The prevalence of diabetes in pregnancy in the USA ranges from 1 % to 14 %, depending on various factors including population, ethnicity, maternal age, BMI, socio-economic status, screening method and diagnostic definition of diabetes( Reference Coustan, Lowe and Metzger 1 , 2 ). The American Diabetes Association suggests that women who screen positive for diabetes before or during their initial prenatal visit be considered as having pregestational diabetes mellitus (PDM), thereby impacting treatment during and following pregnancy( 2 ). Additionally, it recommends that women with glucose intolerance, excluding overt diabetes, with onset in or first recognized in pregnancy be classified as having gestational diabetes mellitus (GDM). Research has shown that the prevalence of both PDM (type 1 and type 2 diabetes) and GDM has been increasing over time in the USA( Reference Dabelea, Snell-Bergeon and Hartsfield 3 – Reference Albrecht, Kuklina and Bansil 6 ).
Maternal diabetes in pregnancy may cause ‘metabolic imprinting’ and result in metabolic dysregulation in infants( Reference Hillier, Pedula and Schmidt 7 ), increasing the risk of the development of neonatal hypoglycaemia in early life and type 2 diabetes, obesity and metabolic syndrome later in life( Reference Metzger 8 – Reference Maayan-Metzger, Lubin and Kuint 12 ). Diabetes in pregnancy increases the risk of premature birth, as well as increases the risk for infant morbidity and mortality( Reference Fadl, Ostlund and Magnuson 13 , Reference Weindling 14 ).
Breast-feeding is a modifiable health behaviour that is health promotional as it augments infant immunoprotection and nurtures growth and development( Reference Labbok, Clark and Goldman 15 ). Lack of breast-feeding is associated with infants’ later risk of developing type 1 diabetes( Reference Patelarou, Girvalaki and Brokalaki 16 ) and type 2 diabetes( Reference Owen, Martin and Whincup 17 ). Early breast-feeding has been associated with improved glycaemic outcomes in infants born to women with diabetes in pregnancy( Reference Cordero, Thung and Landon 18 , Reference Chertok, Raz and Shoham 19 ). On the maternal side, lack of breast-feeding is associated with developing type 2 diabetes( Reference Schwarz, Brown and Creasman 20 ), with a dose-dependent effect for longer duration and higher intensity associated with lower risk of postpartum diabetes among women with GDM history( Reference Stuebe, Rich-Edwards and Willett 21 – Reference Chouinard-Castonguay, Weisnagel and Tchernof 26 ).
Yet, there are few data on differences in breast-feeding initiation and continuation by diabetes status in the USA. A recent Canadian study found that the prevalence of breast-feeding at hospital discharge among women with any type of diabetes was lower than in women without diabetes (NDM) and a higher prevalence of breast-feeding initiation was noted among women with GDM compared with those with PDM( Reference Finkelstein, Keely and Feig 27 ). Studies conducted in Germany also found lower prevalence of breast-feeding initiation and duration among women with type 1 diabetes compared with NDM( Reference Hummel, Winkler and Schoen 28 , Reference Schoen, Sichert-Hellert and Hummel 29 ) and among women with PDM compared with GDM, likely due to various reasons( Reference Soltani and Arden 30 ). For example, compared with NDM, women with type 1 diabetes experienced more birth-related complications such as emergency caesarean delivery, neonatal hypoglycaemia, and interventions such as oral formula supplementation and intravenous glucose administration, which may contribute to the lower prevalence of breast-feeding by hospital discharge( Reference Sparud-Lundin, Wennergren and Elfvin 31 ).
There is also a dearth of information published on reasons for breast-feeding initiation and continuation among women with diabetes in the USA. In a study conducted in the UK, in answer to an open-ended question regarding breast-feeding cessation among women with PDM and GDM, the most common reason for termination was perceived insufficient milk supply( Reference Soltani, Dickinson and Kalk 32 ), although it was not validated by measurement. The perception of delayed lactogenesis II or milk production insufficiency has been found among women with PDM and GDM( Reference Neubauer, Ferris and Chase 33 – Reference Trout, Averbuch and Barowski 36 ). Formula supplementation may be used when women feel that breast-feeding does not satisfy their infants, which may contribute to further reduction in milk production and less reliance on and confidence in breast-feeding, thereby resulting in shortened duration( Reference Gatti 37 – Reference DaMota, Banuelos and Goldbronn 39 ).
As the prevalence of diabetes in the USA increases( Reference Hunt and Schuller 5 ), there is a greater need to address lactation among women with diabetes during pregnancy. The purpose of the present study was to examine (i) the prevalence of and associations between breast-feeding initiation and continuation by maternal diabetes status and (ii) the reasons for not initiating and/or continuing breast-feeding by maternal diabetes status.
Methods
Secondary data analyses were conducted using data from the Pregnancy Risk Assessment Monitoring System (PRAMS), 2009–2011. PRAMS is administered by the Centers for Disease Control and Prevention in collaboration with participating state health departments in thirty-seven states (plus New York City and the Yankton Sioux Tribe in South Dakota). PRAMS methods have been documented in detail previously( 40 – Reference Shulman, Gilbert and Lansky 43 ). Briefly, each month, a stratified random sample of 100 to 300 women with recent live births is selected from the infant birth certificates from each state. A self-administered questionnaire is mailed to each eligible mother at 2 to 4 months postpartum. Data are weighted to adjust for survey design, non-coverage and non-response. Surveys from 2009 to 2011 in thirty states and New York City were included in the current analyses.
The PRAMS questionnaire consists of core questions that appear on all participating state surveys, standard questions that states may select, and state-developed questions tailored to individual states. In 2009–2011, the core questions on self-reported diabetes were modified to better differentiate the pre-pregnancy and gestational diabetes. Self-reported gestational diabetes was obtained by the response to the question, ‘During your most recent pregnancy, were you told by a doctor, nurse, or other health care worker that you had gestational diabetes (diabetes that started during this pregnancy)?’ Self-reported pre-pregnancy diabetes was based on responses to the question, ‘Before you got pregnant with your new baby, were you ever told by a doctor, nurse, or other health care worker that you had type 1 or type 2 diabetes (this is not the same as gestational diabetes or diabetes that starts during pregnancy)?’ Breast-feeding initiation was determined based on yes/no responses to the question, ‘Did you ever breast-feed or pump breast milk to feed your new baby after delivery, even for a short period of time?’ Women who answered ‘yes’ to the initiation question were asked about breast-feeding continuation, ‘How many weeks or months did you breast-feed or pump milk to feed your baby?’ Breast-feeding continuation was defined as breast-feeding for at least 2 months, as this time point has previously been used to define breast-feeding continuation( Reference Sparud-Lundin, Wennergren and Elfvin 31 ) and reflects the minimum amount of time after delivery that the survey is administered.
Women reporting both PDM and GDM were not counted as having either diabetes type and were excluded from analyses (n 425)( Reference Hosler, Nayak and Radigan 44 ). Additionally, women who answered ‘no’ to one of the diabetes questions and had data missing on the other diabetes question were excluded from the analyses (n 360). Finally, analyses were restricted to singleton births.
Bivariate associations between diabetes status and the following variables were tested: maternal age (≤19 years, 20–24 years, 25–29 years, 30–34 years, ≥35 years); race/ethnicity (non-Hispanic White, non-Hispanic Black, Asian, Hispanic, Other); marital status; pre-pregnancy BMI (underweight, BMI<18·5 kg/m2; normal weight, BMI=18·5–24·9 kg/m2; overweight, BMI=25·0–29·9 kg/m2; obese, BMI ≥ 30·0 kg/m2); maternal level of education (<high school, high school, >high school); previous live births; preterm birth (gestational age<37 weeks); intention to become pregnant; current smoking status; participation in the Special Supplemental Nutrition Program for Women, Infants, and Children (which provides nutrition education, breast-feeding education and support; supplemental, highly nutritious foods; and referral to prenatal and paediatric health care and other maternal and child health and human service programmes to income-eligible women); insurance at delivery (Medicaid, other; Medicaid is a human service programme intended to provide income-eligible individuals with health insurance); income (<$US 25000, $US 25000–34999, $US 35000–49999, ≥$US 50000); infant admission to the neonatal intensive care unit; type of delivery (vaginal, caesarean section); adequacy of prenatal care determined by calculating the Kotelchuck index( Reference Kotelchuck 45 ) (a composite score summarizing prenatal care based on the number and timing of prenatal visits); and whether or not breast-feeding was discussed with health-care workers during prenatal care.
Multivariable logistic regression models were built to investigate the associations between breast-feeding initiation and continuation by diabetes status, adjusting for the sixteen potential confounders. Main effects for the multivariable models were chosen using two steps. First, all variables were tested simultaneously, removing variables that were not significant (P>0·05) one at a time with diabetes status modelled as an indicator variable. Second, logistic regression models with all variables were built for each diabetes type as the exposure, separately. If a variable that had previously been removed was significant for at least one diabetes group, it was added back into the main effects model.
Once the main effects were chosen, interactions were examined. Interactions were considered significant at P<0·01. An interaction between diabetes and smoking was tested because previous research has shown smoking to be associated with lack of breast-feeding initiation among PDM( Reference Cordero, Thung and Landon 18 ). We found this interaction was significant in the breast-feeding initiation model (P=0·0008). Significant interactions between other main effects were present but upon further examination, did not meaningfully change the main effects odds ratios and thus were not included in the final model. In the breast-feeding continuation model there were significant interactions present but none that meaningfully changed the main effects odds ratios. As a result no interactions were included in the final continuation model.
The questions regarding reasons for not initiating/not continuing breast-feeding were standard questions voluntarily selected by states for inclusion on the questionnaire. Eight states included the question, ‘What were your reasons for not breast-feeding your new baby?’ All women who did not initiate breast-feeding were included in the analysis of this question. Eleven states and New York City included the question, ‘What were your reasons for stopping breast-feeding?’ All women who initiated breast-feeding were included in the analysis of this question. For both questions, women were asked to check all the reasons that applied for not initiating/not continuing breast-feeding.
Statistical analyses were completed using survey procedures in the SAS statistical software package version 9·3. Nationwide Children’s Hospital’s Institutional Review Board deemed the study as non-human subjects research.
Results
The PRAMS data set contained 72755 observations from thirty states and New York City during 2009–2011, representing a weighted frequency of 3756943 women. Among this national sample of pregnant women, 8·8 % had GDM and 1·7 % had PDM (Table 1). Breast-feeding initiation was similar between GDM and NDM (80·8 % v. 82·2 %, P=0·2) but continuation was lower among GDM (65·7 % v. 68·8 %, P=0·01; Fig. 1). Initiation (78·2 %, P=0·03) and continuation (60·4 %, P<0·01) prevalence was consistently lower among PDM compared with NDM. Prevalence of initiation (P=0·2) and continuation (P=0·06) among GDM compared with PDM was similar.
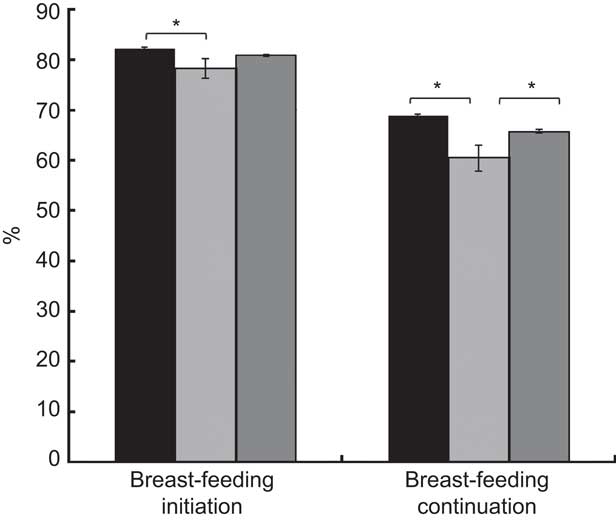
Fig. 1 Breast-feeding initiation and continuation by maternal diabetes status (![]() , no diabetes (NDM);
, no diabetes (NDM); ![]() , pregestational diabetes mellitus (PDM);
, pregestational diabetes mellitus (PDM); ![]() , gestational diabetes mellitus (GDM)); Pregnancy Risk Assessment Monitoring System (PRAMS), 2009–2011. Values are weighted, unadjusted estimates with their standard errors represented by vertical bars (n 72 755 for initiation and n 60 381 for continuation). *Group differences significant at P<0·05
, gestational diabetes mellitus (GDM)); Pregnancy Risk Assessment Monitoring System (PRAMS), 2009–2011. Values are weighted, unadjusted estimates with their standard errors represented by vertical bars (n 72 755 for initiation and n 60 381 for continuation). *Group differences significant at P<0·05
Table 1 Demographic characteristics of women by maternal diabetes status; Pregnancy Risk Assessment Monitoring System (PRAMS), 2009–2011

NDM, no diabetes; PDM, pregestational diabetes mellitus; GDM, gestational diabetes mellitus; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children; NICU, neonatal intensive care unit.
Values are weighted, unadjusted estimates with their standard errors; n 72 755 includes data from thirty states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, WA, WI, WV, WY) and New York City.
χ 2 P values are denoted as follows: *P<0·05, **P<0·01, ***P<0·001.
A significant interaction between current smoking and diabetes was found for the breast-feeding initiation model, resulting in stratified analyses. Non-smokers had higher breast-feeding initiation prevalence compared with smokers, regardless of diabetes status. Specifically, among non-smokers, NDM had the highest breast-feeding initiation prevalence (85·8 %), followed by GDM (82·9 %) and PDM (80·4 %; data not shown). This was confirmed in stratified, adjusted models where, among non-smokers, those with PDM (OR=0·71; 95 % CI 0·54, 0·94) and GDM (OR=0·83; 95 % CI 0·72, 0·97) were significantly less likely to initiate breast-feeding compared with NDM (Table 2). However, among smokers, women with GDM had the highest breast-feeding initiation prevalence (70·0 %) compared with PDM (67·5 %) and NDM (64·0 %; data not shown). In adjusted models, among smokers, there were no differences in breast-feeding initiation between PDM and NDM (OR=1·26; 95 % CI 0·83, 1·93) and those with GDM were more likely to initiate breast-feeding compared with NDM (OR=1·31; 95 % CI 1·03, 1·65; Table 2). There were no differences in breast-feeding initiation between GDM and PDM, regardless of smoking status.
Table 2 Multivariable adjusted associations between breast-feeding initiation and continuation by maternal diabetes status; Pregnancy Risk Assessment Monitoring System (PRAMS), 2009–2011

NDM, no diabetes; PDM, pregestational diabetes mellitus; GDM, gestational diabetes mellitus.
† Adjusted for maternal age, race/ethnicity, marital status, pre-pregnancy BMI, maternal education, previous live births, preterm birth, intention to become pregnant, current smoking status, participation in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), insurance at delivery, infant admission to the neonatal intensive care unit, discussed breast-feeding with a health-care worker during any prenatal visit and type of delivery.
‡ Adjusted for all the same variables as the full model in †, except stratified by current smoking status.
§ Adjusted for maternal age, race/ethnicity, marital status, pre-pregnancy BMI, maternal education, previous live births, preterm birth, current smoking status, participation in WIC and type of delivery.
For breast-feeding continuation at 2 months postpartum, women with PDM were significantly less likely to be breast-feeding compared with NDM (OR=0·71; 95 % CI 0·56, 0·91; Table 2). There were no differences in breast-feeding continuation at 2 months between women with GDM and NDM (OR=0·89; 95 % CI 0·79, 1·00) nor were there differences between women with GDM and PDM (OR=1·25; 95 % CI 0·96, 1·63).
The question regarding reasons for not initiating breast-feeding included 3914 women and the question on not continuing breast-feeding included 10 043 women. Among GDM and NDM, the three most common reasons for not initiating breast-feeding were: ‘I didn’t want to’ (47·1 % and 47·7 %, respectively), ‘I didn’t like breast-feeding’ (30·6 % and 28·9 %, respectively) and ‘I had other children to take care of’ (28·3 % and 20·1 %, respectively; Table 3). Although two of the most common reasons were similar for PDM (52·5 % for ‘I didn’t want to’ and 35·4 % for ‘I didn’t like breast-feeding’), the third most common response among PDM was ‘I was sick or on medicine’ (29·9 %). The three most common reasons for not continuing breast-feeding were similar among GDM, NDM and PDM: ‘I thought I was not producing enough milk’ (27·6 %, 26·8 % and 24·8 %, respectively), ‘Breast milk alone did not satisfy my baby’ (22·8 %, 22·2 % and 14·6 %, respectively) and ‘My baby had difficulty latching or nursing’ (22·1 %, 22·3 % and 19·5 %, respectively; Table 3).
Table 3 Reasons for not initiating or continuing breast-feeding by maternal diabetes status; Pregnancy Risk Assessment Monitoring System (PRAMS), 2009–2011
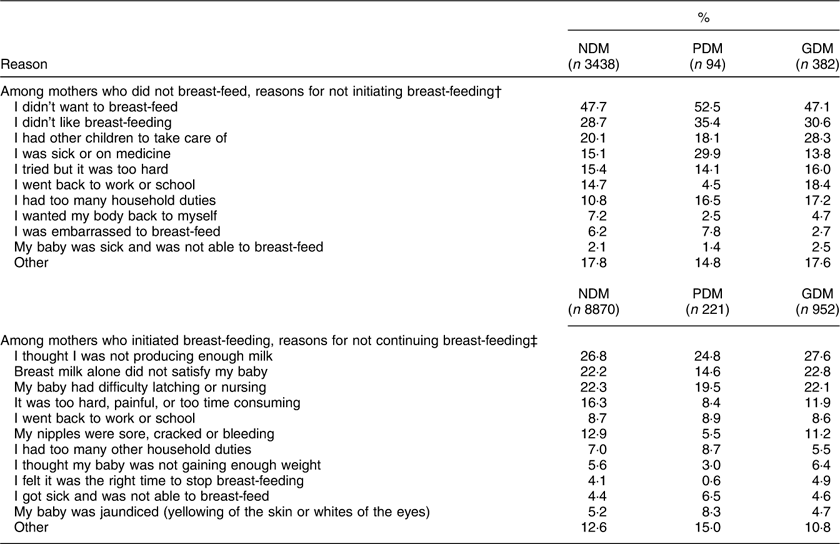
Values are weighted, unadjusted estimates; % represents the proportion of mothers who checked each item; % exceeds 100 % since each was a ‘check all that apply’ question.
† Based on the question ‘What were your reasons for not breast-feeding your new baby?’ and 3914 women from eight states (GA, IL, NH, RI, TN, TX, WI, WV).
‡ Based on the question ‘What were your reasons for stopping breast-feeding?’ and 10 043 women from eleven states (CO, GA, HI, IL, MI, MN, NE, NM, OH, TN, UT) plus New York City.
To assess evidence of selection bias, the 360 women who answered ‘no’ to one of the diabetes questions and had data missing on the other diabetes question that were initially excluded from the analyses were added back and coded as NDM. Results did not change.
Discussion
The present population-based study demonstrated that women with PDM have the lowest breast-feeding initiation and continuation prevalence compared with women with NDM or GDM. The finding regarding lower breast-feeding initiation prevalence for women with PDM compared with NDM is supported by other studies conducted in Canada and Germany, where women with type 1 or insulin-dependent diabetes were found to have lower prevalence of breast-feeding initiation or breast-feeding at time of hospital discharge than women without diabetes( Reference Finkelstein, Keely and Feig 27 , Reference Hummel, Winkler and Schoen 28 ). Similarly, the finding that women with PDM have lower breast-feeding prevalence than women with GDM is also supported by others( Reference Finkelstein, Keely and Feig 27 , Reference Soltani and Arden 30 , Reference Simmons, Conroy and Thompson 46 ). Research in Germany found an increasing trend in breast-feeding among women with PDM, although the prevalence was still lower than in the rest of the population( Reference Hummel, Winkler and Schoen 28 , Reference Schoen, Sichert-Hellert and Hummel 29 ). In a recent study in a university hospital in Ohio, among 392 women with PDM and other chronic metabolic disorders associated with a high risk of GDM, breast-feeding prevalence was 41 %( Reference Cordero, Thung and Landon 18 ), compared with the overall state prevalence of 62 %( 47 ). Possible explanations for the discrepancy in initiation prevalence between PDM and other women may relate to higher rates of caesarean delivery, preterm delivery, infant admission to the neonatal intensive care unit, younger maternal age and higher maternal BMI( Reference Finkelstein, Keely and Feig 27 , Reference Hummel, Winkler and Schoen 28 , Reference Soltani and Arden 30 , Reference Sorkio, Cuthbertson and Barlund 48 ), as well as possible delayed lactogenesis II associated with metabolic issues such as glucose intolerance( Reference Nommsen-Rivers, Dolan and Huang 49 ). These factors and potential maternal–infant separation may negatively impact milk production in the first days postpartum.
The findings of our study show that women with GDM and NDM had similar breast-feeding initiation prevalence, suggesting that having diabetes diagnosed during pregnancy may not negatively affect breast-feeding initiation. A retrospective cohort study of nearly 25 000 deliveries at four hospitals in Canada found lower exclusive breast-feeding prevalence among GDM women compared with NDM women, although any amount of breast-feeding by discharge was not reported( Reference Finkelstein, Keely and Feig 27 ), thereby limiting the comparison of these two studies as the outcome measure differs.
Breast-feeding duration prevalence has been found to be lower among women with type 1 diabetes compared with NDM in international non-intervention studies( Reference Hummel, Winkler and Schoen 28 , Reference Schoen, Sichert-Hellert and Hummel 29 , Reference Sorkio, Cuthbertson and Barlund 48 ). A study examining breast-feeding in 102 women with type 1 diabetes in Denmark found that 68 % of the women were breast-feeding either partially or exclusively at 4 months postpartum, which is lower than the overall national Danish 4-month breast-feeding prevalence of 76 %( Reference Stage, Norgard and Damm 50 ). A retrospective study conducted in the UK of ninety-four women with diabetes found that breast-feeding at 4 months was inversely associated with early prenatal BMI and at 6 months was significantly predicted by higher socio-economic status( Reference Soltani and Arden 30 ). Similarly, in a case–control study in Sweden of 212 women, maternal type 1 diabetes status was not a factor in the adjusted model predicting breast-feeding at 2 months postpartum, while significant predictors were maternal education and having breast-fed at discharge( Reference Sparud-Lundin, Wennergren and Elfvin 31 ). The researchers explained that they excluded women with type 2 diabetes since they are rare in Sweden, as well as women with GDM since there is usually a resolution of diabetes following delivery, which is different from the increasing public health issue of diabetes among women of reproductive age in the USA. It is noteworthy that breast-feeding duration is defined in different manners by the various studies such as breast-feeding at 2, 4 or 6 months, or using maternal report of timing of cessation during the first year of life, as in our study. In general, our findings contribute novel information as our study reflects population-based data of breast-feeding initiation and 2-month duration among women with diabetes in pregnancy, offering stronger analytic power and representativeness than the international studies mentioned above.
Various health, prenatal, postpartum, lactation, sociodemographic, cultural and lifestyle factors have been found to affect breast-feeding initiation and continuation for the general postpartum population. Among women with diabetes, factors other than diabetes status may influence breast-feeding continuation including sociodemographic factors such as maternal education, socio-economic level, maternal BMI and breast-feeding by discharge( Reference Soltani and Arden 30 , Reference Sparud-Lundin, Wennergren and Elfvin 31 , Reference Stage, Norgard and Damm 50 ). In our study, the reasons for not initiating breast-feeding expressed by at least three-quarters of the study population were not wanting to breast-feed and not liking breast-feeding. These reasons suggest that more promotion of breast-feeding and education regarding the importance of breast-feeding prior to delivery, and early postpartum support and encouragement, may help motivate women to initiate breast-feeding. The reasons for not continuing breast-feeding expressed by the majority of the study population were perceived insufficient milk supply, infant not sufficiently satisfied with breast-feeding and difficulty latching or nursing. Perceived insufficient milk supply was also noted as the primary lactation-related reason for breast-feeding termination among women with diabetes in an earlier study( Reference Stage, Norgard and Damm 50 ). The reasons shared by women regarding the decision to terminate breast-feeding suggest the need for health-care providers to provide continued support and guidance in addressing breast-feeding challenges during the postpartum period.
Additionally, research demonstrates a relationship among modifiable risk factors of prenatal smoking and lack of breast-feeding among women in different regions of the USA( Reference Amir and Donath 51 ). In our study, as in another recent study( Reference Cordero, Thung and Landon 18 ), smoking was associated with lower breast-feeding initiation rates among women with diabetes. Among women who were non-smokers, PDM women had the lowest breast-feeding initiation rates compared with GDM and NDM. In contrast, among women who were smokers, those with GDM had higher breast-feeding initiation rates than NDM, which was an unexpected finding. A possible explanation is that women diagnosed with diabetes in pregnancy received comprehensive instruction and recommendations regarding healthy lifestyle changes in an effort to manage their GDM and prevent postpartum diabetes, which may have included the modifiable behaviour of smoking cessation.
The present study was limited in that it was a secondary data analysis, limiting the availability of variables relevant to the questions of interest such as the survey’s definitions of diabetes and breast-feeding initiation and continuation, resulting in reduced comparability across studies due to varying definitions. Additionally, breast-feeding intention, a variable often used to anticipate differences in breast-feeding behaviour, was asked only by one state and one city, and thus could not be used in the analyses. A second limitation was that the findings may not be generalizable beyond the thirty states and one city included in the analyses; however, the study sample extended across the USA and was not limited to one region of the country. Another strength of the study was that the PRAMS survey years used in the analysis (2009–2011) were the first years to include a more explicit question to better differentiate between pre-pregnancy diabetes and gestational diabetes, and also included questions on breast-feeding initiation and continuation. To date, population-based studies in the USA do not ask women both questions within the same survey.
Clinical and policy implications
Pregnancies affected by diabetes have increased risk of adverse outcomes for the woman and her offspring. As such, it is crucial to promote health behaviours such as breast-feeding in an effort to reduce the negative risks and to afford maternal–infant health protection. Beyond prenatal diabetes screening, management and follow-up, postpartum recommendations, support and education regarding the importance and practice of breast-feeding will contribute to the prevention of additional complications associated with diabetes in pregnancy. A prenatal and postpartum breast-feeding intervention for women with type 1 diabetes in Denmark that included early postpartum pumping facilitated breast-feeding initiation and duration, so that the women’s rates were comparable to those of the general Danish population( Reference Stage, Norgard and Damm 50 ). That intervention study provides evidence for breast-feeding support and education delivered by trained health-care providers for women with diabetes during pregnancy( Reference Stage, Norgard and Damm 50 ). Early postpartum breast-feeding support should include encouragement with early and frequent milk expression as well as reducing maternal–infant separation, thereby minimizing delayed lactogenesis II and enhancing breast milk production. It is important to provide practical assistance in overcoming challenges faced by women with diabetes in pregnancy in the early postpartum period to facilitate the transition from breast-feeding initiation to continuation and to assist women in overcoming some of the most commonly expressed reasons for not initiating or continuing breast-feeding.
Future studies on breast-feeding among women with diabetes should differentiate between GDM and PDM as their experiences with breast-feeding differ. The findings of the current study can inform breast-feeding education and promotion activities, particularly related to improving prenatal health education of women with PDM and GDM, to help meet Healthy People 2020 goals for breast-feeding initiation and continuation. Additional support of breast-feeding continuation is needed across groups to meet national goals.
Acknowledgements
Acknowledgements: The authors would like to acknowledge the PRAMS Working Group (Alabama: Izza Afgan, MPH; Alaska: Kathy Perham-Hester, MS, MPH; Arkansas: Mary McGehee, PhD; Colorado: Alyson Shupe, PhD; Delaware: George Yocher, MS; Florida: Cynthia Ulysee, MPH; Georgia: Yan Li, MD, MPH; Hawaii: Emily Roberson, MPH; Illinois: Theresa Sandidge, MA; Louisiana: Amy Zapata, MPH; Maine: Tom Patenaude, MPH; Maryland: Diana Cheng, MD; Massachusetts: Emily Lu, MPH; Michigan: Cristin Larder, MS; Minnesota: Judy Punyko, PhD, MPH; Mississippi: Brenda Hughes, MPPA; Missouri: Venkata Garikapaty, MSc, MS, PhD, MPH; Montana: JoAnn Dotson; Nebraska: Brenda Coufal; New Jersey: Lakota Kruse, MD; New Mexico: Eirian Coronado, MPH; New York State: Anne Radigan-Garcia; New York City: Candace Mulready-Ward, MPH; North Carolina: Kathleen Jones-Vessey, MS; North Dakota: Sandra Anseth; Ohio: Connie Geidenberger, PhD; Oklahoma: Alicia Lincoln, MSW, MSPH; Oregon: Kenneth Rosenberg, MD, MPH; Pennsylvania: Tony Norwood; Rhode Island: Sam Viner-Brown, PhD; South Carolina: Mike Smith, MSPH; Texas: Rochelle Kingsley, MPH; Tennessee: David Law, PhD; Utah: Laurie Baksh; Vermont: Peggy Brozicevic; Virginia: Marilyn Wenner; Washington: Linda Lohdefinck; West Virginia: Melissa Baker, MA; Wisconsin: Katherine Kvale, PhD; Wyoming: Angi Crotsenberg); Centers for Disease Control and Prevention PRAMS Team, Applied Sciences Branch, Division of Reproductive Health. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: R.O.-F. conceived the research questions, drafted the manuscript and supervised the analyses. I.C. drafted the manuscript. A.B. completed the analyses. All authors reviewed and approved the final version of the manuscript prior to submission. Ethics of human subject participation: Nationwide Children’s Hospital’s Institutional Review Board deemed this study as non-human subjects research.






