Growing plants and raising animals were part of a cascade of changes in the social and physical environments of ancient societies that marked the transition to agriculture and pastoralism. How shifts in settlement, subsistence, and labor practices developed within specific societies, often over centuries to millennia, has been the subject of intensive scientific research. Archaeologists use a broad range of approaches to generate new data to test hypotheses about these transitions, including osteology (Mummert et al. Reference Mummert, Esche, Robinson and Armelagos2011; Munoz Reference Munoz2017), paleoethnobotany (Bao et al. Reference Bao, Zhou, Liu, Hu, Zhao, Atahan, Dodson and Li2018; Morales et al. Reference Morales, Pérez-Jordà, Peña-Chocarro, Zapata, Ruíz-Alonso, López-Sáez and Linstädter2013; Snir et al. Reference Snir, Nadel, Groman-Yaroslavski, Melamed, Sternberg, Bar-Yosef and Weiss2015; Winchell et al. Reference Winchell, Stevens, Murphy, Champion and Fuller2017; Zhao Reference Zhao2011), electron and X-ray imaging (Bruno and Whitehead Reference Bruno and Whitehead2003; Murphy and Fuller Reference Murphy and Fuller2017; Smith Reference Smith and Olsen1988), paleogenetics (Bruford and Townsend Reference Bruford, Townsend, Zeder, Bradley, Emshwiller and Smith2006; Dobney and Larson Reference Dobney and Larson2006; Gepts Reference Gepts1993), and zooarchaeology (Bläuer and Kantanen Reference Bläuer and Kantanen2013; Meadow Reference Meadow and Clutton-Brock1989; Zeder Reference Zeder, Zeder, Bradley, Emshwiller and Smith2006).
Over the last 40 years, archaeologists have increasingly analyzed stable isotopes to gain insight into ancient agricultural practices (Araus et al. Reference Araus, Febrero, Catala, Molist, Voltas and Romagosa1999; Barton et al. Reference Barton, Newsome, Chen, Wang, Guilderson and Bettinger2009; Buikstra and Milner Reference Buikstra and Milner1991; Hu et al. Reference Hu, Ambrose and Wang2006) and animal husbandry (Balasse et al. Reference Balasse, Smith, Ambrose and Leigh2003; Ma et al. Reference Ma, Ren, Li, Wang, Zhao and Li2021; Makarewicz and Tuross Reference Makarewicz and Tuross2006; Martín et al. Reference Martín, Tornero, García and Vergès2021; Melton et al. Reference Melton, Alaica, Biwer, González La Rosa, Gordon, Knudson, VanDerwarker and Jennings2023). Indeed, several of the foundational isotopic studies in archaeology used carbon and nitrogen to discriminate signatures associated with early agricultural, pastoral, and hunter-gatherer diets (e.g., Ambrose and DeNiro Reference Ambrose and DeNiro1986; Schoeninger and DeNiro Reference Schoeninger and DeNiro1984).
We report carbon, nitrogen, and sulfur isotope signatures preserved in faunal remains from the site of Upanca, Peru, a site that was occupied for nearly 4,000 years between 3200 BC and AD 650. Camelid, cavid, and cervid bones from across this timespan provide context on the evolution of agropastoral lifeways in the south-central coast of Peru. We add new data to long-standing debates in the Andes regarding the timing of intensive agriculture using field fertilizers (Bruno Reference Bruno2014; Sandor and Eash Reference Sandor and Eash1995; Szpak et al. Reference Szpak, Millaire, White and Longstaffe2012), the incorporation of C4 agricultural products into local economies (Grobman et al. Reference Grobman, Bonavia, Dillehay, Piperno, Iriarte and Holst2012; Haas et al. Reference Haas, Creamer, Mesía, Goldstein, Reinhard and Rodríguez2013; Perry et al. Reference Perry, Sandweiss, Piperno, Rademaker, Malpass, Umire and De la Vera2006), and the rearing and herding of camelids on the coast versus the highlands (Mader Reference Mader2019; Szpak et al. Reference Szpak, Millaire, White and Longstaffe2014, Reference Szpak, Chicoine, Millaire, White, Parry and Longstaffe2016, Reference Szpak, Millaire, Chapdelaine, White and Longstaffe2020).
Background
Upanca is located 30 km east of the modern city of Nasca (Figure 1) and was originally recorded by Schreiber and Isla Cuadrado (1996) as part of a regional survey in the Southern Nasca Region (SNR). With a commanding view of the valley below (Figure 2), the site is located 1,600 m asl in the chaupiyunga ecological zone. About 4 km downslope, this corridor is known as the Tierras Blancas River Valley, whereas the area adjacent to the site and upslope is referred to as the Tambo Quemado River Valley.

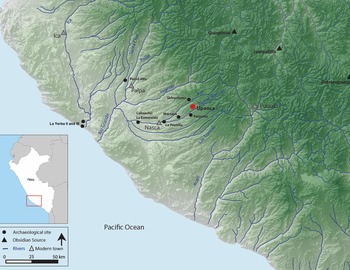
Figure 1. The south coast of Peru. Sites mentioned in text are highlighted: Upanca, Marcaya, Uchuchuma, Cahuachi, La Yerba II, La Yerba III, La Esmeralda, and Pernil Alto (map drawn by Kelsey Sullivan).

Figure 2. View from Upanca down valley toward southwest (left), and stratigraphy of north wall in Unit 29-4 (right) (photographs by Jelmer Eerkens). (Color online)
The valley was a major route between the highlands and coast during the Middle and Late Horizons (Schreiber and Lancho Rojas Reference Schreiber and Rojas2003) and the Late Intermediate period (Conlee Reference Conlee2016). Table 1 provides the local chronology and terms used in this article. The valley is home to sites of major importance to our understanding of regional history, including La Puntilla (Van Gijseghem Reference Van Gijseghem2006), Marcaya (Vaughn Reference Vaughn2009), Pataraya (Edwards and Schreiber Reference Edwards and Schreiber2014), Cahuachi, and Paredones (Menzel Reference Menzel1959; Orefici Reference Orefici2011; Silverman Reference Silverman1993; Strong Reference Strong1957).
Table 1. South Coast of Peru Chronology.

Source: Based on Vaughn and coworkers (Reference Vaughn, Eerkens, Lipo, Sakai and Schreiber2014, Reference Vaughn, Conlee, Whalen and Van Gijseghem2016) and Vaughn and Linares (Reference Vaughn and Grados2006).
Note: There is overlap between the Nasca epochs.
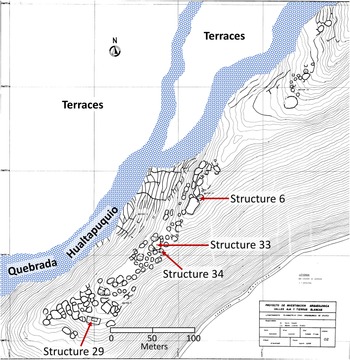
Upanca is divided into five sectors (Vaughn and Linares-Grados Reference Vaughn and Grados2006). Architecture consists of simply constructed circular houses with stone foundations, as well as several large and irregularly shaped patios. Surface pottery is mostly associated with the Late Formative and Nasca periods (approximately 300 BC–AD 600), especially Nasca 2 through Nasca 4 phases, commonly referred to as the Early Nasca epoch dating between AD 100 and 450 (see Vaughn et al. Reference Vaughn, Eerkens, Lipo, Sakai and Schreiber2014). Excavations in 2002 and 2006 in one area of the site revealed an extensive pre-Nasca occupation (Vaughn and Linares-Grados Reference Vaughn and Grados2006). Nearly three-quarters of the animal bones analyzed in this study come from three 2 × 2 m units excavated within Structure 29 on the southwestern edge of the site (Figure 3). These units were up to 2 m deep with a continuous deposit of midden, before reaching bedrock. Four major sedimentary strata occur in these units, labeled from top to bottom A through D. The remaining samples come from three other structures representing Strata A and B only.

Figure 3. Map of surface architecture at Upanca showing location of four structures sampled in this study.
Eleven AMS dates on charcoal within excavation units show that the site was first occupied at the end of the Middle Archaic, more than 5,000 years ago (Table 2). The AMS dates are in chronological order with depth, except for one date (AA72020) from Unit 2 from the 80–100 cm level that was just 40 years older than the date from the deeper 120–140 cm level (AA71804). However, the 95% confidence intervals for these two calibrated dates overlap nearly completely, suggesting that the sediments between 80 and 140 cm, within Stratum C, accumulated over a short period of time. The AMS dates, associated pottery (when present), three luminescence dates on pottery (see Vaughn et al. Reference Vaughn, Eerkens, Lipo, Sakai and Schreiber2014), and obsidian-hydration measurements (Eerkens et al. Reference Eerkens, Vaughn, Carpenter, Conlee, Grados and Schreiber2008) provide a chronological context for the animal bones (Table 3). We did not obtain AMS dates from each stratigraphic level, and in estimating the age of bones from levels between AMS-dated levels, we interpolate and took the average from the overlying and underlying levels.
Table 2. AMS Dates from Upanca.

Note: The two Beta Analytic dates were previously reported in Vaughn and Linares Grados (Reference Vaughn and Grados2006).
Table 3. Interpretation of Age Ranges for Stratigraphic Units A–D at Upanca.

Stable Isotopes and Camelids
In ecological and paleodietary studies, carbon isotope values typically provide an estimate of the consumption of C3 versus C4 plants, although marine foods often overlap with the latter. Most plants are C3 photosynthesizers, which discriminates against 13C, resulting in δ13C values between −30‰ and −22‰. By contrast, a small number of C4 plants have tissues with higher δ13C values typically between −16‰ and −10‰. Several important crop plants fall in the C4 category (Schwarcz and Schoeninger Reference Schwarcz and Schoeninger1991), including maize and some Andean amaranths (Amaranthus sp.; Cadwallader et al. Reference Cadwallader, Beresford-Jones, Whaley and O'Connell2012). In addition, several native C4 South American grasses, such as dropseed (Sporobolus sp.), saltgrass (Distichlis sp.), lovegrass (Eragrostis sp.), and Muhlenbergia sp., were available to wild and herded camelids (Cadwallader et al. Reference Cadwallader, Beresford-Jones, Whaley and O'Connell2012; Shimada and Shimada Reference Shimada and Shimada1985; Thornton et al. Reference Thornton, DeFrance, Krigbaum and Williams2011). Most of these grasses are adapted to warmer and arid climates and hence are mostly available in low-elevation areas along the western flanks of the Andes and coastal Peru. Thus, we expect C4-enriched camelid diets (i.e., higher δ13C) to be found in more lowland and coastal regions and those with greater aridity. For non-ruminant mammals, bone collagen δ13C values are approximately 4‰–5‰ higher than the δ13C of dietary protein (Fernandes et al. Reference Fernandes, Nadeau and Grootes2012; Froehle et al. Reference Froehle, Kellner and Schoeninger2010).
Nitrogen isotopes are strongly correlated with the trophic level of consumed foods (Schoeninger and DeNiro Reference Schoeninger and DeNiro1984): δ15N increases by about 3‰–4‰ with each trophic level. In terrestrial systems, there are essentially three trophic levels: plants, browsers, and carnivores. In aquatic environments there are more trophic levels, resulting in greater enrichment of δ15N at the top of the food chain (typically in large fish, predatory birds, and aquatic mammals).
As terrestrial herbivores, camelids are expected to display relatively low δ15N values. In most Andean environments where the nitrogen cycle is based on nitrogen fixated from the atmosphere, baseline plant δ15N values are between 0‰ and 5‰ (DeNiro and Epstein Reference DeNiro and Epstein1981). Collagen in camelids consuming these plants is expected to be elevated one trophic level above this baseline, or in the 3‰–9‰ range. By contrast, plants grown in fields fertilized with large amounts of organic material, including camelid dung and bird guano, can display significantly elevated δ15N (Szpak et al. Reference Szpak, Millaire, White and Longstaffe2012). This effect can result in isotope values in plant tissues up to 20‰ higher than the nonfertilized baseline. Camelids feeding on plants or plant byproducts from such fields will incorporate this higher δ15N into their own tissues. In most cases, then, δ15N in camelid bone collagen greater than 9‰ suggests at least some fodder from fertilized fields and, by extension, implies that humans were engaging in intensive agricultural practices.
A caveat to the patterns just described concerns plants growing in especially arid and non-irrigated conditions. In these environments, plants show enrichment in both 13C and 15N (Ambrose Reference Ambrose1991; Picon et al. Reference Picon, Guehl and Ferhi1996; Van de Water et al. Reference Van de Water, Leavitt and Betancourt2002; Virginia and Delwich Reference Virginia and Delwiche1982). Empirical data indicate that noncultivated coastal plants (C3 and C4) from southern Peru are often between 6‰ and 12‰ for δ15N (Thornton et al. Reference Thornton, DeFrance, Krigbaum and Williams2011). Camelids feeding on such plants will be one trophic level higher, or between 9‰ and 14‰; theoretically they could be as high as 16‰ if they were feeding exclusively on the 12‰ plants. In short, animals living at higher elevations, under less arid conditions, in the Andes tend to have lower δ13C and δ15N.
In some cases, these contrasts emerge as diachronic differences in camelid isotopes, indicating long-term paleoclimatic changes. For example, holding environment constant, several studies in Argentina show an increase in camelid δ13C (about 1.5‰) and especially δ15N (about 3‰) between 8000 and 5000 cal BP (Grant et al. Reference Grant, Mondini and Panarello2018; Samec et al. Reference Samec, Morales and Yacobaccio2014). This shift is assumed to be the result of large-scale drought (Garvey Reference Garvey2008; Garvey and Bettinger Reference Garvey and Bettinger2018; Gil et al. Reference Gil, Zárate and Neme2005; Neme and Gil Reference Neme and Gil2009).
Studies with both wild and domesticated South American camelids show there are no consistent isotopic differences by species (Gil et al. Reference Gil, Ugan, Otaola, Neme, Giardina and Menéndez2016; Grant et al. Reference Grant, Mondini and Panarello2018; Izeta et al. Reference Izeta, Laguens, Bernarda Marconetto and Scattolín2009; Samec et al. Reference Samec, Yacobaccio and Panarello2018). Instead, environmental factors explain most of the variation in C and N isotopes. Much of this variation is a result of the type of browse consumed by animals, especially the relative proportion of C3 and C4 plants. However, even within a particular browse type, altitude and aridity cause predictable shifts in stable isotope patterns (Gil et al. Reference Gil, Ugan, Otaola, Neme, Giardina and Menéndez2016; Samec et al. Reference Samec, Yacobaccio and Panarello2020).
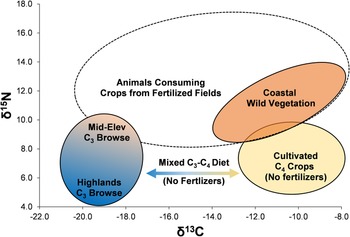
We build on previous models proposed by Thornton and colleagues (Reference Thornton, DeFrance, Krigbaum and Williams2011) and especially Szpak and colleagues (Reference Szpak, Chicoine, Millaire, White, Parry and Longstaffe2016), to contextualize the results from Upanca. The Thornton model recognizes three dietary patterns for camelids, one incorporating primarily C3 plants (low δ13C, low δ15N), one primarily C4 plants (high δ13C, low δ15N), and one a mix of C3 and C4 plants grown in fields with significant inputs of fertilizer (intermediate δ13C, high δ15N). Szpak and colleagues (Reference Szpak, Chicoine, Millaire, White, Parry and Longstaffe2016) propose a similar model, adding a wild, non-irrigated coastal environment. Figure 4 displays these idealized diets in C and N isotopic space as ellipses. Within the C3 ellipse, we add contextual elevational variation due to increasing aridity in lower Andean elevations. We also extend the ellipse along the δ15N axis by 1.5‰ to account for semiarid mid-elevation plants that would be available in the chaupiyunga zone. This extension of the C3 ellipse is particularly relevant given the altitude of Upanca (1,600 m asl). The Szpak model also suggests that a wild-plant coastal signature is likely to overlap completely with a C4 fertilized field signature.

Figure 4. Isotopic model for interpreting Andean camelid stable isotopes. (Color online)
The model is not meant to provide definitive results on a per-animal basis but does help contextualize isotopic variation in animal isotopes from Upanca. We acknowledge the possibility that there could be other natural (i.e., noncultivated) environments in Peru that have not been sampled but could have distinctive isotopic signatures. It is also possible that natural environments existed in the past but have no modern analogs. However, we are only able to construct our models and make our interpretations using available empirical data and must leave open the possibility of alternative scenarios for future research.
Finally, we use δ34S as an additional cross-check on the diet of animals. δ34S reflects biologically available sources of sulfur and is especially effective in discriminating marine versus terrestrial sources of sulfur (Nehlich et al. Reference Nehlich, Borić, Stefanović and Richards2010; Richards et al. Reference Richards, Fuller, Sponheimer, Robinson and Ayliffe2003). In some cases, when animals cross major geological units with different underlying δ34S values, this isotopic system can trace animal mobility (Hobson Reference Hobson1999). Given the lack of regional δ34S databases, we focus on the role of marine-derived sulfur in the diets of animals, including the use of fertilizers from marine environments.
Methods
We selected 67 bone fragments—47 camelids (Llama sp.), 9 cavids (Cavia sp.), and 11 cervids (either Odocoileus virginianus [white-tail deer] or Hippocamelus antisensis [taruca])—from Upanca for stable isotope analysis. Samples were spread across different excavation units and stratigraphic contexts such that each was likely to represent a distinct animal. Approximately 1 g of cortical bone was cleaned of any surface contamination by burring exposed surfaces with a handheld drill and then sonicating the sample in deionized H2O. The sample was dried, weighed, and placed in a 0.5 M hydrochloric acid (HCl) solution to demineralize. The sample was then washed with deionized water and soaked in 0.125 M NaOH (sodium hydroxide) to remove humic acids. After rinsing, slightly acidic pH3 water was added to the vial, and the sample was placed in a 70°C oven to solubilize collagen. Fluid was then pipetted out of the vial to separate it from any remaining solids and placed in a freeze-dryer to remove all remaining water, isolating the “collagen” fraction. We put collagen in quotes here because, even though the vast majority of the resulting pseudomorphs are various peptides of collagen, other proteins and peptides are present in small numbers (Wadsworth and Buckley Reference Wadsworth and Buckley2018).
Of the 67 samples, 46 (69%) produced enough collagen, at least 1 mg, to submit for C and N stable isotope analysis. Total C, total N, δ13C, and δ15N were measured by continuous-flow mass spectrometry on a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (IRMS) at the Stable Isotope Facility (SIF), University of California, Davis. Carbon isotopes ratios (δ13C) are expressed in per-mil notation (parts per thousand) relative to the PeeDee Belemnite standard (arbitrarily set at 0‰), whereas N isotope ratios (δ15N) are expressed against N2 in modern atmospheric air (arbitrarily set to 0‰). Based on repeated measurements of standards, instrument precision is estimated at 0.1‰ for δ13C and 0.2‰ δ15N. DeNiro (Reference DeNiro1985) suggests that collagen samples with reliable stable isotope data should have atomic C/N ratios between 2.9 and 3.6. Following analysis, two samples were rejected from the analysis due to poor C/N ratios. The final sample comprised 30 camelids, seven cavids, and seven cervids.
For samples with high collagen yield, we submitted 10 mg for δ34S analysis to examine the potential contribution of marine resources within the animal foodwebs. Methionine is a sulfur-bearing amino acid present in small amounts in collagen (Eastoe Reference Eastoe1955). Sulfur is taken up by plants from the local soil or water and used to synthesize methionine, which is then passed directly on to animals. Oceanic sulphates (SO4-2) are elevated in 34S relative to 32S, with δ34S typically between +17 and +21‰. By contrast, most terrestrial foods have lower values of δ34S between −7‰ and +8‰ (Chukhrov et al. Reference Chukhrov, Ermilova, Churikov and Nosik1980; Peterson and Fry Reference Peterson and Fry1987); exceptions are geological formations with uplifted marine sediments containing pyrite and sulfur-bearing evaporites, where δ34S is highly variable (−19‰ and +30‰; Peterson and Fry Reference Peterson and Fry1987; Privat et al. Reference Privat, O'Connell and Hedges2007). Terrestrial plants growing near the coast may also have elevated δ34S values due to sea spray. δ34S was measured using an Elementar Vario ISOTOPE cube interfaced to a SerCon 20-22 isotope ratio mass spectrometer and expressed relative to the Vienna Canyon Diablo Troilite. Long-term analyses of standards at the SIF shows that instrument precision for δ34S is 0.4‰.
Results
Supplemental Table 1 provides δ13C, δ15N, C/N, %C, and %N, for the 44 samples that produced more than 1 mg of collagen and had atomic C/N ratios within the 2.9–3.6 range recommended by DeNiro (Reference DeNiro1985). C/N ratios consistently fell between 3.1 and 3.4 (with one sample at 3.6). Also shown are δ34S and ug S/mg collagen for 22 samples that produced more than 11 mg of collagen.
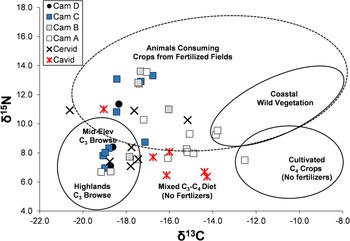
Figure 5 plots δ13C versus δ15N for the Upanca faunal samples, coding the camelids by stratigraphic unit. As shown, there is high isotopic variation for all three species. Relative to camelids, cavids tend to be lower in δ15N, whereas cervids tend to be lower in δ13C. However, the isotopic range of all three species overlaps. Only one of the animals falls within the modeled isotopic range of a pure C4 consumer, and only two for that expected of wild coastal vegetation. The majority fall within the range expected for higher- and mid-elevation C3 consumers, animals feeding from fertilized fields, and animals with a mixed C3–C4 diet (either from fertilized or unfertilized fields). The figure also hints at a time transgressive shift. In particular, two of three Middle Archaic camelids from Stratum D are within the expected range of mid- to high-elevation C3 consumers, as are four of nine Late Archaic camelids from Stratum C. In contrast, only 1 of 5 Stratum B and 3 of 13 Nasca animals fall in this range.

Figure 5. δ13C versus δ15N for camelids (by stratum), cervids, and cavids at Upanca. (Color online)
Figures 6a and b explore this diachronic pattern further, plotting δ13C and δ15N against time. Above each graph we also plot a paleoclimatic reconstruction by Seltzer and colleagues (Reference Seltzer, Rodbell and Burns2000) based on an ice core collected at Nevado Huascarán, the closest paleoclimatic dataset we could locate with the appropriate temporal resolution. Higher values in the paleoclimatic reconstruction indicate greater evaporative conditions (i.e., greater aridity).

Figure 6. (a) δ13C and (b) δ15N over time for camelids, cavids, and cervids from Upanca.
Among camelids, δ13C is generally low from 3000 through 500 BC (averaging −18.1‰) with low inter-animal variation. After 500 BC and through the Nasca period, average values are higher (−16.2‰), but variation is much higher. An F-test comparing variation in δ13C of camelids before and after 500 BC supports greater animal variation later in time (p = 0.001). By contrast, variation in δ15N is lower in the Middle Archaic, before 2500 BC (albeit there are only three samples), and then increases and stays relatively constant after this time. A similar F-test comparing camelid variation in δ15N before and after 500 BC shows little difference (p = 0.72). Significantly, the overall increase in average δ13C cannot be explained by increasing regional aridity, because the paleoclimatic record indicates generally decreasing aridity. This suggests that a shift in cultural practices is responsible for the changing isotopic values.
Figure 7 plots δ34S over time in a similar manner. As shown, δ34S is relatively low, between 4.8‰ and 8.8‰, as expected of terrestrial sources of sulfur and is thus inconsistent with a significant input of marine-derived sulfur. Unfortunately, we lack baseline regional δ34S values to further evaluate these sulfur data; for example, to provide insight into foddering of animals in specific high-elevation or coastal locations. The data do suggest increasing mobility among the camelids over time. Barring one low δ34S value from Stratum C (at 5.1‰), variation seems to be higher in Stratum B and especially Stratum A, suggesting those camelids were eating from a broader range of environmental locations. With inclusion of the 5.1‰ outlier value, an F-test comparing variation in Stratum A with Stratum C shows little difference (p = 0.84); however, removing it produces a much lower p-value (p = 0.02). We note further that this Stratum C outlier is also the lowest among all camelids for δ15N and is close to the C–N values expected of an animal foraging in a high-elevation environment. Limited as the sulfur data currently are, they are consistent with Stratum A camelids consuming foods from a broader range of environments than those in Stratum C.

Figure 7. δ34S over time for camelids, cavids, and cervids from Upanca.
Discussion
Modern sampling of plants and animals from a range of environments in Peru guide our interpretations of ancient camelid (and cavid) foddering and husbanding. As reflected in our model (Figure 4), modern data suggest that δ15N values above 10.5‰, combined with δ13C under −17‰, reflect diets with significant input of C3 crops from fertilized fields. Such values imply the existence of local agricultural systems, thereby providing information not only on animal diets but also on human activities. In this section, we combine the isotopic data from camelid, cavid, and cervid remains with other contextual data from the site of Upanca to help us understand broad changes in human settlement and subsistence behavior in the SNR. We examine each period in turn, relating the results from Upanca to what is known regionally from other sites.
Middle Archaic (6000–3000 BC)
Stratum D at Upanca is represented by a series of thin occupation layers separated by sterile sediment, suggesting sporadic and short-term occupation events. The stone artifact assemblage is dominated by highland obsidian, especially from the Quispisisa source (Burger and Glasock Reference Burger and Glascock2000; Tripcevich and Contreras Reference Tripcevich and Contreras2011), some 80 km from the site. Obsidian comprises 93% of the stone tools and 71% of the waste flakes, with local cherts and coarse-grained volcanic materials (e.g., basalt, andesite) making up the remainder. Together with the stratigraphy, the emphasis on nonlocal toolstone suggests that Upanca was occupied by residentially mobile groups; perhaps it was a stopping point on seasonal transhumance between the uplands and lowlands. Paleobotanical remains from flotation samples include small numbers of chenopods (Chenopodium sp. and Amaranthus sp.), Solanum sp., guava (Psidium guajava), native cacti (Acanthocereus sp., Haagacereus sp., and Espostoa sp.), and cotton (Gossypium barbadense). Although many of these remains may represent wild varieties of species that were later domesticated, cotton particularly suggests access to at least some industrial agricultural products.
The Middle Archaic faunal isotopic sample at Upanca is represented by only three camelid isotopic samples, which all appear to represent animals consuming predominantly C3 plants. δ15N values for two samples, at 7.1‰ and 8.4‰, are most consistent with foraging of wild foods from mid-elevation zones, although they are just within the upper range of high-elevation browse (cf., Thornton et al. Reference Thornton, DeFrance, Krigbaum and Williams2011). The third has a δ15N value (11.4‰) that falls within our predicted range for animals consuming some crops from fertilized fields and is just outside the range predicted for mid-elevation wild browse. The sample size is small, but the values are consistent with a mix of hunting of locally available wild camelids by more mobile groups (two of three animals) and herding or access to domesticated animals fed small amounts of fertilized C3 crops (one of three animals).
Regionally, a handful of Middle Archaic sites have been excavated in the Nasca region. Located on the Ica River estuary in the Northern Nasca Region (NNR), La Yerba II (5571–4674 cal BC) was a logistical base camp of complex hunter-gatherers exploiting high-return foods (especially marine resources) during seasonal rounds (Beresford-Jones et al. Reference Beresford-Jones, Pullen, Chauca, Cadwallader, García, Salvatierra and Whaley2018). La Yerba III (4485–3893 cal BC), situated just upriver from La Yerba II, seems to have been a permanently settled village whose inhabitants relied on local cultivars in addition to locally foraged resources. As at Upanca, abundant Quispisisa obsidian suggests spheres of interaction extending into the highlands.
La Esmeralda, located 40 km west in the lower valley of Nasca, was interpreted as a temporary summer camp of hunter-gatherers who foraged along the coast and lomas environment (Isla Cuadrado Reference Isla Cuadrado1990). Highland obsidian also suggests seasonal transhumance. A single radiocarbon date places this site at approximately 5200 cal BC, making it about 2,000 years older than Upanca. It is likely that groups such as those from La Esmeralda made temporary stops at places like Upanca during their seasonal movements from the lowlands to highlands.
By contrast, the Middle Archaic component at Pernil Alto, located 60 km northwest of Upanca, was interpreted as a sedentary village with pithouses and subsistence based on small-scale horticulture supplemented by some hunting and gathering (Gorbahn Reference Gorbahn2020; Reindel and Isla Cuadrado Reference Reindel and Isla2009). Sixty-nine radiocarbon dates place this oldest occupation at Pernil Alto much closer in time to Upanca, between about 3000 and 2000 cal BC.
Late Archaic (3000–1800 BC)
Stratum C at Upanca is much more substantial, representing more than 1 m of deposit and suggesting more intense and sustained occupation. Obsidian still dominates the toolstone, comprising 90% of tools and more than 50% of the debitage. Geochemical analyses demonstrate that a range of distant obsidian sources were accessed, including Potreropampa (Burger et al. Reference Burger, Fajardo Rios and Glascock2006), which is more than 140 km from the site, and an unknown source; however, most of the obsidian is from the closer Quispisisa source (Eerkens et al. Reference Eerkens, Vaughn, Linares-Grados, Conlee, Schreiber, Glascock and Tripcevich2010). A richer paleobotanical assemblage was recovered from Stratum C, which included charred fragments of achira (Canna edulis), Solanum sp., a range of chenopods, cacti, guava, mallow (Sida sp.), calabaza (Cucurbita sp.), and cotton, as well as a charred seed belonging to the Fabaceae (bean) family. These plants suggest people were engaged in small-scale agriculture at the site or had regular access to agricultural products.
Many more faunal remains from Upanca Stratum C were analyzed for stable isotopes. Of the nine camelids, four show low δ13C and δ15N, very similar to two of the Middle Archaic samples, and another one shows slightly elevated δ15N similar to the third Middle Archaic camelid. These five animals could be wild, consuming mostly C3 plants from mid- and higher-elevation zones. However, three camelids show elevated δ15N as if they had been eating significant amounts of C3 plants grown in fertilized fields, with two of these also displaying slightly higher δ13C consistent with small amounts of C4 plants (i.e., falling into the “mixed” dietary zone). The final Stratum C camelid shows only moderate δ15N but more elevated δ13C, just outside the range we expect for wild C3 plants. It, too, could have been consuming small amounts of C4 plants, either from lower-elevation coastal environments or from cultivated foods.
Sulfur isotope values for seven of eight Stratum C camelids are tightly clustered between 8.2‰ and 8.8‰. This may indicate the “local” value for the mid-elevation zone around Upanca (note also that δ34S values for the three cervids in our sample are close to this range). However, one camelid displays an unusually low δ34S value, which may indicate it was recently eating in a nonlocal region. Judging by the C and N values, this region could have been at high elevation, consistent with some herding of camelids in highland pastures.
The slightly elevated δ13C in several of the Stratum C camelid bones may indicate the introduction of maize or kiwicha (Amaranthus caudatus) in the region. Late Archaic maize is certainly known in coastal Peru. On the north coast, maize kernels, husks, and cobs are directly dated to at least 4500 BC (Grobman et al. Reference Grobman, Bonavia, Dillehay, Piperno, Iriarte and Holst2012; Haas et al. Reference Haas, Creamer, Mesía, Goldstein, Reinhard and Rodríguez2013; Kistler et al. Reference Kistler, Yoshi Maezumi, de Souza, Przelomska, Costa, Smith and Loiselle2018). Closer to Upanca, a maize cob was found within an Archaic context at the site of La Tiza (Conlee Reference Conlee2016:57–60), and cobs dating to 1000 BC were found in Initial period deposits at Pernil Alto (Reindel and Isla Reference Reindel and Isla2009), slightly later than our dating of Stratum C at Upanca. Gorbahn (Reference Gorbahn2020) reports a single “probable” maize stem fragment from the Middle Archaic at Pernil Alto but suggested that, if this fragment is maize, this crop was unimportant at that time. Less is known about the domestication history of kiwicha; however, a small number of Amaranthus sp. seeds were recovered at Upanca in both Strata C and D. These seeds could represent kiwicha or another species of amaranth with a C4 pathway. In any case, the three Stratum C camelids from Upanca with elevated δ13C may have been consuming a mixed diet with primarily C3 and small amounts of C4 plants.
The apparent foddering of some camelids with foods from fertilized fields is consistent with regional findings suggesting intensive agriculture using irrigation during the Late Archaic. Hesse and Baade (Reference Hesse and Baade2009) report irrigation agriculture by at least 1500 BC, and possibly as early as 1800 BC in the Palpa Valley, some 40 km northwest of Upanca. Horn and colleagues (Reference Horn, Hölzl, Rummel, Åberg, Schiegl, Biermann, Struck, Rossmann, Reindel and Wagner2009) report isotopic analyses on archaeological maize fragments from the Palpa region. Their article does not indicate the age of these fragments, though we suspect they date after the Late Archaic (given that most other samples in their study date after 1000 BC). Significant, however, is that δ15N values in some maize fragments are as high as 16‰, providing unambiguous evidence of fertilization. Horn and colleagues (Reference Horn, Hölzl, Rummel, Åberg, Schiegl, Biermann, Struck, Rossmann, Reindel and Wagner2009) suspect fields were fertilized with dung, rather than seaweed or guano. They also report maize with δ15N around 1‰, showing that not all maize was grown in fertilized fields.
Finally, two Stratum C cavids show similar and low δ13C values, indicating consumption of C3 foods, but they have markedly different δ15N values. One has elevated δ15N (11.0‰) within the range of what we expect for an animal consuming food from fertilized fields; the other value is 3‰, as if the cavid were raised on legume protein only from an unfertilized field. As noted earlier, a charred bean was recovered from this level.
Initial and Formative Periods (1800 BC–AD 100)
Materials associated with Stratum B at Upanca are consistent with a pottery-producing sedentary society. Although nearly all the flaked stone tools are still made from obsidian (94%), two-thirds of waste flakes (66%) are from local materials, indicating significant on-site production using nearby toolstone. Fewer paleobotanical remains were recovered but include chenopod seeds.
Three of five camelids from Stratum B at Upanca display elevated δ15N, indicative of foddering from fertilized fields. By contrast, four of five δ13C values are between −17‰ and −18‰, indicating only small amounts of C4 plants. Two cavids, however, show much higher δ13C—between −16‰ and −14‰—suggesting that maize- or kiwicha-based foods were more often fed to guinea pigs than to llamas and alpacas.
On the north coast of Peru, Takigami and colleagues (Reference Takigami, Uzawa, Seki, Morales Chocano and Yoneda2020) show that maize foddering of domesticated camelids was rare between 1200 and 800 BC (defined as the Initial period here), but significantly increased between 800 and 500 BC (our Early Formative). The data from Upanca are consistent with this pattern: the four camelids dating between 700 and 900 BC have low δ13C, with the single camelid dating to 300 BC showing higher δ13C at −16‰. Likewise, Mader and colleagues (Reference Mader, Hölzl, Heck, Reindel and Isla2018) suggest that many camelids in Palpa were subject to a diversified range of management strategies in the Late Formative. Our data are consistent with this finding.
The Nasca Period (AD 100–650)
Stratum A from Upanca is associated with the Nasca period, and particularly the Early Nasca epoch, during which sedentary agropastoral societies flourished. There are fewer flaked stone tools overall, but obsidian still dominates as a raw material among the formal tools (93%); however, debitage is dominated by local non-obsidian toolstone (64%). Paleobotanical remains from Stratum A are minimal but include some chenopod seeds. Isotopic signatures in human bone from other Nasca-period sites indicate that maize, kiwicha, or both were clearly staples of the diet at this time (Kellner and Schoeninger Reference Kellner and Schoeninger2012).
Isotopic values in Upanca camelids from Stratum A are highly variable, especially δ13C, suggesting a range of mixtures of C3 and C4 plants in fodder. Three of the camelids fall within the range expected for animals consuming highland browse, two fall within the range expected of wild coastal vegetation, one falls within the range expected of cultivated C4 crops, five are within the range expected of mixed C3–C4 diets with little or no fertilization, and two are in the range expected of C3 diets with fertilization. Similar high isotopic variation is observed among contemporaneous camelid remains from the north coast of Peru. Szpak and colleagues (Reference Szpak, Millaire, White and Longstaffe2014) show high inter- but low intra-animal variation in the Viru Valley, with little directional change between AD 1 and 1500. Their pattern suggests a consistent diet within the lifetime of an individual animal but significant variation in husbanding practices across animals. Together, the isotopic data imply that Nasca period inhabitants at Upanca used a wide range of strategies to raise camelids. An interesting pattern that needs further research is also highlighted in Figure 5, where higher values in δ13C are correlated with lower values in δ15N. This seems to suggest that even though C3 agricultural products were likely grown in fertilized fields, the C4 plants (most likely maize or kiwicha) were grown under different, likely unfertilized, conditions.
The three cavids from Stratum A suggest a mixed C3–C4 diet with little input of food from fertilized fields. Finally, five Stratum A cervid bones also show high inter-animal variation. This could represent deer browsing in fields near Upanca (e.g., Cormie and Schwarcz Reference Cormie and Schwarcz1994). Such a pattern has been observed among archaeological (Emery et al. Reference Emery, Wright and Schwarcz2000) and modern (Can-Hernández et al. Reference Can-Hernández, Villanueva-García, Gordillo-Chávez, Pacheco-Figueroa, Pérez-Netzahual and García-Morales2019; Flores-Armillas et al. Reference Flores-Armillas, López-Medellín, Barrios, MacGregor-Fors and Valenzuela-Galván2020; Hermira and Michalski Reference Hermira and Michalski2022) deer elsewhere in the Americas.
Conclusions
Data presented here paint a picture of evolving animal husbandry and agricultural practices in the Southern Nasca Region. Results demonstrate that isotopic variation increased markedly over time, reflecting wider diversity in camelid use, herding, and foddering in the Nasca period than in earlier Archaic periods. We highlight notable findings in this section.
First, the appearance of camelids with higher δ15N (>10.5‰), above what is predicted for natural local environments, begins in small numbers in the Middle Archaic Stratum D (n = 1 of 3) but increases markedly in the Late Archaic Stratum C (n = 4 of 9). We believe these values mark the onset of more intensive C3 agriculture in the region, with fertilization of fields by 2200 cal BC in the Late Archaic. These percentages continue to climb in Stratum B in the Initial/Formative period (n = 4 of 5) but decrease again in the Stratum A Nasca period (n = 2 of 11), when significant diversification in camelid husbanding practices emerged (see the later discussion). δ34S values indicate that this Late Archaic fertilizer was not marine derived (e.g., bird guano or marine shell) but likely was local animal or perhaps human waste.
Second, increasing δ13C values over time are inconsistent with broad paleoenvironmental changes, which should have resulted in decreasing δ13C. Instead, increasing δ13C must reflect a cultural process of intensifying C4 crop cultivation. No isotopic evidence for consumption of C4 plants is evident in Middle Archaic animals (though the sample size is small, n = 3; average δ13C = −18.6‰). Instead, this process begins slowly in the Late Archaic when average camelid δ13C increases to −18.2‰, and three of nine camelids, but none of two cavids, show slightly higher δ13C (> −17.5‰). This trend increases in Stratum B, where average camelid δ13C rises again to −17.2‰, and four of five camelids and both cavids display values over −17.5‰. Average camelid δ13C reaches its highest in the Nasca period (−15.9‰) when 11 of 13 camelids and all three cavids have values over −17.5‰. We believe much of this increase is caused by the introduction of maize and/or kiwicha farming. In short, the data suggest that limited maize or kiwicha farming began around 4200 cal BC, with increasing reliance on these crops through and into the Nasca period. Data also show that Nasca period camelid diets were most diverse, suggesting the widest range of feeding strategies to maintain flocks of animals later in time.
Third, variation in δ13C increases more slowly and at a later date than that of δ15N, suggesting that crop fertilization and intensification of C3 plants appeared first and that maize and/or kiwicha were integrated later and more slowly into the local economy. Significant consumption of C4 foods seems to have occurred first with cavids, likely as leftover food from human meals, and only later appeared in large amounts in the camelid diet. By the Nasca period, even some cervids have higher δ13C values and moderately high δ15N, above that expected of wild browse. This suggests that cervids may have been obtaining some of their food by grazing in Nasca fields, some with fertilized C3 and others with unfertilized C4 plants. Although deer are extirpated in the region today, deer field grazing and crop damage are well known in other areas of Latin America (Can-Hernández et al. Reference Can-Hernández, Villanueva-García, Gordillo-Chávez, Pacheco-Figueroa, Pérez-Netzahual and García-Morales2019; Flores-Armillas et al. Reference Flores-Armillas, López-Medellín, Barrios, MacGregor-Fors and Valenzuela-Galván2020; Hermira and Michalski Reference Hermira and Michalski2022). Unfortunately, we did not have enough older cervid samples to evaluate this behavior before the Nasca period.
Fourth, though we have fewer samples measured for δ34S, here too we see increasing variation over time, culminating in a peak in the Nasca period. As with δ13C and δ15N, this is consistent with a diversification process wherein Nasca people were moving camelids across the widest range of environments and feeding or foddering them on the broadest suite of foods. By contrast, the majority of Late Archaic camelids have what we believe to be a local mid-elevation sulfur isotopic signature, with one notable exception that may be from the highlands, indicating predominantly hunting of wild animals or foddering with local crops. This is also consistent with the C and N isotopes, which indicate mid- rather than high-elevation foraging. This is in marked contrast to the pattern in Palpa, where researchers suggest that Paracas populations, during what we refer to as the Formative period, were exploiting camelids that were mostly reared in the highlands (Mader Reference Mader2019; Mader et al. Reference Mader, Hölzl, Heck, Reindel and Isla2018). Our data from Upanca suggest less reliance on high-elevation foddering.
Ultimately, our work adds data to a poorly understood period in south coast archaeology. Although previous work in Ica, Palpa, and Nasca added to the archaeological understanding of diet, the isotopic data here demonstrate specific practices related to agricultural intensification and pastoralism that paint a different picture than what was previously understood.
Acknowledgments
We thank Wyndom Wescott, Susan Talcott, and Samantha Cramer for assistance in preparing samples for analysis; Joy Matthews and the UC Davis Stable Isotope Facility for assistance in running samples; and local Upanca community members for permission and assistance during excavations. We thank the three anonymous reviewers for their comments and insights on a previous draft.
Funding Statement
Funding for this research was provided, in part, by a grant from the Heinz Latin American Archaeology Program to JWE and KJV, and a Small Grant in Aid of Research from the UC Davis Academic Senate to JWE.
Data Availability Statement
All the original data used in this article are published in Table 2 and Supplemental Table 1.
Competing interests
The authors declare none.
Supplemental Material
For supplemental material accompanying this article, visit https://doi.org/10.1017/laq.2023.44.
Supplemental Table 1. Stratigraphic, Chronological, and Stable Isotope Data for Animal Remains Included in Study.