathlon, canyoning, kayaking, etc.)
Besides an improvement of the surveillance system, several measures were proposed in order to prevent infection such as (i) the compulsory use a full neoprene swimming suit, (ii) protection of wounds before the race and (iii) an immediate shower together with disinfection of wounds at the end of competition. It was recommended to organizers to delay events in cases of heavy rainfall, and whenever possible to organize the events during the dry season.
Last, a communication effort towards a better protection and sensitization to leptospirosis risk was undertaken. Organizers were exhorted to raise awareness on leptospirosis risk in participants and to encourage them to seek medical advice in case of sickness in the weeks following the event. In 2014, these measures, including the implementation of the new form, have allowed the identification of one cluster associated with kayaking within the first days of onset of the index case. In keeping with the increased sensitization of organizers and participants, competitors with fever following the 2014 triathlon event spontaneously joined emergency units and suspected cases were quickly reported to the health authorities. None of these cases were confirmed by PCR.
Outbreaks of acute leptospirosis occur less commonly after classical occupational exposure, but are increasingly encountered in recreational settings that involve exposure to contaminated soil or water. It is thus necessary to improve the surveillance system to allow quick identification of putative clusters of cases in order to limit the burden of the disease. In addition, multidisciplinary investigations involving practitioners, epidemiologists, veterinaries and academic researchers should be quickly implemented following such outbreaks in order to produce comprehensive epidemiological, molecular and serological data that will give access to a complete picture of leptospirosis
ACKNOWLEDGEMENTS
We acknowledge the Genotyping of Pathogens and Public Health Platform (Institut Pasteur, Paris, France). We also thank all the staff of the CVAGS and LAV for their daily work in the monitoring and the fight against leptospirosis in Réunion Island. We thank all the staff of Cire OI for their help in the interviewing of competitors.
The environmental part of this work was supported by the CPER/Regional Council/European Regional Development Funds ERDF – POCT; Réunion, LeptOI (no. 32913).
DECLARATION OF INTEREST
None.








